Abstract
An international consensus document was recently published and provides guidance on the diagnosis, treatment and monitoring of late-onset hypogonadism (LOH) in men. The diagnosis of LOH requires biochemical and clinical components. Controversy in defining the clinical syndrome continues due to the high prevalence of hypogonadal symptoms in the aging male population and the non-specific nature of these symptoms. Further controversy surrounds setting a lower limit of normal testosterone, the limitations of the commonly available total testosterone result in assessing some patients and the unavailability of reliable measures of bioavailable or free testosterone for general clinical use. As with any clinical intervention testosterone treatment should be judged on a balance of risk versus benefit. The traditional benefits of testosterone on sexual function, mood, strength and quality of life remain the primary goals of treatment but possible beneficial effects on other parameters such as bone density, obesity, insulin resistance and angina are emerging and will be reviewed. Potential concerns regarding the effects of testosterone on prostate disease, aggression and polycythaemia will also be addressed. The options available for treatment have increased in recent years with the availability of a number of testosterone preparations which can reliably produce physiological serum concentrations.
Keywords:
Introduction
The prevalence of biochemical testosterone deficiency increases with age. This is partly due to decreasing testosterone levels associated with illness or debility but there is also convincing epidemiological data to show that serum free and total testosterone levels also fall with normal aging (CitationHarman et al 2001; CitationFeldman et al 2002). The symptoms of aging include tiredness, lack of energy, reduced strength, frailty, loss of libido, decreased sexual performance depression and mood change. Men with hypogonadism experience similar symptoms. This raises the question of whether some symptoms of aging could be due to relative androgen deficiency. On the other hand, similarities between normal aging and the symptoms of mild androgen deficiency make the clinical diagnosis of hypogonadism in aging men more challenging.
Epidemiological studies suggest that many significant clinical findings and important disease states are linked to low testosterone levels. These include osteoporosis (CitationCampion and Maricic 2003), Alzheimer’s disease (CitationMoffat et al 2004), frailty, obesity (CitationSvartberg, von Muhlen, Sundsfjord et al 2004), diabetes (CitationBarrett-Connor 1992), hypercholesterolemia (CitationHaffner et al 1993; CitationVan Pottelbergh et al 2003), hypertension (CitationPhillips et al 1993), cardiac failure (CitationTappler and Katz 1979; CitationKontoleon et al 2003) and ischemic heart disease (CitationBarrett-Connor and Khaw 1988). The extent to which testosterone deficiency is involved in the pathogenesis of these conditions, or to which testosterone supplementation could be useful in their treatment is an area of great interest with many unanswered questions.
This paper will aim to review the current evidence of clinical effects of testosterone treatment within an aging male population. As with any other clinical intervention a decision to treat patients with testosterone requires a balance of risk versus benefit. We shall try to facilitate this by examining the effects of testosterone on the various symptoms and organs involved.
Diagnosing late-onset hypogonadism
‘A clinical and biochemical syndrome associated with advancing age and characterised by typical symptoms and a deficiency in serum testosterone levels. It [late-onset hypogonadism] may result in significant detriment in the quality of life and adversely affect the function of multiple organ systems’ (CitationNieschlag et al 2005).
— ISA, ISSAM, and EAU recommendations.
Male hypogonadism is a clinical syndrome caused by a lack of androgens or their action. Causes of hypogonadism may reflect abnormalities of the hypothalamus, pituitary, testes or target tissues. Increases in the amount of testosterone converted to estrogen under the action of the enzyme aromatase may also contribute to hypogonadism. Most aspects of the clinical syndrome are unrelated to the location of the cause. A greater factor in the production of a clinical syndrome is the age of onset. The development of hypogonadism with aging is known as late-onset hypogonadism and is characterised by loss of vitality, fatigue, loss of libido, erectile dysfunction, somnolence, depression and poor concentration. Hypogonadal ageing men also gain fat mass and lose bone mass, muscle mass and strength.
The diagnosis of late-onset hypogonadism requires the combination of low serum testosterone levels with symptoms of hypogonadism. Questionnaires are available which check for the symptoms of hypogonadism. These have been validated for the assessment of aging patients with hypogonadism (CitationMorley et al 2000; CitationMoore et al 2004) but have a low specificity. In view of the overlap in symptoms between hypogonadism, aging and other medical conditions it is wise to use a formal method of symptom assessment which can be used to monitor the effects of testosterone replacement.
In order to discuss the biochemical diagnosis of hypogonadism it is necessary to outline the usual carriage of testosterone in the blood. Total serum testosterone consists of free testosterone (2%–3%), testosterone bound to sex hormone binding globulin (SHBG) (45%) and testosterone bound to other proteins (mainly albumin −50%) (CitationDunn et al 1981). Testosterone binds only loosely to albumin and so this testosterone as well as free testosterone is available to tissues and is termed bioavailable testosterone. Testosterone bound to SHBG is tightly bound and is biologically inactive. Bioavailable and free testosterone are known to correlate better than total testosterone with clinical sequelae of androgenization such as bone mineral density and muscle strength (CitationKhosla et al 1998; CitationRoy et al 2002). There is diurnal variation in serum testosterone levels with peak levels seen in the morning following sleep, which can be maintained into the seventh decade (CitationDiver et al 2003). Samples should always be taken in the morning before 11 am to allow for standardization.
The reliable measurement of serum free testosterone requires equilibrium dialysis. This is not appropriate for clinical use as it is very time consuming and therefore expensive. The amount of bioavailable testosterone can be measured as a percentage of the total testosterone after precipitation of the SHBG bound fraction using ammonium sulphate. The bioavailable testosterone is then calculated from the total testosterone level. This method has an excellent correlation with free testosterone (CitationTremblay and Dube 1974) but is not widely available for clinical use. In most clinical situations the available tests are total testosterone and SHBG which are both easily and reliably measured. Total testosterone is appropriate for the diagnosis of overt male hypogonadism where testosterone levels are very low and also in excluding hypogonadism in patients with normal/high-normal testosterone levels. With increasing age, a greater number of men have total testosterone levels just below the normal range or in the low-normal range. In these patients total testosterone can be an unreliable indicator of hypogonadal status. There are a number of formulae that calculate an estimated bioavailable or free testosterone level using the SHBG and total testosterone levels. Some of these have been shown to correlate well with laboratory measures and there is evidence that they more reliably indicate hypogonadism than total testosterone in cases of borderline biochemical hypogonadism (CitationVermeulen et al 1971; CitationMorris et al 2004). It is important that such tests are validated for use in patient populations relevant to the patient under consideration.
Recently, a panel with cooperation from international andrology and urology societies, published specific recommendations with regard to the diagnosis of Late-onset Hypogonadism (CitationNieschlag et al 2005). These are summarized in the following text. It is advised that at least two serum testosterone measurements, taken before 11 am on different mornings, are necessary to confirm the diagnosis. The second sample should also include measurement of gonadotrophin and prolactin levels, which may indicate the need for further investigations for pituitary disease. Patients with serum total testosterone consistently below 8 nmol/l invariably demonstrate the clinical syndrome of hypogonadism and are likely to benefit from treatment. Patients with serum total testosterone in the range 8–12 nmol/l often have symptoms attributable to hypogonadism and it may be decided to offer either a clinical trial of testosterone treatment or to make further efforts to define serum bioavailable or free testosterone and then reconsider treatment. Patients with serum total testosterone persistently above 12 nmol/l do not have hypogonadism and symptoms are likely to be due to other disease states or ageing per se so testosterone treatment is not indicated.
Changes in testosterone levels with aging
Longitudinal studies in male aging studies have shown that serum testosterone levels decline with age (CitationHarman et al 2001; CitationFeldman et al 2002). Total testosterone levels fall at an average of 1.6% per year whilst free and bioavailable levels fall by 2%–3% per year. The reduction in free and bioavailable testosterone levels is larger because aging is also associated with increases in SHBG levels (CitationFeldman et al 2002). Cross-sectional data supports these trends but has usually shown smaller reductions in testosterone levels with aging (CitationFeldman et al 2002). This is likely to reflect strict entry criteria to cross-sectional studies so that young healthy men are compared to older healthy men. During the course of longitudinal studies some men may develop pathologies which accentuate decreases in testosterone levels.
The changes in average serum testosterone levels with aging mean that the proportion of men fulfilling a biochemically defined diagnosis of hypogonadism increases with aging. Twenty percent of men aged over 60 have total testosterone levels below the normal range and the figure rises to 50% in those aged over 80. The figures concerning free testosterone are even higher as would be expected in view of the concurrent decrease in SHBG levels (CitationHarman et al 2001).
The mechanism of age related decreases in serum testosterone levels has also been the subject of investigation. Metabolic clearance declines with age but this effect is less pronounced than a reduction in testosterone production, so the overall effect is to reduce serum testosterone levels. Gonadotrophin levels rise during aging (CitationFeldman et al 2002) and testicular secretory responses to recombinant human chorionic gonadotrophin (hCG) are reduced (CitationMulligan et al 1999, Citation2001). This implies that the reduced production may be caused by primary testicular failure but in fact these changes are not adequate to fully explain the fall in testosterone levels. There are changes in the lutenising hormone (LH) production which consist of decreased LH pulse frequency and amplitude, (CitationVeldhuis et al 1992; CitationPincus et al 1997) although pituitary production of LH in response to pharmacological stimulation with exogenous GnRH analogues is preserved (CitationMulligan et al 1999). It therefore seems likely that there are changes in endogenous production of GnRH which underlie the changes in LH secretion and have a role in the age related decline in testosterone. Thus the decreases in testosterone levels with aging seem to reflect changes at all levels of the hypothalamic-pituitary-testicular axis. With advancing age there is also a reduction in androgen receptor concentration in some target tissues and this may contribute to the clinical syndrome of LOH (CitationOno et al 1988; CitationGallon et al 1989).
Interpretation of clinical trials of testosterone treatment
Before assessing the evidence of testosterone’s action in the aging male it is important to note certain methodological considerations which are common to the interpretation of any clinical trial of testosterone replacement. Many interventional trials of the effects of testosterone on human health and disease have been conducted. There is considerable heterogenicity in terms of study design and these differences have a potential to significantly affect the results seen in various studies. Gonadal status at baseline and the testosterone level produced by testosterone treatment in the study are of particular importance because the effects of altering testosterone from subphysiological to physiological levels may be different from those of altering physiological levels to supraphysiological. Another important factor is the length of treatment. Randomised controlled trials of testosterone have ranged from one to thirty-six months in duration (CitationIsidori et al 2005) although some uncontrolled studies have lasted up to 42 months. Many effects of testosterone are thought to fully develop in the first few months of treatment but effects on bone, for example, have been shown to continue over two years or more (CitationSnyder et al 2000; CitationWang, Cunningham et al 2004).
Late onset hypogonadism reflects a particular pathophysiology and it may not be appropriate to extrapolate results from studies concerning the effects of testosterone in treating hypogonadism of other etiology to aging males. For this reason, the age of men treated in clinical trials is certainly relevant. Other important factors include patient comorbidities and the preparation and route of testosterone replacement used in the study, which can affect the production of estrogen and dihydrotestosterone, testosterone’s active metabolites
Effects on bone and osteoporosis
Osteoporosis refers to pathological loss of bone density and strength. It is an important condition due to its prevalence and association with bone fractures; most commonly of the hip, vertebra and forearm. Men are relatively protected from the development of osteoporosis by a higher peak bone mass compared with women (CitationCampion and Maricic 2003). Furthermore, women lose bone at an accelerated rate immediately following the menopause. Nevertheless, men start to lose bone mass during early adult life and experience an increase in the rate of bone loss with age (CitationScopacasa et al 2002). Women of a given age have a higher prevalence of osteoporosis in comparison to men but the prevalence increases with age in both sexes. As a result, men have a lower incidence of osteoporotic fractures than women of a given age but the gap between the sexes narrows with advancing age (CitationChang et al 2004) and there is evidence that hip fractures in men are associated with greater mortality than in women (CitationCampion and Maricic 2003).
A number of epidemiological studies have found that bone mineral density in the aging male population is positively associated with endogenous androgen levels (CitationMurphy et al 1993; CitationOngphiphadhanakul et al 1995; CitationRucker et al 2004). Testosterone levels in young men have been shown to correlate with bone size, indicating a role in determination of peak bone mass and protection from future osteoporosis (CitationLorentzon et al 2005). Male hypogonadism has been shown to be a risk factor for hip fracture (CitationJackson et al 1992) and a recent study showed a high prevalence of hypogonadism in a group of male patients with average age 75 years presenting with minimal trauma fractures compared to stroke victims who acted as controls (CitationLeifke et al 2005). Estrogen is a well known determinant of bone density in women and some investigators have found serum estrogen to be a strong determinant of male bone density (CitationKhosla et al 1998; CitationKhosla et al 2001). Serum estrogen was also found to correlate better than testosterone with peak bone mass (CitationKhosla et al 2001) but this is in contradiction of a more recent study showing a negative correlation of estrogen with peak bone size (CitationLorentzon et al 2005). Men with aromatase deficiency (CitationCarani et al 1997) or defunctioning estrogen receptor mutations (CitationSmith et al 1994) have been found to have abnormally low bone density despite normal or high testosterone levels which further emphasizes the important influence of estrogen on male bone density.
A large number of trials have demonstrated a positive effect of testosterone treatment on bone mineral density (CitationKatznelson et al 1996; CitationBehre et al 1997; CitationLeifke et al 1998; CitationSnyder et al 2000; CitationZacharin et al 2003; CitationWang, Cunningham et al 2004; CitationAminorroaya et al 2005; CitationBenito et al 2005) and bone architecture (CitationBenito et al 2005). These effects are often more impressive in longer trials, which have shown that adequate replacement will lead to near normal bone density but that the full effects may take two years or more (CitationSnyder et al 2000; CitationWang, Cunningham et al 2004; CitationAminorroaya et al 2005). Three randomized placebo-controlled trials of testosterone treatment in aging males have been conducted (CitationSnyder et al 1999; CitationKenny et al 2001; CitationAmory et al 2004). One of these studies concerned men with a mean age of 71 years with two serum testosterone levels less than 12.1nmol/l. After 36 months of intramuscular testosterone treatment or placebo, there were significant increases in vertebral and hip bone mineral density. In this study, there was also a significant decrease in the bone resorption marker urinary deoxypyridinoline with testosterone treatment (CitationAmory et al 2004). The second study contained men with low bioavailable testosterone levels and an average age of 76 years. Testosterone treatment in the form of transdermal patches was given for 1 year. During this trial there was a significant preservation of hip bone mineral density with testosterone treatment but testosterone had no effect on bone mineral density at other sites including the vertebrae. There were no significant alterations in bone turnover markers during testosterone treatment (CitationKenny et al 2001). The remaining study contained men of average age 73 years. Men were eligible for the study if their serum total testosterone levels were less than 16.5 nmol/L, meaning that the study contained men who would usually be considered eugonadal. The beneficial effects of testosterone on bone density were confined to the men who had lower serum testosterone levels at baseline and were seen only in the vertebrae. There were no significant changes in bone turnover markers. Testosterone in the trial was given via scrotal patches for a 36 month duration (CitationSnyder et al 1999). A recent meta-analysis of the effects on bone density of testosterone treatment in men included data from these studies and two other randomized controlled trials. The findings were that testosterone produces a significant increase of 2.7% in the bone mineral density at the lumber spine but no overall change at the hip (CitationIsidori et al 2005). These results from randomized controlled trials in aging men show much smaller benefits of testosterone treatment on bone density than have been seen in other trials. This could be due to the trials including patients who are not hypogonadal and being too short to allow for the maximal effects of testosterone. The meta-analysis also assessed the data concerning changes of bone formation and resorption markers during testosterone treatment. There was a significant decrease in bone resorption markers but no change in markers of bone formation suggesting that reduction of bone resorption may be the primary mode of action of testosterone in improving bone density (CitationIsidori et al 2005).
Overall, it seems that both estrogen and testosterone are important for normal bone growth and maintenance. Deficiency or failure of action of the sex hormones is associated with osteoporosis and minimal trauma fractures. Estrogen in males is produced via metabolism of testosterone by aromatase and it is therefore important that androgens used for the treatment of hypogonadism be amenable to the action of aromatase to yield maximal positive effects on bone. There is data showing that testosterone treatment increases bone mineral density in aging males but that these benefits are confined to hypogonadal men. The magnitude of this improvement is greater in the spine than in the hip and further studies are warranted to confirm or refute any differential effects of testosterone at these important sites. Improvements seen in randomized controlled trials to date may underestimate true positive effects due to relatively short duration and/or baseline characteristics of the patients involved. There is no data as yet to confirm that the improvement in bone density with testosterone treatment reduces fractures in men and this is an important area for future study.
Testosterone and body composition
Changes in body composition are seen with aging. In general terms, aging males are prone to loss of muscle mass and a gain in fat mass, especially in the form of visceral or central fat. An epidemiological study of community dwelling men aged between 24 and 85 years has confirmed that total and free testosterone levels are inversely correlated with waist circumference and that testosterone levels are specifically related to this measure of central obesity rather than general obesity (CitationSvartberg, von Muhlen, Sundsfjord et al 2004). Prospective studies show that testosterone levels predict future development of central obesity (CitationKhaw and Barrett-Connor 1992; CitationTsai et al 2000). Reductions in free testosterone also correlate with age related declines in fat free mass (muscle mass) and muscle strength (CitationBaumgartner et al 1999; CitationRoy et al 2002). Studies in hypogonadal men confirm an increase in fat mass and decrease in fat free mass versus comparable eugonadal men (CitationKatznelson et al 1998). Taken together, the epidemiological data suggest that a hypogonadal state promotes loss of muscle mass and a gain in fat mass, particularly visceral fat and therefore mimics the changes of ‘normal’ aging.
A number of research groups have tried to further define the relationship of testosterone and body composition by artificial alteration of testosterone levels in eugonadal populations. Induction of a hypogonadal state in healthy men (CitationMauras et al 1998) or men with prostate cancer (CitationSmith et al 2001) using a gonadotrophin-releasing-hormone (GnRH) analogue was shown to produce increases in fat mass and decreased fat free mass. Another experimental approach in healthy men featured suppression of endogenous testosterone production with a GnRH analogue, followed by treatment with different doses of weekly intramuscular testosterone esters for 20 weeks. Initially the experiments involved men aged 18–35 years (CitationBhasin et al 2001) but subsequently the study was repeated with a similar protocol in men aged 60–75 years (CitationBhasin et al 2005). The different doses given were shown to produce a range of serum concentrations from subphysiological to supraphysiological (CitationBhasin et al 2001). A given testosterone dose produced higher serum concentrations of testosterone in the older age group (CitationBhasin et al 2005). Subphysiological dosing of testosterone produced a gain in fat mass and loss of fat free mass during the study. There were sequential decreases in fat mass and increases in fat free mass with each increase of testosterone dose. These changes in body composition were seen in physiological and supraphysiological treatment doses. The trend was similar in younger versus older men but the gain of fat mass at the lowest testosterone dose was less prominent in older patients (CitationBhasin et al 2001; CitationBhasin et al 2005). With regard to muscle function, the investigators showed dose dependent increases in leg strength and power with testosterone treatment in young and older men but there was no improvement in fatigability (CitationStorer et al 2003; CitationBhasin et al 2005).
Many clinical studies have looked at the effect of testosterone treatment on body composition in hypogonadal men or men with borderline low testosterone levels. Some of these studies specifically examine these changes in older men (CitationTenover 1992; CitationMorley et al 1993; CitationUrban et al 1995; CitationSih et al 1997; CitationSnyder et al 1999; CitationKenny et al 2001; CitationFerrando et al 2002; CitationSteidle et al 2003; CitationPage et al 2005). The data from studies, on patients from all age groups, are consistent in showing an increase in fat free mass and decrease in fat mass or visceral adiposity with testosterone treatment. A recent meta-analysis of 16 randomized controlled trials of testosterone treatment effects on body composition confirms this pattern (CitationIsidori et al 2005). There have been less consistent results with regard to the effects of testosterone treatment of muscle strength. Some studies have shown an increase in muscle strength (CitationFerrando et al 2002; CitationPage et al 2005) with testosterone whilst others have not (CitationSnyder et al 1999). Within the same trial some muscle group strengths may improve whilst others do not (CitationLy et al 2001). It is likely that the differences are partly due to the methodological variations in assessing strength, but it also possible that testosterone has different effects on the various muscle groups. The meta-analysis found trends toward significant improvements in dominant knee and hand grip strength only (CitationIsidori et al 2005).
Overall there is evidence that testosterone treatment increases lean body mass and reduces obesity, particularly visceral obesity, in a variety of populations including aging men. With regard to muscle changes, some studies demonstrate improvements in maximal strength but the results are inconsistent and it has not been demonstrated that these changes lead to clinically important improvements in mobility, endurance or quality of life. Studies are needed to clarify this. Changes in abdominal obesity are particularly important as visceral fat is now recognised as predisposing the metabolic syndrome, diabetes and cardiovascular disease.
Testosterone, diabetes and the metabolic syndrome
Type 2 diabetes is an important condition in terms of morbidity and mortality, and the prevalence is increasing in the developed and developing world. The prevalence also increases with age. Insulin resistance is a primary pathological feature of type 2 diabetes and predates the onset of diabetes by many years, during which time raised serum insulin levels compensate and maintain normoglycemia. Insulin resistance and/or impaired glucose tolerance are also part of the metabolic syndrome which also comprises an abnormal serum lipid profile, central obesity and hypertension. The metabolic syndrome can be considered to be a pre-diabetic condition and is itself linked to cardiovascular mortality. shows the three commonly used definitions of the metabolic syndrome as per WHO, NCEPIII and IDF respectively (CitationWHO 1999; CitationNCEPIII 2001; CitationZimmet et al 2005).
Table 1 Definition of the metabolic syndrome
Epidemiological evidence supports a link between testosterone and glucose metabolism. Studies in non-diabetic men have found an inverse correlation of total or free testosterone with glucose and insulin levels (CitationSimon et al 1992; CitationHaffner et al 1994) and studies show lower testosterone levels in patients with the metabolic syndrome (CitationLaaksonen et al 2003; CitationMuller et al 2005; CitationKupelian et al 2006) or diabetes (CitationBarrett-Connor 1992; CitationAndersson et al 1994; CitationRhoden et al 2005). A study of patients with type 2 diabetes using measurement of serum free testosterone by the gold standard method of equilibrium dialysis, found a 33% prevalence of biochemical hypogonadism (CitationDhindsa et al 2004). The Barnsley study demonstrated a high prevalence of clinical and biochemical hypogonadism with 19% having total testosterone levels below 8 nmol/l and a further 25% between 8–12 nmol/l (CitationKapoor, Aldred et al 2007). There are also a number longitudinal studies linking low serum testosterone levels to the future development of the metabolic syndrome (CitationLaaksonen et al 2004) or type 2 diabetes (CitationHaffner et al 1996; CitationTibblin et al 1996; CitationStellato et al 2000; CitationOh et al 2002; CitationLaaksonen et al 2004), indicating a possible role of hypogonadism in the pathogenesis of type 2 diabetes in men. Alternatively, it has been postulated that obesity may be the common link between low testosterone levels and insulin resistance, diabetes and cardiovascular disease (CitationPhillips et al 2003; CitationKapoor et al 2005). With regard to this hypothesis, study findings vary as to whether the association of testosterone with diabetes occurs independently of obesity (CitationHaffner et al 1996; CitationLaaksonen et al 2003; CitationRhoden et al 2005).
Clinical trials of the effect of testosterone on glucose metabolism in men have occurred in diabetic and non-diabetic populations. Data specific to aging males is not available. A series of studies investigated the effects of testosterone or dihydrotestosterone given for 6 weeks or 3 months to middle aged, non-diabetic obese men (CitationMarin, Holmang et al 1992; CitationMarin, Krotkiewski et al 1992; CitationMarin et al 1993). It was found that physiological treatment doses led to improved insulin resistance, as measured by the gold standard technique using a euglycemic clamp and/or serum glucose and insulin responses during glucose tolerance test. These improvements were associated with decreased central obesity, measured by computered tomography (CT) or waist-hip ratio, without reduced total fat mass. Insulin resistance improved more with testosterone than dihydrotestosterone treatment and beneficial effects were greater in men with lower baseline testosterone levels. Increasing testosterone levels into the supraphysiological range lead to decreased glucose tolerance.
Trials of testosterone treatment in men with type 2 diabetes have also taken place. A recent randomized controlled crossover trial assessed the effects of intramuscular testosterone replacement to achieve levels within the physiological range, compared with placebo injections in 24 men with diabetes, hypogonadism and a mean age of 64 years (CitationKapoor et al 2006). Ten of these men were insulin treated. Testosterone treatment led to a significant reduction in glycated hemoglobin (HbA1C) and fasting glucose compared to placebo. Testosterone also produced a significant reduction in insulin resistance, measured by the homeostatic model assessment (HOMA), in the fourteen non-insulin treated patients. It is not possible to measure insulin resistance in patients treated with insulin but five out of ten of these patients had a reduction of insulin dose during the study. Other significant changes during testosterone treatment in this trial were reduced total cholesterol, waist circumference and waist-hip ratio. Similarly, a placebo-controlled but non-blinded trial in 24 men with visceral obesity, diabetes, hypogonadism and mean age 57 years found that three months of oral testosterone treatment led to significant reductions in HbA1C, fasting glucose, post-prandial glucose, weight, fat mass and waist-hip ratio (CitationBoyanov et al 2003). In contrast, an uncontrolled study of 150 mg intramuscular testosterone given to 10 patients, average age 64 years, with diabetes and hypogonadism found no significant change in diabetes control, fasting glucose or insulin levels (CitationCorrales et al 2004). Another uncontrolled study showed no beneficial effect of testosterone treatment on insulin resistance, measured by HOMA and ‘minimal model’ of area under acute insulin response curves, in 11 patients with type 2 diabetes aged between 33 and 73 years (CitationLee et al 2005). Body mass index was within the normal range in this population and there was no change in waist-hip ratio or weight during testosterone treatment. Baseline testosterone levels were in the low-normal range and patients received a relatively small dose of 100 mg intramuscular testosterone every three weeks. A good increase in testosterone levels during the trial is described but it is not stated at which time during the three week cycle the testosterone levels were tested, so the lack of response could reflect an insufficient overall testosterone dose in the trial period.
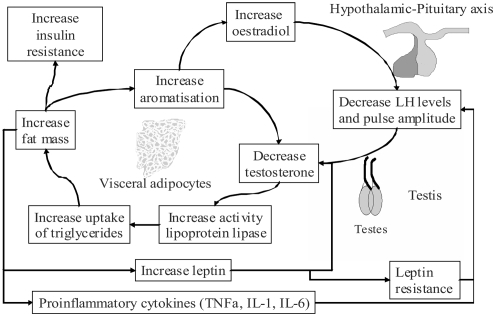
Findings that improvements in serum glucose, serum insulin, insulin resistance or glycemic control, in men treated with testosterone are accompanied by reduced measures of central obesity, are in line with other studies showing a specific effect of testosterone in reducing central or visceral obesity (CitationRebuffe-Scrive et al 1991; CitationMarin, Holmang et al 1992). Furthermore, studies that have shown neutral effects of testosterone on glucose metabolism have not measured (CitationCorrales et al 2004), or shown neutral effects (CitationLee et al 2005) (CitationTripathy et al 1998; CitationBhasin et al 2005) on central obesity. Given the known association of visceral obesity with insulin resistance, it is possible that testosterone treatment of hypogonadal men acts to improve insulin resistance and diabetes through an effect in reducing central obesity. This effect can be explained by the action of testosterone in inhibiting lipoprotein lipase and thereby reducing triglyceride uptake into adipocytes (CitationSorva et al 1988), an action which seems to occur preferentially in visceral fat (CitationMarin et al 1995; CitationMarin et al 1996). Visceral fat is thought to be more responsive to hormonal changes due to a greater concentration of androgen receptors and increased vascularity compared with subcutaneous fat (CitationBjorntorp 1996). Further explanation of the links between hypogonadism and obesity is offered by the hypogonadal-obesity-adipocytokine cycle hypothesis (see ). In this model, increases in body fat lead to increases in aromatase levels, in addition to insulin resistance, adverse lipid profiles and increased leptin levels. Increased action of aromatase in metabolizing testosterone to estrogen, reduces testosterone levels which induces further accumulation of visceral fat. Higher leptin levels and possibly other factors, act at the pituitary to suppress gonadotrophin release and exacerbate hypogonadism (CitationCohen 1999; CitationKapoor et al 2005). Leptin has also been shown to reduce testosterone secretion from rodent testes in vitro (CitationTena-Sempere et al 1999). A full review of the relationship between testosterone, insulin resistance and diabetes can be found elsewhere (CitationKapoor et al 2005; CitationJones 2007).
Figure 1 The hypogonadal-obesity-adipocytokine cycle hypothesis. Adipose tissue contains the enzyme aromatase which metabolises testosterone to oestrogen. This results in reduced testosterone levels, which increase the action of lipoprotein lipase and increase fat mass, thus increasing aromatisation of testosterone and completing the cycle. Visceral fat also promotes lower testosterone levels by reducing pituitary LH pulse amplitude via leptin and/or other factors. In vitro studies have shown that leptin also inhibits testosterone production directly at the testes. Visceral adiposity could also provide the link between testosterone and insulin resistance (CitationJones 2007).
Testosterone, cardiovascular disease, and atherosclerosis
Cardiovascular disease, and its underlying pathological process atherosclerosis, is an important cause of morbidity and mortality in the developed and developing world. Coronary heart disease in particular is the commonest cause of death worldwide (CitationAHA 2002; CitationMacKay and Mensah 2004). As well as increasing with age, this disease is more common in the male versus female population internationally, which has led to interest in the potential role of sex hormones in modulating risk of development of atherosclerosis. Concerns about the potential adverse effects of testosterone treatment on cardiovascular disease have previously contributed to caution in prescribing testosterone to those who have, or who are at risk of, cardiovascular disease. Contrary to fears of the potential adverse effects of testosterone on cardiovascular disease, there are over forty epidemiological studies which have examined the relationship of testosterone levels to the presence or development of coronary heart disease, and none have shown a positive correlation. Many of these studies have found the presence of coronary heart disease to be associated with low testosterone levels (Reviews: CitationJones, Jones et al 2003; CitationJones et al 2005).
Epidemiological studies have also assessed links between serum testosterone and non-coronary atherosclerosis. A study of over 1000 people aged 55 years and over found an inverse correlation between serum total and bioavailable testosterone and the amount of aortic atherosclerosis in men, as assessed by radiological methods (CitationHak et al 2002). Increased intima-media thickness (IMT) is an early sign of atherosclerosis and has also been shown to predict cardiovascular mortality (CitationMurakami et al 2005). Cross-sectional studies have found that testosterone levels are negatively correlated with carotid IMT in independently living men aged 74–93 years (Citationvan den Beld et al 2003), diabetic men (CitationFukui et al 2003) and young obese men (CitationDe Pergola et al 2003). A 4-year follow up study of the latter population showed that free testosterone was also inversely correlated with the rate of increase of IMT (CitationMuller et al 2004).
This evidence, together with the beneficial effects of testosterone replacement on central obesity and diabetes, raises the question whether testosterone treatment could be beneficial in preventing or treating atherosclerosis. No trial of sufficient size or duration has investigated the effect of testosterone replacement in primary or secondary prevention cardiovascular disease. The absence of such data leads us to examine the relationship of testosterone to other cardiovascular risk factors, such as adverse lipid parameters, blood pressure, endothelial dysfunction, coagulation factors, inflammatory markers and cytokines. This analysis can supply evidence of the likely effects of testosterone on overall cardiovascular risk. This has limitations, however, including the potential for diverging effects of testosterone on the various factors involved and the resultant impossibility of accurately predicting the relative impact of such changes.
Epidemiological data has associated low testosterone levels with atherogenic lipid parameters, including lower HDL cholesterol (CitationLichtenstein et al 1987; CitationHaffner et al 1993; CitationVan Pottelbergh et al 2003) and higher total cholesterol (CitationHaffner et al 1993; CitationVan Pottelbergh et al 2003), LDL cholesterol (CitationHaffner et al 1993) and triglyceride levels (CitationLichtenstein et al 1987; CitationHaffner et al 1993). Furthermore, these relationships are independent of other factors such as age, obesity and glucose levels (CitationHaffner et al 1993; CitationVan Pottelbergh et al 2003). Interventional trails of testosterone replacement have shown that treatment causes a decrease in total cholesterol. A recent meta-analysis of 17 randomized controlled trials confirmed this and found that the magnitude of changes was larger in trials of patients with lower baseline testosterone levels (CitationIsidori et al 2005). The same meta-analysis found no significant overall change in LDL or HDL cholesterol levels but in trials with baseline testosterone levels greater than 10 nmol/l, there was a small reduction in HDL cholesterol with testosterone treatment.
Studies also show a consistent negative correlation of testosterone with blood pressure (CitationBarrett-Connor and Khaw 1988; CitationKhaw and Barrett-Connor 1988; CitationSvartberg, von Muhlen, Schirmer et al 2004). Data specific to the ageing male population suggests that this relationship is particularly powerful for systolic hypertension (CitationFogari et al 2005). Interventional trials have not found a significant effect of testosterone replacement on blood pressure (CitationKapoor et al 2006).
There is a negative correlation of testosterone levels with plasminogen activator inhibitor-1 (PAI-1) (CitationGlueck et al 1993; CitationPhillips 1993), which is a major prothrombotic factor and known to be associated with progression of atherosclerosis, as well as other prothrombotic factors fibrinogen, α2-antiplasmin and factor VII (CitationBonithon-Kopp et al 1988; CitationGlueck et al 1993; CitationPhillips 1993; CitationDe Pergola et al 1997). There is a positive correlation with tissue plasminogen activator (tPA) which is one of the major fibrinolytic agents (CitationGlueck et al 1993). Interventional trials have shown a neutral effect of physiological testosterone replacement on the major clotting factors (CitationSmith et al 2005) but supraphysiological androgen administration can produce a temporary mild pro-coagulant effect (CitationAnderson et al 1995).
A recent study compared total and bioavailable testosterone levels with inflammatory cytokines in men aged 65 and over. There was an inverse correlation with the pro-inflammatory soluble interleukin-6 receptor, but no association with interleukin-6 (IL-6), highly sensitive CRP (hsCRP), tumor necrosis factor-α (TNF-α) or interleukin-1β (IL-1β (CitationMaggio et al 2006). Another trial found that young men with idiopathic hypogonadotrophic hypogonadism had higher levels of proinflammatory factors interleukin-2 (IL-2), interleukin-4 (IL-4), complement C3c and total immunoglobulin in comparison to controls (CitationYesilova et al 2000). Testosterone treatment in a group of hypogonadal men, mostly with known coronary artery disease, induced anti-inflammatory changes in the cytokine profile of reduced IL-1β and TNF-α and increased IL-10 (CitationMalkin, Pugh, Jones et al 2004).
A related issue is the potential use of testosterone as a coronary vasodilator and anti-anginal agent. Testosterone has been shown to act as a vasodilator of coronary arteries at physiological concentrations during angiography (CitationWebb, McNeill et al 1999). Furthermore men given a testosterone injection prior to exercise testing showed improved performance, as assessed by ST changes compared to placebo (CitationRosano et al 1999; CitationWebb, Adamson et al 1999). Administration of one to three months of testosterone treatment has also been shown to improve symptoms of angina and exercise test performance (CitationWu and Weng 1993; CitationEnglish et al 2000; CitationMalkin, Pugh, Morris et al 2004). Longer term studies are underway. It is thought that testosterone improves angina due its vasodilatory action, which occurs independently of the androgen receptor, via blockade of L-type calcium channels at the cell membrane of the vascular smooth muscle in an action similar to the dihydropyridine calcium-channel blockers such as nifedipine (CitationHall et al 2006).
In summary, low testosterone levels are linked to the presence of numerous cardiovascular risk factors. Testosterone treatment acts to improve some of these factors, but effects may vary according to pre- and post-treatment testosterone levels, as well as other factors. There is little data from trials specific to aging males. Appropriately-powered randomized controlled trials, with cardiovascular disease primary endpoints, are needed to clarify the situation, but in the meantime the balance of evidence is that testosterone has either neutral or beneficial effects on the risk of cardiovascular disease in men. It is particularly important to define the effect of testosterone treatment on cardiovascular disease in view of its potential use as an anti-anginal agent.
Testosterone and cardiac failure
Studies have demonstrated reduced testosterone levels in men with heart failure as well as other endocrine changes (CitationTappler and Katz 1979; CitationKontoleon et al 2003). Treatment of cardiac failure with chronic mechanical circulatory support normalizes many of these changes, including testosterone levels (CitationNoirhomme et al 1999). More recently, two double-blind randomized controlled trials of testosterone treatment for men with low or low-normal serum testosterone levels and heart failure have shown improvements in exercise capacity and symptoms (CitationPugh et al 2004; CitationMalkin et al 2006). The mechanism of these benefits is currently unclear, although a study of the acute effects of buccal testosterone given to men with chronic cardiac failure under invasive monitoring showed that testosterone increased cardiac index and reduced systemic vascular resistance (CitationPugh et al 2003). Testosterone may prove useful in the management of cardiac failure but further research is needed.
Testosterone and the prostate
The normal development of the prostate gland is dependent on the action of testosterone via the androgen receptor, and abnormal biosynthesis of the hormone or inactivating mutations of the androgen receptor are associated with a rudimentary prostate gland. Testosterone also requires conversion to dihydrotestosterone in the prostate gland for full activity. In view of this link between testosterone and prostate development, it is important to consider the impact that testosterone replacement may have on the prevalence and morbidity associated with benign prostatic hypertrophy (BPH) and prostate cancer, which are the common conditions related to pathological growth of the prostate gland.
Cross-sectional studies conducted at the time of diagnosis of BPH have failed to show consistent differences in testosterone levels between patients and controls. A prospective study also failed to demonstrate a correlation between testosterone and the development of BPH (CitationGann et al 1995). Clinical trials have shown that testosterone treatment of hypogonadal men does cause growth of the prostate, but only to the size seen in normal men, and also causes a small increase in prostate specific antigen (PSA) within the normal range (CitationRhoden and Morgentaler 2005). Despite growth of the prostate a number of studies have failed to detect any adverse effects on symptoms of urinary obstruction or physiological measurements such as flow rates and residual volumes (CitationSnyder et al 1999; CitationKenny et al 2000, Citation2001). Despite the lack of evidence linking symptoms of BPH to testosterone treatment, it remains important to monitor for any new or deteriorating problems when commencing patients on testosterone treatment, as the small growth of prostate tissue may adversely affect a certain subset of individuals.
Autopsy studies have found histological prostate cancer to be very common, with one series showing a prevalence of greater than fifty percent in men over age sixty (CitationHolund 1980). The majority of histological cancers go undetected so that the clinical incidence of the disease is much lower, but it is still the most prevalent non-skin cancer in men (CitationJemal et al 2003). Prostate cancer is also unusual in comparison to other adult cancers in that the majority of those with the disease will die of other causes. Treatment of prostate cancer with androgen deprivation is known to be successful and is widely practiced, indicating an important role for testosterone in modifying the behavior of prostate cancer. In view of this, testosterone treatment is absolutely contraindicated in any case of known or suspected prostate cancer. The question of whether testosterone treatment could cause new cases of prostate cancer, or more likely cause progression of undiagnosed histological prostate cancer that would otherwise have remained occult, is an important consideration when treating ageing males with testosterone.
Cross-sectional studies have not shown raised testosterone levels at the time of diagnosis of prostate cancer, and in fact, low testosterone at the time of diagnosis has been linked with more locally aggressive and malignant tumors (CitationMassengill et al 2003; CitationImamoto et al 2005; CitationIsom-Batz et al 2005). This may reflect loss of hormone related control of the tumor or the effect of a more aggressive tumor in decreasing testosterone levels. One study found that 14% of hypogonadal men, with normal digital rectal examination and PSA levels, had histological prostate cancer on biopsy. It is possible that low androgen levels masked the usual evidence of prostate cancer in this population (CitationMorgentaler et al 1996). Most longitudinal studies have not shown a correlation between testosterone levels and the future development of prostate cancer (CitationCarter et al 1995; CitationHeikkila et al 1999; CitationStattin et al 2004) but a recent study did find a positive association (CitationParsons et al 2005). Interpretation of such data requires care, as the presentation of prostate cancer could be altered or delayed in patients with lower testosterone levels.
There is a polymorphic CAG repeat sequence in the androgen receptor gene, which codes for a variable number of glutamine amino acids in the part of the receptor affecting gene transcription. A receptor with a short CAG sequence produces greater activity when androgens attach, and men with shorter CAG polymorphisms exhibit androgenic traits, such as preserved bone density (CitationZitzmann et al 2001) and prostate growth during testosterone treatment (CitationZitzmann et al 2003). Indirect evidence of the importance of androgens in the development of prostate cancer is provided by case control study findings of a shorter, more active CAG repeat sequence in the androgen receptor gene of patients with prostate cancer compared with controls (CitationHsing et al 2000, Citation2002).
More can be learned from a large, randomized, placebo-controlled trial of finasteride treatment in 18,800 men aged 55 or more. Finasteride is a 5α-reductase inhibitor which acts to prevent the metabolism of testosterone to dihydrotestosterone (DHT) – the most active androgen in the prostate. The trial showed a greater overall incidence of prostate cancer in the control group, but men treated with finasteride were more likely to have high grade tumors (CitationThompson et al 2003), suggesting that reduced androgen exposure of the prostate may delay the presentation of prostate cancer and/or promote advanced disease in some other way.
There have been case reports of development of prostate cancer in patients during treatment with testosterone, including one case series of twenty patients (CitationGaylis et al 2005). It is not known whether this reflects an increase in incidence, as prostate cancer is very common and because the monitoring for cancer in patients treated with testosterone is greater. Randomized controlled trials of testosterone treatment have found a low incidence of prostate cancer and they do not provide evidence of a link between testosterone treatment and the development of prostate cancer (CitationRhoden and Morgentaler 2004). More large scale clinical trials of longer durations of testosterone replacement are required to confirm that testosterone treatment does not cause prostate cancer. Overall, it is not known whether testosterone treatment of aging males with hypogonadism increases the risk of prostate cancer, but monitoring for the condition is clearly vital. This should take the form of PSA blood test and rectal examination every three months for the first year of treatment and yearly thereafter (CitationNieschlag et al 2005). Age adjusted PSA reference ranges should be used to identify men who require further assessment. The concept of PSA velocity is also important and refers to the rate of increase in PSA per year. Patients with abnormal rectal examination suggestive of prostate cancer, PSA above the age specific reference range or a PSA velocity greater than 0.75 ng/ml/yr should be referred to a urologist for consideration of prostate biopsy.
Testosterone and erectile dysfunction
Erectile dysfunction is a common finding in the aging male. A prevalence of over 70% was found in men older than 70 in a recent cross-sectional study (CitationPonholzer et al 2005). Treatment with phosphodiesterase-5 (PDE-5) inhibitors is proven to be effective for the majority of men but some do not respond (CitationShabsigh and Anastasiadis 2003). The condition is multi-factorial, with contributions from emotional, vascular, neurological and pharmacological factors. The concept of erectile dysfunction as a vascular disease is particularly interesting in view of the evidence presented above, linking testosterone to atherosclerosis and describing its action as a vasodilator.
In a recent study of male workers, men with low testosterone levels had an increased chance of severe erectile dysfunction (CitationKratzik et al 2005), although such a link had not been found previously (CitationRhoden et al 2002). Certainly erectile dysfunction is considered part of the clinical syndrome of hypogonadism, and questions regarding erectile dysfunction form part of the clinical assessment of patients with hypogonadism (CitationMorley et al 2000; CitationMoore et al 2004).
A previous meta-analysis has confirmed that treatment of hypogonadal patients with testosterone improves erections compared to placebo (CitationJain et al 2000). A number of studies have investigated the effect of testosterone levels on erectile dysfunction in normal young men by inducing a hypogonadal state, for example by using a GnRH analogue, and then replacing testosterone at varying doses to produce levels ranging from low-normal to high (CitationBuena et al 1993; CitationHirshkowitz et al 1997). These studies have shown no significant effects of testosterone on erectile function. These findings contrast with a similar study conducted in healthy men aged 60–75, showing that free testosterone levels achieved with treatment during the study correlate with overall sexual function, including morning erections, spontaneous erections and libido (CitationGray et al 2005). This suggests that the men in this older age group are particularly likely to suffer sexual symptoms if their testosterone is low. Furthermore, the severity of erectile dysfunction positively correlates with lower testosterone levels in men with type 2 diabetes (CitationKapoor, Clarke et al 2007).
There is increasing interest in the group of patients who fail to respond to treatment with PDE-5 inhibitors and have low serum testosterone levels. Evidence from placebo-controlled trials in this group of men shows that testosterone treatment added to PDE-5 inhibitors improves erectile function compared to PDE-5 inhibitors alone (CitationAversa et al 2003; CitationShabsigh et al 2004).
It seems that adequate testosterone levels are an important influence on sexual symptoms in the aging male and also influence the response of men to PDE-5 inhibitors, the first line treatment for erectile dysfunction in men. Many would now suggest screening for testosterone deficiency in all men presenting with erectile dysfunction (CitationGore and Rajfer 2004; CitationShabsigh 2005). This would seem appropriate because, in addition to benefits on sexual function, identification and treatment of hypogonadal men with testosterone could improve other symptoms of hypogonadism and protect against other conditions such as osteoporosis.
Testosterone, mood, and cognition
Cognitive abilities differ between males and females and these differences are present from childhood. In broad terms, girls have stronger verbal skills than boys who tend to have stronger skills related to spatial ability (CitationLinn and Petersen 1985). It is thought that the actions of sex hormones have a role in these differences. Reviewing different cognitive strengths of male versus female humans is not within the scope of this article but the idea that cognition could be altered by testosterone deserves attention.
Cross-sectional studies have found a positive association between serum testosterone and some measures of cognitive ability in men (CitationBarrett-Connor, Goodman-Gruen et al 1999; CitationYaffe et al 2002). Longitudinal studies have found that free testosterone levels correlate positively with future cognitive abilities and reduced rate of cognitive decline (CitationMoffat et al 2002) and that, compared with controls, testosterone levels are reduced in men with Alzheimer’s disease at least 10 years prior to diagnosis (CitationMoffat et al 2004). Studies of the effects of induced androgen deficiency in patients with prostate cancer have shown that profoundly lowering testosterone leads to worsening cognitive functions (CitationAlmeida et al 2004; CitationSalminen et al 2004) and increased levels of serum amyloid (CitationGandy et al 2001; CitationAlmeida et al 2004), which is central to the pathogenesis of Alzheimer’s disease (CitationParihar and Hemnani 2004). Furthermore, testosterone reduces amyloid-induced hippocampal neurotoxity in vitro (CitationPike 2001) as well as exhibiting other neuroprotective effects (CitationPouliot et al 1996). The epidemiological and experimental data propose a potential role of testosterone in protecting cognitive function and preventing Alzheimer’s disease.
Studies of the effects on cognition of testosterone treatment in non-cognitively impaired eugonadal and hypogonadal ageing males have shown varying results, with some showing beneficial effects on spatial cognition (CitationJanowsky et al 1994; CitationCherrier et al 2001), verbal memory (CitationCherrier et al 2001) and working memory (CitationJanowsky et al 2000), and others showing no effects (CitationSih et al 1997; CitationKenny et al 2002). Other trials have examined the effects of testosterone treatment in older men with Alzheimer’s disease or cognitive decline. Results have been promising, with two studies showing beneficial effects of testosterone treatment on spatial and verbal memory (CitationCherrier et al 2005b) and cognitive assessments including visual-spatial memory (CitationTan and Pu 2003), and a recent randomized controlled trial comparing placebo versus testosterone versus testosterone and an aromatase inhibitor suggesting that testosterone treatment improves spatial memory directly and verbal memory after conversion to estrogen (CitationCherrier et al 2005a). Not all studies have shown positive results (CitationKenny et al 2004; CitationLu et al 2005), and variations could be due to the different measures of cognitive abilities that were used and the cognitive state of men at baseline. The data from clinical trials offers evidence that testosterone may be beneficial for certain elements of cognitive function in the aging male with or without cognitive decline. Larger studies are needed to confirm and clarify these effects.
Mood disturbance and dysthymia are part of the clinical syndrome of hypogonadism. Epidemiological studies have found a positive association between testosterone levels and mood, and depressed aging males have lower testosterone levels than controls (CitationBarrett-Connor, Von Muhlen et al 1999). Furthermore, induction of a hypogonadal state during treatment of men for prostate cancer leads to an increase in depression scores (CitationAlmeida et al 2004). Trials of testosterone treatment effects on mood have varied in outcome. Data on the effects on men with depression are conflicting (CitationSeidman et al 2001; CitationPope et al 2003) but there is evidence that testosterone treatment of older hypogonadal men does result in improvements in mood (CitationWang et al 1996) and that this may occur through changes in regional brain perfusion (CitationAzad et al 2003).
Treating hypogonadism – general considerations
The aim of treatment for hypogonadism is to normalize serum testosterone levels and abolish symptoms or pathological states that are due to low testosterone levels. The exact target testosterone level is a matter of debate, but current recommendations advocate levels in the mid-lower normal adult range (CitationNieschlag et al 2005). Truly physiological testosterone replacement would require replication of the diurnal rhythm of serum testosterone levels, but there is no current evidence that this is beneficial (CitationNieschlag et al 2005).
At the present time, it is suggested that androgen replacement should take the form of natural testosterone. Some of the effects of testosterone are mediated after conversion to estrogen or dihydrotestosterone by the enzymes aromatase and 5a-reductase enzymes respectively. Other effects occur independently of the traditional action of testosterone via the classical androgen receptor- for example, its action as a vasodilator via a cell membrane action as described previously. It is therefore important that the androgen used to treat hypogonadism is amenable to the action of these metabolizing enzymes and can also mediate the non-androgen receptor actions of testosterone. Use of natural testosterone ensures this and reduces the chance of non-testosterone mediated adverse effects. There are now a number of testosterone preparations which can meet these recommendations and the main factor in deciding between them is patient choice.
Regardless of the method of testosterone treatment chosen, patients will require regular monitoring during the first year of treatment in order to monitor clinical response to testosterone, testosterone levels and adverse effects, including prostate cancer (see ). It is recommended that patients should be reviewed at least every three months during this time. Once treatment has been established, less frequent review is appropriate but the care of the patient should be the responsibility of an appropriately trained specialist with sufficient experience of managing patients treated with testosterone.
Table 2 The advantages and disadvantages of available testosterone preparations with advantages and disadvantages of each
Testosterone preparations
Currently available testosterone preparations in common use include intramuscular injections, subcutaneous pellets, buccal tablets, transdermal gels and patches (see ). Oral testosterone is not widely used. Unmodified testosterone taken orally is largely subject to first-pass metabolism by the liver. Oral doses 100 fold greater than physiological testosterone production can be given to achieve adequate serum levels. Methyl testosterone esters have been associated with hepatotoxicity. There has been some use of testosterone undecanoate, which is an esterified derivative of testosterone that is absorbed via the lymphatic system and bypasses the liver. Unfortunately, it produces unpredictable testosterone levels and increases testosterone levels for only a short period after each oral dose (CitationSchurmeyer et al 1983).
Intramuscular testosterone injections were first used around fifty years ago. Commercially available preparations contain testosterone esters in an oily vehicle. Esterification is designed to retard the release of testosterone from the depot site into the blood because the half life of unmodified testosterone would be very short. For many years intramuscular preparations were the most commonly used testosterone therapy and this is still the case in some centers. Pain can occur at injection sites, but the injections are generally well tolerated and free of major side effects. Until recently, the available intramuscular injections were designed for use at a frequency of between weekly and once every four weeks. These preparations are the cheapest mode of testosterone treatment available, but often cause supraphysiological testosterone levels in the days immediately following injection and/or low trough levels prior to the next injection during which time the symptoms of hypogonadism may return (CitationNieschlag et al 1976). More recently, a commercial preparation of testosterone undecanoate for intramuscular injection has become available. This has a much longer half life and produces testosterone levels in the physiological range throughout each treatment cycle (CitationSchubert et al 2004). The usual dose frequency is once every three months. This is much more convenient for patients but does not allow prompt cessation of treatment if a contraindication to testosterone develops. The most common example of this would be prostate cancer and it has therefore been suggested that shorter acting testosterone preparations should preferably used for treating older patients (CitationNieschlag et al 2005). Similar considerations apply to the use of subcutaneous implants which take the form of cylindrical pellets injected under the skin of the abdominal wall and steadily release testosterone to provide physiological testosterone levels for up to six months. Problems also include pellet extrusion and infection (CitationHandelsman et al 1997).
Transdermal preparations of testosterone utilize the fact that the skin readily absorbs steroid hormones. Initial transdermal preparations took the form of scrotal patches with testosterone loaded on to a membranous patch. Absorption from the scrotal skin was particularly good and physiological levels of testosterone with diurnal variation were reliably attained. The scrotal patches are now rarely used because they require regular shaving or clipping of scrotal hair and because they produce rather high levels of dihydrotestosterone compared to testosterone (CitationBehre et al 1999). Subsequently, non-scrotal patches were developed but the absorptive capacity of non-scrotal skin is much lower, so these patches contain additional chemicals which enhance absorption. The non-scrotal skin patches produce physiological testosterone levels without supraphysiological dihydrotestosterone levels. Unfortunately, the patches produce a high rate of local skin reactions often leading to discontinuation (CitationParker and Armitage 1999). In the last few years, transdermal testosterone gel preparations have become available. These require daily application by patients and produce steady state physiological testosterone levels within a few days in most patients (CitationSwerdloff et al 2000; CitationSteidle et al 2003). The advantages compared with testosterone patches include invisibility, reduced skin irritation and the ability to adjust dosage, but concerns about transfer to women and children on close skin contact necessitate showering after application or coverage with clothes.
Another recent development is the production of adhesive tablets which are applied twice daily to the buccal mucosa on the gum above the incisor teeth. The tablets gradually release testosterone into the systemic venous circulation and steady state physiological concentrations are achieved in most patients within two days (CitationRoss et al 2004). Some patients do not like the feeling of the tablet in the mouth or find that there is an abnormal taste in the mouth, but local adverse effects are usually mild and transient (CitationWang, Swerdloff et al 2004).
A full review of available testosterone preparations can be found elsewhere (CitationNieschlag et al 2004).
Testosterone treatment – adverse events, contraindications
A large number of side-effects have been attributed to testosterone. In our clinical experience, the incidence of significant adverse effects with treatment producing physiological testosterone levels is low, and many side effects attributed to testosterone are mainly relevant to supraphysiological replacement. Some adverse effects are specific to a given mode of delivery and have already been described. Potential adverse effects concerning the prostate have also been discussed and require appropriate monitoring of symptoms, PSA and digital rectal examination. Other tumors which may be androgen responsive include cancer of the breast and primary liver tumors, and these are both contraindications to testosterone treatment
Another effect that can limit treatment is polycythemia, which occurs due to various stimulatory effects of testosterone on erythropoiesis (CitationZitzmann and Nieschlag 2004). Polycythemia is known to produce increased rates of cerebral ischemia and there have been reports of stroke during testosterone induced polycythaemia (CitationKrauss et al 1991). It is necessary to monitor hematocrit during testosterone treatment, and hematocrit greater than 50% should prompt either a reduction of dose if testosterone levels are high or high-normal, or cessation of treatment if levels are low-normal. On the other hand, late onset hypogonadism frequently results in anemia which will then normalize during physiological testosterone replacement.
Sleep apnea is another frequently listed contraindication to testosterone treatment. There have been a few reports of the development, or worsening, of sleep apnea during testosterone therapy (CitationMatsumoto et al 1985) but sleep apnea is actually associated with lower serum testosterone levels (CitationLuboshitzky et al 2002). The reduction in fat mass during treatment with testosterone could potentially be beneficial for sleep apnea, so many specialists will still consider patients for treatment with appropriate monitoring. It is wise to take a clinical history for sleep apnea during testosterone treatment in all men and perform sleep studies in those who develop symptoms.
Mental status changes including excess aggression are a well known phenomenon in the context of anabolic steroid abuse (CitationPerry et al 1990). An increase in self-reported aggressive behaviors have also been reported in one double blind placebo controlled trial of testosterone in young hypogonadal men (CitationFinkelstein et al 1997), but this has not been confirmed in other studies (CitationSkakkebaek et al 1981; CitationO’Connor et al 2002). Aggression should therefore be monitored but in our experience is rarely a significant problem during testosterone replacement producing physiological levels.
Overall, few patients have a compelling contraindication to testosterone treatment. The majority of men with late onset hypogonadism can be safely treated with testosterone but all will require monitoring of prostate parameters HDL cholesterol, hematocrit and psychological state. It is also wise to monitor symptoms of sleep apnea. Other specific concerns may be raised by the mode of delivery such as local side effects from transdermal testosterone.
Conclusion
Male hypogonadism becomes more common with increasing age and is currently an under-treated condition. The diagnosis of hypogonadism in the aging male requires a combination of symptoms and low serum testosterone levels. The currently available testosterone preparations can produce consistent physiological testosterone levels and provide for patient preference.
Some of the effects of testosterone treatment are well recognised and it seems clear that testosterone treatment for aging hypogonadal men can be expected to increase lean body mass, decrease visceral fat mass, increase bone mineral density and decrease total cholesterol. Beneficial effects have been seen in many trials on other parameters such as glycemic control in diabetes, erectile dysfunction, cardiovascular risk factors, angina, mood and cognition. These potentially important effects require confirmation in larger clinical trials. Indeed, it is apparent that longer duration randomized controlled trials of testosterone treatment in large numbers of men are needed to confirm the effects of testosterone on many aspects of aging male health including cardiovascular health, psychiatric health, prostate cancer and functional capacity. In the absence of such studies, it is necessary to balance risk and benefit on the best available data. At the present time the data supports the treatment of hypogonadal men with testosterone to normalize testosterone levels and improve symptoms. Most men with hypogonadism do not have a contraindication to treatment, but it is important to monitor for adverse consequences including prostate complications and polycythemia.
Important future developments will include selective androgen receptor modulators (SARMs). These drugs will be able to produce isolated effects of testosterone at androgen receptors. They are likely to become useful clinical drugs, but their initial worth may lie in facilitating research into the relative importance of testosterone’s action at the androgen receptor compared to at other sites or after conversion to other hormones. Testosterone will remain the treatment of choice for late onset hypogonadism for some time to come.
References
- [AHA] American Heart AssociationHeart disease and stroke statistics2002DallasAmerican Heart Association
- AlmeidaOPWaterreusASpryNOne year follow-up study of the association between chemical castration, sex hormones, beta-amyloid, memory and depression in menPsychoneuroendocrinology20042910718115219659
- AminorroayaAKelleherSConwayAJAdequacy of androgen replacement influences bone density response to testosterone in androgen-deficient menEur J Endocrinol2005152881615941928
- AmoryJKWattsNBEasleyKAExogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosteroneJ Clin Endocrinol Metab2004895031014764753
- AndersonRALudlamCAWuFCHaemostatic effects of supraphysiological levels of testosterone in normal menThromb Haemost19957469378585008
- AnderssonBMarinPLissnerLTestosterone concentrations in women and men with NIDDMDiabetes Care199417405118062607
- AversaAIsidoriAMSperaGAndrogens improve cavernous vasodilation and response to sildenafil in patients with erectile dysfunctionClin Endocrinol (Oxf)200358632812699447
- AzadNPitaleSBarnesWETestosterone treatment enhances regional brain perfusion in hypogonadal menJ Clin Endocrinol Metab2003883064812843144
- Barrett-ConnorELower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitusAnn Intern Med1992117807111416554
- Barrett-ConnorEGoodman-GruenDPatayBEndogenous sex hormones and cognitive function in older menJ Clin Endocrinol Metab1999843681510523014
- Barrett-ConnorEKhawKTEndogenous sex hormones and cardiovascular disease in men. A prospective population-based studyCirculation198878539453409497
- Barrett-ConnorEVon MuhlenDGKritz-SilversteinDBioavailable testosterone and depressed mood in older men: the Rancho Bernardo StudyJ Clin Endocrinol Metab199984573710022418
- BaumgartnerRNWatersDLGallagherDPredictors of skeletal muscle mass in elderly men and womenMech Ageing Dev19991071233610220041
- BehreHMKlieschSLeifkeELong-term effect of testosterone therapy on bone mineral density in hypogonadal menJ Clin Endocrinol Metab1997822386909253305
- BehreHMvon EckardsteinSKlieschSLong-term substitution therapy of hypogonadal men with transscrotal testosterone over 7–10 yearsClin Endocrinol (Oxf)1999506293510468929
- BenitoMVasilicBWehrliFWEffect of testosterone replacement on trabecular architecture in hypogonadal menJ Bone Miner Res20052017859116160736
- BhasinSWoodhouseLCasaburiRTestosterone dose-response relationships in healthy young menAm J Physiol Endocrinol Metab2001281E11728111701431
- BhasinSWoodhouseLCasaburiROlder men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscleJ Clin Endocrinol Metab2005906788815562020
- BjorntorpPThe regulation of adipose tissue distribution in humansInt J Obes Relat Metab Disord1996202913028680455
- Bonithon-KoppCScarabinPYBaraLRelationship between sex hormones and haemostatic factors in healthy middle-aged menAtherosclerosis1988717163377881
- BoyanovMABonevaZChristovVGTestosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiencyAging Male200361712809074
- BuenaFSwerdloffRSSteinerBSSexual function does not change when serum testosterone levels are pharmacologically varied within the normal male rangeFertil Steril1993591118238486184
- CampionJMMaricicMJOsteoporosis in menAm Fam Physician2003671521612722852
- CaraniCQinKSimoniMEffect of testosterone and estradiol in a man with aromatase deficiencyN Engl J Med19973379159211678
- CarterHBPearsonJDMetterEJLongitudinal evaluation of serum androgen levels in men with and without prostate cancerProstate19952725317541528
- ChangKPCenterJRNguyenTVIncidence of hip and other osteoporotic fractures in elderly men and women: Dubbo Osteoporosis Epidemiology StudyJ Bone Miner Res200419532615005838
- CherrierMMAsthanaSPlymateSTestosterone supplementation improves spatial and verbal memory in healthy older menNeurology20015780811445632
- CherrierMMMatsumotoAMAmoryJKThe role of aromatization in testosterone supplementation: effects on cognition in older menNeurology2005a64290615668427
- CherrierMMMatsumotoAMAmoryJKTestosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairmentNeurology2005b642063815985573
- CohenPGThe hypogonadal-obesity cycle: role of aromatase in modulating the testosterone-estradiol shunt – a major factor in the genesis of morbid obesityMed Hypotheses199952495110342671
- CorralesJJBurgoRMGarca-BerrocalBPartial androgen deficiency in aging type 2 diabetic men and its relationship to glycemic controlMetabolism2004536667215131775
- De PergolaGDe MitrioVSciaraffiaMLower androgenicity is associated with higher plasma levels of prothrombotic factors irrespective of age, obesity, body fat distribution, and related metabolic parameters in menMetabolism1997461287939361687
- De PergolaGPannacciulliNCicconeMFree testosterone plasma levels are negatively associated with the intima-media thickness of the common carotid artery in overweight and obese glucose-tolerant young adult menInt J Obes Relat Metab Disord200327803712821965
- DhindsaSPrabhakarSSethiMFrequent occurrence of hypogonadotropic hypogonadism in type 2 diabetesJ Clin Endocrinol Metab2004895462815531498
- DiverMJImtiazKEAhmadAMDiurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young menClin Endocrinol (oxf)2003587101712780747
- DunnJFNisulaBCRodbardDTransport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasmaJ Clin Endocrinol Metab19815358687195404
- EnglishKMSteedsRPJonesTHLow-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: A randomized, double-blind, placebo-controlled studyCirculation200010219061111034937
- FeldmanHALongcopeCDerbyCAAge trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging studyJ Clin Endocrinol Metab2002875899811836290
- FerrandoAASheffield-MooreMYeckelCWTestosterone administration to older men improves muscle function: molecular and physiological mechanismsAm J Physiol Endocrinol Metab2002282E601711832363
- FinkelsteinJWSusmanEJChinchilliVMEstrogen or testosterone increases self-reported aggressive behaviors in hypogonadal adolescentsJ Clin Endocrinol Metab199782243389253313
- FogariRPretiPZoppiASerum testosterone levels and arterial blood pressure in the elderlyHypertens Res2005286253016392765
- FukuiMKitagawaYNakamuraNAssociation between serum testosterone concentration and carotid atherosclerosis in men with type 2 diabetesDiabetes Care20032618697312766125
- GallonCVeyssiereGBergerMAge-related changes in the concentration of cytosolic androgen receptors in the epididymis, vas deferens and seminal vesicle of maturing male miceJ Androl198910188942745231
- GandySAlmeidaOPFonteJChemical andropause and amyloid-beta peptideJAMA20012852195611325319
- GannPHHennekensCHLongcopeCA prospective study of plasma hormone levels, nonhormonal factors, and development of benign prostatic hyperplasiaProstate1995264097531326
- GaylisFDLinDWIgnatoffJMProstate cancer in men using testosterone supplementationJ Urol20051745348 discussion 53816006887
- GlueckCJGlueckHIStroopDEndogenous testosterone, fibrinolysis, and coronary heart disease risk in hyperlipidemic menJ Lab Clin Med1993122412208228555
- GoreJRajferJThe role of serum testosterone testing: routine hormone analysis is an essential part of the initial screening of men with erectile dysfunctionRev Urol200462071016985604
- GrayPBSinghABWoodhouseLJDose-dependent effects of testosterone on sexual function, mood, and visuospatial cognition in older menJ Clin Endocrinol Metab20059038384615827094
- HaffnerSMMykkanenLValdezRARelationship of sex hormones to lipids and lipoproteins in nondiabetic menJ Clin Endocrinol Metab1993771610158263149
- HaffnerSMShatenJSternMPLow levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. MRFIT Research Group. Multiple Risk Factor Intervention TrialAm J Epidemiol1996143889978610702
- HaffnerSMValdezRAMykkanenLDecreased testosterone and dehydroepiandrosterone sulfate concentrations are associated with increased insulin and glucose concentrations in nondiabetic menMetabolism1994435996038177048
- HakAEWittemanJCde JongFHLow levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam studyJ Clin Endocrinol Metab2002873632912161487
- HallJJonesRDJonesTHSelective inhibition of L-type Ca2+channels in A7r5 cells by physiological levels of testosteroneEndocrinology200614726758016527846
- HandelsmanDJMackeyMAHoweCAn analysis of testosterone implants for androgen replacement therapyClin Endocrinol (Oxf)199747311169373452
- HarmanSMMetterEJTobinJDLongitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of AgingJ Clin Endocrinol Metab2001867243111158037
- HeikkilaRAhoKHeliovaaraMSerum testosterone and sex hormone-binding globulin concentrations and the risk of prostate carcinoma: a longitudinal studyCancer1999863121510421267
- HirshkowitzMMooreCAO’ConnorSAndrogen and sleep-related erectionsJ Psychosom Res19974254169226601
- HolundBLatent prostatic cancer in a consecutive autopsy seriesScand J Urol Nephrol19801429356154966
- HsingAWChokkalingamAPGaoYTPolymorphic CAG/CAA repeat length in the AIB1/SRC-3 gene and prostate cancer risk: a population-based case-control studyCancer Epidemiol Biomarkers Prev2002113374111927493
- HsingAWGaoYTWuGPolymorphic CAG and GGN repeat lengths in the androgen receptor gene and prostate cancer risk: a population-based case-control study in ChinaCancer Res20006051111611016637
- ImamotoTSuzukiHFukasawaSPretreatment serum testosterone level as a predictive factor of pathological stage in localized prostate cancer patients treated with radical prostatectomyEur Urol2005473081215716191
- IsidoriAMGiannettaEGrecoEAEffects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysisClin Endocrinol (Oxf)2005632809316117815
- Isom-BatzGBiancoFJJrKattanMWTestosterone as a predictor of pathological stage in clinically localized prostate cancerJ Urol20051731935715879785
- JacksonJARiggsMWSpiekermanAMTestosterone deficiency as a risk factor for hip fractures in men: a case-control studyAm J Med Sci1992304481642253
- JainPRademakerAWMcVaryKTTestosterone supplementation for erectile dysfunction: results of a meta-analysisJ Urol2000164371510893588
- JanowskyJSChavezBOrwollESex steroids modify working memoryJ Cogn Neurosci2000124071410931767
- JanowskyJSOviattSKOrwollESTestosterone influences spatial cognition in older menBehav Neurosci1994108325328037876
- JemalAMurrayTSamuelsACancer statistics, 2003CA Cancer J Clin20035352612568441
- JonesRDNettleshipJEKapoorDTestosterone and atherosclerosis in aging men: purported association and clinical implicationsAm J Cardiovasc Drugs200551415415901202
- JonesRDPughPJJonesTHThe vasodilatory action of testosterone: a potassium-channel opening or a calcium antagonistic action?Br J Pharmacol20031387334412642373
- JonesTHTestosterone associations with erectile dysfunction, diabetes and the metabolic syndromeEur Urol Suppl2007684757
- JonesTHJonesRDChannerKSTestosterone and cardiovascular disordersRecent Research Developments in Endocrinology and Metabolism20031Kerala, IndiaTransworld Research Network143167
- KapoorDAldredHClarkSClinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposityDiabetes Care2007309111717392552
- KapoorDClarkeSChannerKSErectile dysfunction is associated with low bioactive testosterone levels and visceral adiposity in men with type 2 diabetesInt J Androl200730500718028199
- KapoorDGoodwinEChannerKSTestosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetesEur J Endocrinol200615489990616728551
- KapoorDMalkinCJChannerKSAndrogens, insulin resistance and vascular disease in menClin Endocrinol (Oxf)2005632395016117808
- KatznelsonLFinkelsteinJSSchoenfeldDAIncrease in bone density and lean body mass during testosterone administration in men with acquired hypogonadismJ Clin Endocrinol Metab1996814358658954042
- KatznelsonLRosenthalDIRosolMSUsing quantitative CT to assess adipose distribution in adult men with acquired hypogonadismAJR Am J Roentgenol199817042379456958
- KennyAMBellantonioSGrumanCAEffects of transdermal testosterone on cognitive function and health perception in older men with low bioavailable testosterone levelsJ Gerontol A Biol Sci Med Sci200257M321511983727
- KennyAMFabregasGSongCEffects of testosterone on behavior, depression, and cognitive function in older men with mild cognitive lossJ Gerontol A Biol Sci Med Sci20045975814718489
- KennyAMPrestwoodKMGrumanCAEffects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levelsJ Gerontol A Biol Sci Med Sci200156M2667211320105
- KennyAMPrestwoodKMRaiszLGShort-term effects of intramuscular and transdermal testosterone on bone turnover, prostate symptoms, cholesterol, and hematocrit in men over age 70 with low testosterone levelsEndocr Res2000261536810921445
- KhawKTBarrett-ConnorEBlood pressure and endogenous testosterone in men: an inverse relationshipJ Hypertens19886329323379300
- KhawKTBarrett-ConnorELower endogenous androgens predict central adiposity in menAnn Epidemiol19922675821342319
- KhoslaSMeltonLJ3rdAtkinsonEJRelationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly menJ Clin Endocrinol Metab20018635556111502778
- KhoslaSMeltonLJ3rdAtkinsonEJRelationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogenJ Clin Endocrinol Metab1998832266749661593
- KontoleonPEAnastasiou-NanaMIPapapetrouPDHormonal profile in patients with congestive heart failureInt J Cardiol2003871798312559538
- KratzikCWSchatzlGLunglmayrGThe impact of age, body mass index and testosterone on erectile dysfunctionJ Urol2005174240315947646
- KraussDJTaubHALantingaLJRisks of blood volume changes in hypogonadal men treated with testosterone enanthate for erectile impotenceJ Urol19911461566701942342
- KupelianVPageSTAraujoABLow SHBG, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in non-obese menJ Clin Endocrinol Metab2006
- LaaksonenDENiskanenLPunnonenKSex hormones, inflammation and the metabolic syndrome: a population-based studyEur J Endocrinol2003149601814641004
- LaaksonenDENiskanenLPunnonenKTestosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged menDiabetes Care20042710364115111517
- LeeCHKuoSWHungYJThe effect of testosterone supplement on insulin sensitivity, glucose effectiveness, and acute insulin response after glucose load in male type 2 diabeticsEndocr Res2005311394816353672
- LeifkeEKornerHCLinkTMEffects of testosterone replacement therapy on cortical and trabecular bone mineral density, vertebral body area and paraspinal muscle area in hypogonadal menEur J Endocrinol19981385189461316
- LeifkeEWichersCGorenoiVLow serum levels of testosterone in men with minimal traumatic hip fracturesExp Clin Endocrinol Diabetes20051132081315891956
- LichtensteinMJYarnellJWElwoodPCSex hormones, insulin, lipids, and prevalent ischemic heart diseaseAm J Epidemiol1987126647573307391
- LinnMCPetersenACEmergence and characterization of sex differences in spatial ability: a meta-analysisChild Dev1985561479984075870
- LorentzonMSwansonCAnderssonNFree testosterone is a positive, whereas free estradiol is a negative, predictor of cortical bone size in young Swedish men: the GOOD studyJ Bone Miner Res20052013344116007330
- LuPHMastermanDAMulnardREffects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly menArch Neurol2005
- LuboshitzkyRAvivAHefetzADecreased pituitary-gonadal secretion in men with obstructive sleep apneaJ Clin Endocrinol Metab2002873394812107256
- LyLPJimenezMZhuangTNA double-blind, placebo-controlled, randomized clinical trial of transdermal dihydrotestosterone gel on muscular strength, mobility, and quality of life in older men with partial androgen deficiencyJ Clin Endocrinol Metab20018640788811549629
- MacKayJDMensahGAThe atlas of heart disease and stroke2004GenevaWHO
- MaggioMBasariaSBleACorrelation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older menJ Clin Endocrinol Metab200691345716263825
- MalkinCJPughPJJonesRDThe effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal menJ Clin Endocrinol Metab20048933131815240608
- MalkinCJPughPJMorrisPDTestosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of lifeHeart200490871615253956
- MalkinCJPughPJWestJNTestosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trialEur Heart J200627576416093267
- MarinPHolmangSGustafssonCAndrogen treatment of abdominally obese menObes Res199312455116350577
- MarinPHolmangSJonssonLThe effects of testosterone treatment on body composition and metabolism in middle-aged obese menInt J Obes Relat Metab Disord19921699171335979
- MarinPKrotkiewskiMBjorntorpPAndrogen treatment of middle-aged, obese men: effects on metabolism, muscle and adipose tissuesEur J Med19921329361341460
- MarinPLonnLAnderssonBAssimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosteroneJ Clin Endocrinol Metab1996811018228772568
- MarinPOdenBBjorntorpPAssimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgensJ Clin Endocrinol Metab199580239437829619
- MassengillJCSunLMoulJWPretreatment total testosterone level predicts pathological stage in patients with localized prostate cancer treated with radical prostatectomyJ Urol20031691670512686805
- MatsumotoAMSandblomRESchoeneRBTestosterone replacement in hypogonadal men: effects on obstructive sleep apnoea, respiratory drives, and sleepClin Endocrinol (Oxf)198522713214017261
- MaurasNHayesVWelchSTestosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposityJ Clin Endocrinol Metab1998831886929626114
- MoffatSDZondermanABMetterEJLongitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly menJ Clin Endocrinol Metab2002875001712414864
- MoffatSDZondermanABMetterEJFree testosterone and risk for Alzheimer disease in older menNeurology2004621889314745052
- MooreCHueblerDZimmermannTThe Aging Males’ Symptoms scale (AMS) as outcome measure for treatment of androgen deficiencyEur Urol20044680715183551
- MorgentalerABruningCO3rdDeWolfWCOccult prostate cancer in men with low serum testosterone levelsJAMA1996276190468968017
- MorleyJECharltonEPatrickPValidation of a screening questionnaire for androgen deficiency in aging malesMetabolism20004912394211016912
- MorleyJEPerryHM3rdKaiserFEEffects of testosterone replacement therapy in old hypogonadal males: a preliminary studyJ Am Geriatr Soc199341149528426037
- MorrisPDMalkinCJChannerKSA mathematical comparison of techniques to predict biologically available testosterone in a cohort of 1072 menEur J Endocrinol2004151241915296480
- MullerMGrobbeeDEden TonkelaarIEndogenous sex hormones and metabolic syndrome in aging menJ Clin Endocrinol Metab20059026182315687322
- MullerMvan den BeldAWBotsMLEndogenous sex hormones and progression of carotid atherosclerosis in elderly menCirculation20041092074915096452
- MulliganTIranmaneshAKerznerRTwo-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig cell) defects in the healthy aging male gonadotropic axisEur J Endocrinol19991412576610474123
- MulliganTIranmaneshAVeldhuisJDPulsatile iv infusion of recombinant human LH in leuprolide-suppressed men unmasks impoverished Leydig-cell secretory responsiveness to midphysiological LH drive in the aging maleJ Clin Endocrinol Metab20018655475311701734
- MurakamiSOtsukaKHottaNCommon carotid intima-media thickness is predictive of all-cause and cardiovascular mortality in elderly community-dwelling people: Longitudinal Investigation for the Longevity and Aging in Hokkaido County (LILAC) studyBiomed Pharmacother200559Suppl 1S495316275507
- MurphySKhawKTCassidyASex hormones and bone mineral density in elderly menBone Miner199320133408453329
- NCEPIIIExecutive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III)JAMA200128524869711368702
- NieschlagEBehreHMBouchardPTestosterone replacement therapy: current trends and future directionsHum Reprod Update2004104091915297434
- NieschlagECuppersHJWiegelmannWBioavailability and LH-suppressing effect of different testosterone preparations in normal and hypogonadal menHorm Res19767138451002121
- NieschlagESwerdloffRBehreHMInvestigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, and EAU recommendationsInt J Androl200528125715910536
- NoirhommePJacquetLUnderwoodMThe effect of chronic mechanical circulatory support on neuroendocrine activation in patients with end-stage heart failureEur J Cardiothorac Surg19991663710456405
- O’ConnorDBArcherJHairWMExogenous testosterone, aggression, and mood in eugonadal and hypogonadal menPhysiol Behav2002755576612062320
- OhJYBarrett-ConnorEWedickNMEndogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo studyDiabetes Care200225556011772901
- OngphiphadhanakulBRajatanavinRChailurkitLSerum testosterone and its relation to bone mineral density and body composition in normal malesClin Endocrinol (Oxf)199543727338736276
- OnoKHajiMNawataHAge-related changes in glucocorticoid and androgen receptors of cultured human pubic skin fibroblastsGerontology198834128333262075
- PageSTAmoryJKBowmanFDExogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum TJ Clin Endocrinol Metab20059015021015572415
- PariharMSHemnaniTAlzheimer’s disease pathogenesis and therapeutic interventionsJ Clin Neurosci2004114566715177383
- ParkerSArmitageMExperience with transdermal testosterone replacement therapy for hypogonadal menClin Endocrinol (Oxf)199950576210341856
- ParsonsJKCarterHBPlatzEASerum testosterone and the risk of prostate cancer: potential implications for testosterone therapyCancer Epidemiol Biomarkers Prev20051422576016172240
- PerryPJAndersenKHYatesWRIllicit anabolic steroid use in athletes. A case series analysisAm J Sports Med19901842282403192
- PhillipsGBRelationship between serum sex hormones and the glucose-insulin-lipid defect in men with obesityMetabolism199342116208446038
- PhillipsGBJingTHeymsfieldSBRelationships in men of sex hormones, insulin, adiposity, and risk factors for myocardial infarctionMetabolism2003527849012800107
- PhillipsGBJingTYResnickLMSex hormones and hemostatic risk factors for coronary heart disease in men with hypertensionJ Hypertens1993116997028228187
- PikeCJTestosterone attenuates beta-amyloid toxicity in cultured hippocampal neuronsBrain Res2001919160511689174
- PincusSMVeldhuisJDMulliganTEffects of age on the irregularity of LH and FSH serum concentrations in women and menAm J Physiol1997273E989959374686
- PonholzerATemmlCMockKPrevalence and risk factors for erectile dysfunction in 2869 men using a validated questionnaireEur Urol200547805 discussion 85–615582253
- PopeHGJrCohaneGHKanayamaGTestosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trialAm J Psychiatry20031601051112505808
- PouliotWAHandaRJBeckSGAndrogen modulates N-methyl-D-aspartate-mediated depolarization in CA1 hippocampal pyramidal cellsSynapse19962310198723131
- PughPJJonesRDWestJNTestosterone treatment for men with chronic heart failureHeart200490446715020527
- PughPJJonesTHChannerKSAcute haemodynamic effects of testosterone in men with chronic heart failureEur Heart J2003249091512714022
- Rebuffe-ScriveMMarinPBjorntorpPEffect of testosterone on abdominal adipose tissue in menInt J Obes19911579151778664
- RhodenELMorgentalerARisks of testosterone-replacement therapy and recommendations for monitoringN Engl J Med20043504829214749457
- RhodenELMorgentalerAInfluence of demographic factors and biochemical characteristics on the prostate-specific antigen (PSA) response to testosterone replacement therapyInt J Impot Res2005
- RhodenELRibeiroEPTelokenCDiabetes mellitus is associated with subnormal serum levels of free testosterone in menBJU Int2005968677016153219
- RhodenELTelokenCMafessoniRIs there any relation between serum levels of total testosterone and the severity of erectile dysfunction?Int J Impot Res2002141677112058243
- RosanoGMLeonardoFPagnottaPAcute anti-ischemic effect of testosterone in men with coronary artery diseaseCirculation19999916667010190874
- RossRJJabbarAJonesTHPharmacokinetics and tolerability of a bioadhesive buccal testosterone tablet in hypogonadal menEur J Endocrinol2004150576314713280
- RoyTABlackmanMRHarmanSMInterrelationships of serum testosterone and free testosterone index with FFM and strength in aging menAm J Physiol Endocrinol Metab2002283E2849412110533
- RuckerDEzzatSDiamandiAIGF-I and testosterone levels as predictors of bone mineral density in healthy, community-dwelling menClin Endocrinol (Oxf)200460491915049965
- SalminenEKPortinRIKoskinenAAssociations between serum testosterone fall and cognitive function in prostate cancer patientsClin Cancer Res20041075758215569988
- SchubertMMinnemannTHublerDIntramuscular testosterone undecanoate: pharmacokinetic aspects of a novel testosterone formulation during long-term treatment of men with hypogonadismJ Clin Endocrinol Metab20048954293415531493
- SchurmeyerTWickingsEJFreischemCWSaliva and serum testosterone following oral testosterone undecanoate administration in normal and hypogonadal menActa Endocrinol (Copenh)1983102456626402875
- ScopacasaFWishartJMNeedAGBone density and bone-related biochemical variables in normal men: a longitudinal studyJ Gerontol A Biol Sci Med Sci200257M3859112023268
- ScraggJLJonesRDChannerKSTestosterone is a potent inhibitor of L-type Ca(2+) channelsBiochem Biophys Res Commun2004318503615120629
- SeidmanSNSpatzERizzoCTestosterone replacement therapy for hypogonadal men with major depressive disorder: a randomized, placebo-controlled clinical trialJ Clin Psychiatry2001624061211465516
- ShabsighRTestosterone therapy in erectile dysfunction and hypogonadismJ Sex Med200527859216422803
- ShabsighRAnastasiadisAGErectile dysfunctionAnnu Rev Med2003541536812525671
- ShabsighRKaufmanJMSteidleCRandomized study of testosterone gel as adjunctive therapy to sildenafil in hypogonadal men with erectile dysfunction who do not respond to sildenafil aloneJ Urol20041726586315247755
- SihRMorleyJEKaiserFETestosterone replacement in older hypogonadal men: a 12-month randomized controlled trialJ Clin Endocrinol Metab199782166179177359
- SimonDPreziosiPBarrett-ConnorEInterrelation between plasma testosterone and plasma insulin in healthy adult men: the Telecom StudyDiabetologia19923517371547923
- SkakkebaekNEBancroftJDavidsonDWAndrogen replacement with oral testosterone undecanoate in hypogonadal men: a double blind controlled studyClin Endocrinol (Oxf)19811449617014044
- SmithAMEnglishKMMalkinCJTestosterone does not adversely affect fibrinogen or tissue plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1) levels in 46 men with chronic stable anginaEur J Endocrinol20051522859115745938
- SmithEPBoydJFrankGREstrogen resistance caused by a mutation in the estrogen-receptor gene in a manN Engl J Med19943311056618090165
- SmithJCBennettSEvansLMThe effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancerJ Clin Endocrinol Metab2001864261711549659
- SnyderPJPeacheyHBerlinJAEffects of testosterone replacement in hypogonadal menJ Clin Endocrinol Metab2000852670710946864
- SnyderPJPeacheyHHannoushPEffect of testosterone treatment on bone mineral density in men over 65 years of ageJ Clin Endocrinol Metab19998419667210372695
- SorvaRKuusiTTaskinenMRTestosterone substitution increases the activity of lipoprotein lipase and hepatic lipase in hypogonadal malesAtherosclerosis19886919173126749
- StattinPLummeSTenkanenLHigh levels of circulating testosterone are not associated with increased prostate cancer risk: a pooled prospective studyInt J Cancer20041084182414648709
- SteidleCSchwartzSJacobyKAA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual functionJ Clin Endocrinol Metab20038826738112788872
- StellatoRKFeldmanHAHamdyOTestosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging studyDiabetes Care200023490410857940
- StorerTWMaglianoLWoodhouseLTestosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tensionJ Clin Endocrinol Metab20038814788512679426
- SvartbergJvon MuhlenDSchirmerHAssociation of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromso StudyEur J Endocrinol20041501657114713281
- SvartbergJvon MuhlenDSundsfjordJWaist circumference and testosterone levels in community dwelling men. The Tromso studyEur J Epidemiol2004196576315461197
- SwerdloffRSWangCCunninghamGLong-term pharmacokinetics of transdermal testosterone gel in hypogonadal menJ Clin Endocrinol Metab20008545001011134099
- TanRSPuSJA pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s diseaseAging Male20036131712809076
- TapplerBKatzMPituitary-gonadal dysfunction in low-output cardiac failureClin Endocrinol (Oxf)19791021926455737
- Tena-SempereMPinillaLGonzalezLCLeptin inhibits testosterone secretion from adult rat testis in vitroJ Endocrinol19991612111810320818
- TenoverJSEffects of testosterone supplementation in the aging maleJ Clin Endocrinol Metab199275109281400877
- ThompsonIMGoodmanPJTangenCMThe influence of finasteride on the development of prostate cancerN Engl J Med20033492152412824459
- TibblinGAdlerberthALindstedtGThe pituitary-gonadal axis and health in elderly men: a study of men born in 1913Diabetes199645160598866567
- TremblayRRDubeJYPlasma concentrations of free and non-TeBG bound testosterone in women on oral contraceptivesContraception1974105996054448070
- TripathyDShahPLakshmyREffect of testosterone replacement on whole body glucose utilisation and other cardiovascular risk factors in males with idiopathic hypogonadotrophic hypogonadismHorm Metab Res19983064259851674
- TsaiECBoykoEJLeonettiDLLow serum testosterone level as a predictor of increased visceral fat in Japanese-American menInt J Obes Relat Metab Disord2000244859110805506
- UrbanRJBodenburgYHGilkisonCTestosterone administration to elderly men increases skeletal muscle strength and protein synthesisAm J Physiol1995269E82067491931
- van den BeldAWBotsMLJanssenJAEndogenous hormones and carotid atherosclerosis in elderly menAm J Epidemiol2003157253112505887
- Van PottelberghIBraeckmanLDe BacquerDDifferential contribution of testosterone and estradiol in the determination of cholesterol and lipoprotein profile in healthy middle-aged menAtherosclerosis20031669510212482555
- VeldhuisJDUrbanRJDufauMLEvidence that androgen negative feedback regulates hypothalamic gonadotropin-releasing hormone impulse strength and the burst-like secretion of biologically active luteinizing hormone in menJ Clin Endocrinol Metab1992741227351592863
- VermeulenAStoicaTVerdonckLThe apparent free testosterone concentration, an index of androgenicityJ Clin Endocrinol Metab197133759675125384
- WangCAlexanderGBermanNTestosterone replacement therapy improves mood in hypogonadal men – a clinical research center studyJ Clin Endocrinol Metab1996813578838855804
- WangCCunninghamGDobsALong-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal menJ Clin Endocrinol Metab20048920859815126525
- WangCSwerdloffRKipnesMNew testosterone buccal system (Striant) delivers physiological testosterone levels: pharmacokinetics study in hypogonadal menJ Clin Endocrinol Metab2004893821915292312
- WebbCMAdamsonDLde ZeiglerDEffect of acute testosterone on myocardial ischemia in men with coronary artery diseaseAm J Cardiol1999834379A910072236
- WebbCMMcNeillJGHaywardCSEffects of testosterone on coronary vasomotor regulation in men with coronary heart diseaseCirculation19991001690610525487
- WHODefinition, Diagnosis and classification of Diabetes Mellitus1999GenevaWHO
- WuSZWengXZTherapeutic effects of an androgenic preparation on myocardial ischemia and cardiac function in 62 elderly male coronary heart disease patientsChin Med J (Engl)1993106415188222891
- YaffeKLuiLYZmudaJSex hormones and cognitive function in older menJ Am Geriatr Soc2002507071211982672
- YesilovaZOzataMKocarIHThe effects of gonadotropin treatment on the immunological features of male patients with idiopathic hypogonadotropic hypogonadismJ Clin Endocrinol Metab200085667010634365
- ZacharinMRPuaJKanumakalaSBone mineral density outcomes following long-term treatment with subcutaneous testosterone pellet implants in male hypogonadismClin Endocrinol (Oxf)200358691512780744
- ZimmetPMaglianoDMatsuzawaYThe metabolic syndrome: a global public health problem and a new definitionJ Atheroscler Thromb20051229530016394610
- ZitzmannMBruneMKornmannBThe CAG repeat polymorphism in the androgen receptor gene affects bone density and bone metabolism in healthy malesClin Endocrinol (Oxf)2001556495711894977
- ZitzmannMDepenbuschMGromollJProstate volume and growth in testosterone-substituted hypogonadal men are dependent on the CAG repeat polymorphism of the androgen receptor gene: a longitudinal pharmacogenetic studyJ Clin Endocrinol Metab20038820495412727953
- ZitzmannMNieschlagEBehreHMAndrogens and erythropoiesisTestosterone. Action, deficiency, substitution2004CambridgeUniversity of Cambridge28396
