Abstract
Background
Several studies have shown an association between chronic obstructive pulmonary disease (COPD) and cognitive impairment. These studies have been limited by methodological issues such as diagnostic uncertainty, cross-sectional design, small sample size, or lack of appropriate referent group. This study aimed to elucidate the association between COPD and the risk of cognitive impairment compared to referent subjects without COPD. In patients with established COPD, we evaluated the impact of disease severity and impairment of respiratory physiology on cognitive impairment and the potential mitigating role of oxygen therapy.
Methods
We used the Function, Living, Outcomes and Work (FLOW) cohort study of adults with COPD (n = 1202) and referent subjects matched by age, sex, and race (n = 302) to study the potential risk factors for cognitive impairment among subjects with COPD. Cognitive impairment was defined as a Mini-Mental State Exam score of <24 points. Disease severity was using Forced Expiratory Volume in one second (FEV1); the validated COPD Severity Score; and the BMI (Body Mass Index), Obstruction, Dyspnea, Exercise Capacity (BODE) Index. Multivariable analysis was used to control for confounding by age, sex, race, educational attainment, and cigarette smoking.
Results
COPD was associated with a substantive risk of cognitive impairment compared to referent subjects (odds ratio [OR] 2.42; 95% confidence interval [CI] 1.043–6.64). Among COPD patients, none of the COPD severity measures were associated with the risk of cognitive impairment (P > 0.20 in all cases). Low baseline oxygen saturation was related to increased risk of cognitive impairment (OR for oxygen saturation ≤88% (OR 5.45; 95% CI 1.014–29.2; P = 0.048). Conversely, regular use of supplemental oxygen therapy decreased the risk for cognitive impairment (OR 0.14; 95% CI 0.07–0.27; P < 0.0001).
Conclusion
COPD is a major risk factor for cognitive impairment. Among patients with COPD, hypoxemia is a major contributor and regular use of home oxygen is protective. Health care providers should consider screening their COPD patients for cognitive impairment.
Keywords:
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by the irreversible loss of lung function. Although COPD has been traditionally considered as a disease primarily affecting the lungs, its systemic effects have been increasingly recognized with diverse manifestations involving body systems distant from the lung.Citation1,Citation2 The brain, in particular, may be vulnerable to the systemic effects of COPD.Citation3–Citation8 Several features of the disease may contribute to impaired cognitive function, including hypoxemia and comorbid cardiovascular disease.Citation7–Citation9 In addition, COPD may lead patients to curtail their physical activity which may, in turn, further increase the risk of cognitive impairment.Citation10
Although there is a plausible link between COPD and cognitive impairment, the existing literature is limited by methodological issues such as diagnostic uncertainty, cross-sectional design, small sample size, or lack of appropriate referent group.Citation11–Citation14 In a large cohort study of COPD, we aimed to elucidate the impact of COPD on the risk of cognitive impairment compared to a matched referent group without disease. Among patients with established COPD, we evaluated the impact of disease severity and impairment of respiratory physiology on cognitive impairment and the potential mitigating role of oxygen therapy.
Methods
Recruitment of the cohort and referent subjects
The current study is a matched case-referent analysis conducted within the Function, Living, Outcomes, and Work (FLOW) study of COPD. The FLOW study is an ongoing prospective cohort study of adult members of an integrated health care delivery system with a physician’s diagnosis of COPD with an accompanying matched control group. Recruitment methods have been previously reported in detail.Citation1,Citation15–Citation17 We recruited a cohort of 1,202 Kaiser Permanente Medical Care Program (KPMCP) members who were recently treated for COPD using a validated algorithm based both on health care utilization and pharmacy dispensing for COPD.Citation18 A control group of 302 subjects without COPD were recruited who were matched by age, sex, and race to the cohort members. Spirometry confirmed that none of the controls met Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria for COPD. At baseline assessment, we conducted structured telephone interviews that ascertained sociodemographic characteristics, COPD clinical history, and health status.Citation1,Citation15,Citation16 Research clinic visits included spirometry, other physical assessments, and cognitive testing. The study was approved both by the University of California, San Francisco Committee on Human Research and the Kaiser Foundation Research Institute’s institutional review board and all participants provided written informed consent.
Structured telephone interviews
All subjects (COPD subjects and non-COPD referents) underwent 30–40 minute structured telephone interviews that ascertained sociodemographic characteristics, cigarette smoking, health status, and home oxygen use. As in our previous studies, we defined educational attainment as high school or less, some college, or college/graduate degree.Citation19 Race-ethnicity was categorized as white non-Hispanic, African American, Asian/Pacific Islander, Hispanic, or other.Citation19 Cigarette smoking was measured using questions developed for the National Health Interview Survey (NHIS) and was defined as current smoking, past smoking, or never smoked.Citation20 We assessed comorbid cardiovascular conditions using survey items modified from the NHIS.Citation21 These include a reported physician’s diagnosis of coronary artery disease, congestive heart failure, or stroke based on our previous work.Citation1
Assessment of pulmonary physiology
Baseline oxygen saturation was measured at rest in the seated position using the Nellcor N-180 (Covidien-Nellcor, Boulder, CO). Most subjects were measured on room air; 61 subjects had oxygen saturation measured on their prescribed supplemental oxygen (range 1–4 liters/minute).
To measure pulmonary function, we conducted spirometry according to American Thoracic Society (ATS) Guidelines.Citation22,Citation23 We used the EasyOne™ Frontline spirometer (ndd Medical Technologies, Chelmsford, MA, USA), which is known for its reliability, accuracy, and durability and has been widely used in epidemiologic research.Citation24,Citation25 Because research clinic examinations were conducted by trained nonmedical personnel, we did not administer bronchodilators for study purposes. However, 90% of subjects had taken their own short-acting bronchodilator within 4 hours of spirometry or had taken a long-acting bronchodilator earlier in the same day.
Measurement of COPD severity
We used a combined approach to measure COPD severity. We used a disease-specific COPD severity score that we had previously developed and validated for use in epidemiologic and outcomes research.Citation26,Citation27 Based on survey responses, the COPD severity score is comprised of five overall aspects of COPD severity: respiratory symptoms, systemic corticosteroid use, other COPD medication use, previous hospitalization or intubation for respiratory disease, and home oxygen use. Each item was weighted based on clinical aspects of the disease and its expected contribution to overall COPD severity. Possible total scores range from 0 to 35, with higher scores reflecting more severe COPD.
We also used the validated BMI (Body Mass Index), Obstruction, Dyspnea, Exercise Capacity (BODE) Index, which is a multi-modal measure of disease severity.Citation28 The BODE Index is based on the body-mass index (B), the degree of airflow obstruction (O) measured by Forced Expiratory Volume in one second (FEV1), grade of dyspnea (D) assessed by the modified Medical Research Council (MRC) Dyspnea Scale, and exercise capacity (E) measured by the six-minute-walk test. Each component is assigned a specific score and the total score ranges from 0 to 10 points (higher scores indicate greater severity). The BODE index predicts death and other poor outcomes in COPD.Citation28–Citation30
Cognitive assessment
At the time of the research clinic visit, cognitive function was measured using the Mini-Mental State Examination, which is the most commonly used screening test for cognitive impairment in North America.Citation31 The 11-item instrument assesses orientation, recall ability, short-term memory, and arithmetic ability.Citation32 It evaluates most of the main domains of cognitive status and has been extensively validated.Citation31–Citation36 We used the recommended cut-point score of <24 points out of the total possible 30 points to indicate cognitive impairment.Citation37
Statistical analysis
Statistical analysis was conducted using SAS software, version 9.1 (SAS Institute, Inc, Cary, NC, USA) and Stata 10 (College Park, TX, USA). Bivariate comparisons were carried out using t-test for continuous variables and the chi-square test for categorical variables. We used logistic regression analysis to examine the association between COPD (vs referent) and the risk of cognitive impairment, controlling for age, sex, race, educational attainment, and smoking history (past smoking and current smoking as indicator variables).
In the COPD cohort, we used multivariable logistic regression analysis to elucidate the association between COPD severity measures and the risk of cognitive impairment. Risk estimates (ie, odds ratios) were expressed per a 0.5 standard deviation of each predictor variable, an approximation of the minimal clinically important difference.Citation38 Multivariable analysis was used to control for age, sex, race, educational attainment, and smoking history.
We also evaluated the impact of home oxygen use on the risk of cognitive impairment. Because oxygen therapy is prescribed to patients with more severe disease, confounding by indication will likely bias the unadjusted results in the direction of no effect. We used two approaches to control for confounding. First, we used standard multivariable logistic regression. Second, we used propensity scores to correct for confounding by indication. Propensity scores were developed from a multivariable logistic regression model to predict home oxygen use conditional on observed covariates. Variables included were age, sex, race, smoking history, BODE Score, FEV1, baseline oxygen saturation (≤88%), and desaturation during Six Minute Walk Test (≥4%). Area under receiver operator characteristic curve for this model was excellent (0.92). Multivariable logistic regression analysis was then used to evaluate the impact of home oxygen use on the risk of cognitive impairment using inverse probability-weighted estimators derived from the propensity scores to control for confounding.Citation39
The LOWESS (locally weighted regression scatter plot smoother) procedure was used to graphically depict the relationship between oxygen saturation and the logit (ie, log odds) of cognitive impairment. This method fitted a flexible smoothed curve that did not impose a linear relationship.Citation40
Results
Baseline characteristics of subjects with and without COPD
Subjects with and without COPD were similar in age, sex, and race-ethnicity, which reflected matching (P > 0.20 in all cases) (). The prevalence of lifetime smoking was higher among those with COPD (87%) than in the referent group (48%) (P < 0.0001). Persons with COPD also had lower educational attainment than referents.
Table 1 Baseline characteristics of FLOW cohort of COPD vs referent subjects
COPD and the risk of cognitive impairment
The prevalence of cognitive impairment was much higher among COPD subjects compared to the matched referent group without COPD (5.5% vs 2.0%, P = 0.0051). In multivariable analysis, COPD was associated with a greater risk of cognitive impairment function even after controlling for age, sex, race, smoking history, and educational attainment (OR 2.42, 95% CI 1.043–6.64) ().
Table 2 COPD and the risk of cognitive impairment
Risk factors for cognitive impairment among subjects with COPD
Among patients with COPD, respiratory impairment, as measured by FEV1, was not associated with cognitive impairment (). Low baseline oxygen saturation (≤88%) was strongly related to cognitive impairment (adjusted OR 5.45; 95% CI 1.014–29.2). A small minority of subjects (n = 61) used prescribed supplemental oxygen. When oxygen use was added to the multivariable model, low oxygen saturation remained associated with a greater risk of cognitive impairment (OR 5.46; 95% CI 1.007–30). demonstrates that the risk of cognitive impairment increases with decreasing oxygen saturation, but that the relationship is non-linear.
Table 3 Relationship between COPD severity and cognitive impairment
Figure 1 Relationship between resting oxygen saturation and the risk of cognitive impairment. Oxygen saturation was measured using pulse oximetry. The LOWESS (locally weighted regression scatter plot smoother) procedure was used to graphically depict the relationship between oxygen saturation and the logit (ie, log odds) of cognitive impairment. This method fitted a flexible smoothed curve that did not impose a linear relationship.
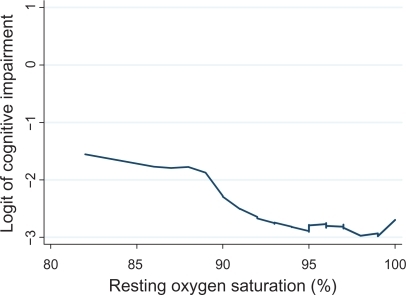
The risk of cognitive impairment was not associated with COPD severity as defined by either the COPD severity score or BODE score (). Stroke and other cardiovascular disease were also not related to cognitive impairment ().
Table 4 Cardiovascular disease and the risk of cognitive impairment in COPD
Home oxygen therapy and cognitive impairment in COPD
Home oxygen therapy was associated with a decreased risk of cognitive impairment among persons with COPD (). The unadjusted estimate, which was likely confounded by indication, did not exclude the ‘no effect’ level. In the most adjusted model, using inverse probability-weighted estimators derived from the propensity scores to control for confounding, home oxygen use was strongly associated with a decreased risk of cognitive impairment (OR 0.14; 95% CI 0.01–0.27).
Table 5 Home oxygen use and the risk of cognitive impairment in COPD
Discussion
COPD is often considered a disease that solely affects the lungs; however, its extrapulmonary effects are profound and disabling. Our study indicates that COPD is strongly associated with cognitive impairment in a large cohort of patients with a broad range of COPD severity. Resting hypoxemia was the single most important risk factor for cognitive impairment; treatment with supplemental oxygen markedly decreased the risk. Lung function impairment, COPD severity, and cardiovascular disease did not appear to explain the development of cognitive impairment in COPD.
We used a rigorous and multifaceted approach to measuring COPD severity (lung function, COPD Severity Score, and BODE Index). Despite this approach, we found no impact of these indicators of disease severity on the risk of cognitive impairment. This differs from some previous work,Citation41 but is similar to other studies that found no association between disease severity and cognitive impairment.Citation42 Our results reveal a disconnect between global measures of COPD severity, which were not related to cognitive impairment, and decreased resting oxygenation, which was strongly associated with decreased cognitive function.
The specific role of cardiovascular comorbidities as a risk factor for cognitive impairment in COPD is difficult to ascertain as both conditions share risk factors (eg, smoking) and COPD patients may be predisposed to cardiovascular events.Citation18 When cardiovascular comorbidities were evaluated as a risk factor for cognitive impairment, no association was found among the COPD cohort. The point estimate for cardiovascular disease was elevated, however, in the unadjusted analysis, so lack of statistical power may be one explanation for this lack of effect. Controlling for covariates, such as smoking, reduced the effect estimate, so that any association between cardiovascular disease and cognitive impairment may be mediated by smoking and other factors.
Cognitive impairment may interfere with patients’ ability to adhere to their medication regimen, adjust their medications in response to respiratory symptoms, and perform other aspects of self-management. Given that many of the medications for COPD are self-activated, even mild degrees of cognitive impairment could have significant effects on disease management. More broadly, cognitive impairment may create difficulties with performing daily activities, especially those that involve memory or complex reasoning.Citation43 It is likely that cognitive impairment has major effects on many aspects of patient functioning and health status.
The MMSE is a widely used measure of cognitive impairment but it is not sensitive for mild cognitive dysfunction.Citation44,Citation45 Consequently, our methods could have missed cases of mild cognitive impairment. It remains possible, therefore, that COPD severity could predict milder degrees of cognitive dysfunction. A future analysis of cognitive impairment using a detailed neuropsychologic battery of tests is required to evaluate milder cognitive impairment in COPD.
Our study is subject to several other limitations. Because our primary study focus was development of disability in COPD, we enrolled younger patients (45–65 years). Consequently, our study may underestimate the impact of COPD on cognitive impairment in older patients. Misclassification of COPD could also have occurred. Our COPD definition required concomitant treatment with COPD medications to increase the specificity of our definition. In addition, all patients had a physician diagnosis of COPD and reported having the condition. The lifetime smoking prevalence was similar to that in other population-based epidemiologic studies of COPD, supporting the diagnosis of COPD rather than asthma.Citation46,Citation47 We also previously demonstrated the validity of our approach using medical record review.Citation18 Nonetheless, we acknowledge this potential limitation.
Another limitation is the small proportion of subjects who were using home oxygen. Consequently, the confidence intervals around our estimates of the effectiveness of oxygen for preventing cognitive impairment are wide in some cases. As the FLOW cohort continues longitudinal follow-up, we will further evaluate the impact of oxygen therapy on cognitive function.
COPD is a multisystem disease with extrapulmonary sequelae. It is strongly associated with an increased risk of cognitive impairment, especially among hypoxemic patients. Clinicians should evaluate their COPD patients for cognitive impairment in order to identify those who may benefit from interventions such as medication assistance or supplemental oxygen therapy. Future research should use detailed neuropsychological testing to carefully evaluate multiple cognitive domains among subjects with a broad range of ages and disease severity.
Acknowledgements
Funded by: NHLBI/NIH R01HL077618 and K24 HL 097245.
Disclosure
The authors report no conflicts of interest in this work.
References
- EisnerMDBlancPDYelinEHCOPD as a systemic disease: impact on physical functional limitationsAm J Med200812178979618724969
- Rodriguez Gonzalez-MoroJMde Lucas RamosPIzquierdo AlonsoJLImpact of COPD severity on physical disability and daily living activities: EDIP-EPOC I and EDIP-EPOC II studiesInt J Clin Pract20096374275019392924
- GrantIPrigatanoGPHeatonRKMcSweenyAJWrightECAdamsKMProgressive neuropsychologic impairment and hypoxemia. Relationship in chronic obstructive pulmonary diseaseArch Gen Psychiatry19874499910063675139
- IncalziRAGemmaAMarraCMuzzolonRCapparellaOCarboninPChronic obstructive pulmonary disease. An original model of cognitive declineAm Rev Respir Dis19931484184248342906
- OzgeCOzgeAUnalOCognitive and functional deterioration in patients with severe COPDBehav Neurol20061712113016873924
- OrtapamukHNaldokenSBrain perfusion abnormalities in chronic obstructive pulmonary disease: comparison with cognitive impairmentAnn Nucl Med2006209910616615418
- HeatonRKGrantIMcSweenyAJAdamsKMPettyTLPsychologic effects of continuous and nocturnal oxygen therapy in hypoxemic chronic obstructive pulmonary diseaseArch Intern Med1983143194119476625781
- KropHDBlockAJCohenENeuropsychologic effects of continuous oxygen therapy in chronic obstructive pulmonary diseaseChest1973643173224749376
- FillitHNashDTRundekTZuckermanACardiovascular risk factors and dementiaAm J Geriatr Pharmacother2008610011818675769
- LautenschlagerNTCoxKLFlickerLEffect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trialJAMA20083001027103718768414
- HungWWWisniveskyJPSiuALRossJSCognitive decline among patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med200918013413719423714
- MossMFranksMBriggsPKennedyDScholeyACompromised arterial oxygen saturation in elderly asthma sufferers results in selective cognitive impairmentJ Clin Exp Neuropsychol20052713915015903147
- IncalziRAChiappiniFFusoLTorriceMPGemmaAPistelliRPredicting cognitive decline in patients with hypoxaemic COPDRespir Med1998925275339692117
- OhruiTTanakaKChibaKCognitive decline in patients with long-term domiciliary oxygen therapyTohoku J Exp Med200520634735215997207
- EisnerMDIribarrenCYelinEHPulmonary function and the risk of functional limitation in chronic obstructive pulmonary diseaseAm J Epidemiol20081671090110118343879
- BlancPDIribarrenCTrupinLOccupational exposures and the risk of COPD: dusty trades revisitedThorax20096461218678700
- OmachiTAKatzPPYelinEHDepression and health-related quality of life in chronic obstructive pulmonary diseaseAm J Med2009122778.e9e1519635280
- SidneySSorelMQuesenberryCPJrDeLuiseCLanesSEisnerMDCOPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care ProgramChest20051282068207516236856
- EisnerMDYelinEHTrupinLBlancPDThe influence of chronic respiratory conditions on health status and work disabilityAm J Public Health2002921506151312197984
- Cigarette smoking among adults – United States, 1997MMWR Morb Mortl Wkly Rep199948993996
- National Health Interview Survey Accessed3282008 at http://www.cdc.gov/nchs/about/major/nhis/quest_data_related_1997_forward.htm.
- American Thoracic SocietyStandardization of spirometry – 1987 update. Statement of the American Thoracic SocietyAm Rev Respir Dis1987136128512983674589
- Standardization of Spirometry, 1994 Update. American Thoracic SocietyAm J Respir Crit Care Med1995152110711367663792
- WaltersJAWood-BakerRWallsJJohnsDPStability of the EasyOne ultrasonic spirometer for use in general practiceRespirology20061130631016635089
- Perez-PadillaRVazquez-GarciaJCMarquezMNThe long-term stability of portable spirometers used in a multinational study of the prevalence of chronic obstructive pulmonary diseaseRespir Care2006511167117117005063
- EisnerMDTrupinLKatzPPDevelopment and validation of a survey-based COPD severity scoreChest20051271890189715947299
- OmachiTAYelinEHKatzPPBlancPDEisnerMDThe COPD Severity Score: a dynamic prediction tool for health care utilizationCOPD2008533934619353347
- CelliBRCoteCGMarinJMThe body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary diseaseN Engl J Med20043501005101214999112
- MartinezFJHanMKAndreiACLongitudinal change in the BODE index predicts mortality in severe emphysemaAm J Respir Crit Care Med200817849149918535255
- OngKCLuSJSohCSDoes the multidimensional grading system (BODE) correspond to differences in health status of patients with COPD?Int J Chron Obstruct Pulmon Dis20061919618046907
- McDowellINewellCMeasuring health: A guide to rating scales and questionnairesNew YorkOxford University Press1996
- FolsteinMFFolsteinSEMcHughPR“Mini-mental state”. A practical method for grading the cognitive state of patients for the clinicianJ Psychiatr Res1975121891981202204
- MolloyDWAlemayehuERobertsRReliability of a Standardized Mini-Mental State Examination compared with the traditional Mini-Mental State ExaminationAm J Psychiatry19911481021051984692
- FillenbaumGGHeymanAWilkinsonWEHaynesCSComparison of two screening tests in Alzheimer’s disease. The correlation and reliability of the Mini-Mental State Examination and the modified Blessed testArch Neurol1987449249273619711
- FaustmanWOMosesJAJrCsernanskyJGLimitations of the Mini-Mental State Examination in predicting neuropsychological functioning in a psychiatric sampleActa Psychiatr Scand1990811261312327274
- MitrushinaMSatzPReliability and validity of the Mini-Mental State Exam in neurologically intact elderlyJ Clin Psychol1991475375431939698
- RaitGFletcherASmeethLPrevalence of cognitive impairment: results from the MRC trial of assessment and management of older people in the communityAge Ageing20053424224815863409
- NormanGRSloanJAWyrwichKWInterpretation of changes in health-related quality of life: The remarkable universality of half a standard deviationMed Care20034158259212719681
- CurtisLHHammillBGEisensteinELKramerJMAnstromKJUsing inverse probability-weighted estimators in comparative effectiveness analyses with observational databasesMed Care200745S103S10717909367
- ClevelandWSRobust locally weighted regression and smoothing scatterplotsJ Am Stat Assoc197974829836
- LieskerJJPostmaDSBeukemaRJCognitive performance in patients with COPDRespir Med20049835135615072176
- IncalzilRABelliaVMaggiSMild to moderate chronic airways disease does not carry an excess risk of cognitive dysfunctionAging Clin Exp Res20021439540112602575
- PerneczkyRPohlCSorgCImpairment of activities of daily living requiring memory or complex reasoning as part of the MCI syndromeInt J Geriatr Psychiatry20062115816216416470
- KukullWALarsonEBTeriLBowenJMcCormickWPfanschmidtMLThe Mini-Mental State Examination score and the clinical diagnosis of dementiaJ Clin Epidemiol199447106110677730909
- KauferDIWilliamsCSBraatenAJGillKZimmermanSSloanePDCognitive screening for dementia and mild cognitive impairment in assisted living: Comparison of 3 testsJ Am Med Dir Assoc2008958659319083293
- EisnerMDBalmesJKatzPPTrupinLYelinEHBlancPDLifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary diseaseEnviron Health20054715890079
- ManninoDMHomaDMAkinbamiLJFordESReddSCChronic obstructive pulmonary disease surveillance – United States, 1971–2000MMWR Surveill Summ200251116
