Abstract
Aim
This observational study with tiotropium Respimat® was performed in a real-life setting to investigate its effectiveness with regard to physical functioning and tolerability.
Methods
Patients with chronic obstructive pulmonary disease (COPD; n = 1,230; mean age, 65.5 years) received tiotropium 5 μg once daily via Respimat® Soft Inhaler for 6 weeks in an open-label observational study. At baseline and week 6, patients completed the Physical Function subdomain [PF-10] of the Short Form (SF) 36 questionnaire.
Results
Improvement in standardized PF-10 score of ≥10 points was achieved by 61.5% of patients. Mean (SD) standardized PF-10 scores improved by 13.4 (15.9) points, from 49.0 (24.5) to 62.3 points (23.5; P < 0.001). Results in smokers (n = 435) were not significantly different to those in nonsmokers. The general condition of patients improved during treatment. Adverse events were reported by 4.0% of patients and were chiefly respiratory symptoms and dry mouth.
Conclusion
In COPD patients receiving tiotropium Respimat® in daily practice, physical function improved rapidly within 6 weeks of treatment, irrespective of smoking status.
Keywords:
Introduction
Assessment of disease severity in patients with chronic obstructive pulmonary disease (COPD) centers on measures of airflow limitation, such as forced expiratory volume.Citation1 However, patients with COPD also suffer considerably with extrapulmonary effects of the disease, notably a loss of skeletal muscle mass (cachexia), which in tandem with poor pulmonary function, results in reduced physical activity.Citation2 Cardiovascular disorders and depression, also common in COPD patients,Citation3 place further constraints on physical function, the deterioration of which contributes to the decline in the patient’s health status and quality of life.
In controlled trials in COPD patients, treatment with the inhaled long-acting anticholinergic tiotropium (Spiriva®) not only improved lung function and symptoms, and prevented exacerbations, but also improved health-related quality of life (HRQoL).Citation4–Citation8 The instrument used to measure HRQoL in these studies was the St George’s Respiratory Questionnaire (SGRQ),Citation9 but in two of the studies,Citation4,Citation5 an additional measure was used, ie, the Short Form (SF)-36 questionnaire. The SF-36 is a general instrument for measuring health status and is not aligned to any specific disease or patient population,Citation10–Citation12 and selected subdomains of this questionnaire, including the 10-item Physical Function Questionnaire (PF-10), have been shown to perform just as reliably when administered separately as when administered as part of the entire instrument.Citation13 The PF-10 score constitutes a validated patient-relevant measure of physical activity in daily living that can be easy applied in day-to-day clinical practice.Citation14
The type of COPD patients who participate in observational studies often differ from those in randomized controlled clinical trials where more rigorous exclusion and inclusion criteria are defined. Observational studies performed in routine clinical practice provide more realistic evidence as to how a treatment will perform in a general clinical setting. As a consequence, the results from these studies may have a greater external validity than randomized controlled clinical trials.Citation15 In addition it should be noted that neither specific diagnostic nor therapeutic interventions are allowed in these trials. Noninterventional studies can provide additional information about the efficacy and safety of already registered drugs. The aim of this noninterventional trial with tiotropium Respimat® was to use the PF-10 questionnaire to measure changes in physical functioning of smokers and nonsmokers with COPD in everyday practice. Respimat, a multidose propellant-free active inhalation device, generates a fine, slow-moving cloud with a high fine particle fraction.Citation16 We also sought to assess the tolerability of treatment in these patients.
Methods
Study design
This noninterventional study employed a prospective design, in which patients received treatment with tiotropium Respimat for approximately six weeks. Patients were enrolled by 230 off ice-based pulmonologists in Germany. The study was conducted according to the German Medicines Act (Article 4, Section 23 and Article 67, Section 6), with the approval of the Baden-Württemberg Medical Association Ethics Committee and notified to the national authorities. All patients gave informed consent to participate.
Patients and treatments
Patients with a diagnosis of COPD who had not been treated with tiotropium in the six weeks before baseline and who required treatment with a long-acting bronchodilator were eligible for inclusion in the study.
At the first clinic visit, data were collected on demographics, smoking history, coexisting diseases, and current medications. All eligible patients started treatment with tiotropium 5 μg once daily (two puffs of 2.5 μg) via the Respimat inhaler. Throughout the study period, patients were allowed to take any other pulmonary medications. After six weeks of study treatment, patients returned for a second clinic visit.
Assessments and endpoints
At baseline and week 6, physical function was measured by the 10 self-administered questions in the PF-10 of the SF-36 questionnaire. The PF-10 questionnaire relates to whether patients are restricted in the following activities:
Vigorous activities (eg, running, lifting heavy objects)
Moderate activities (eg, moving a table, bowling)
Lifting or carrying groceries
Climbing several flights of stairs
Climbing one flight of stairs
Bending, kneeling, or stooping
Walking more than one kilometer
Walking several hundred meters
Walking one hundred meters
Bathing or dressing yourself
The sum of scores for the 10 items was standardized to a range of 0–100 points for analysis. The primary efficacy endpoint was the proportion of patients achieving “therapeutic success”, which was defined as a 10-point increase in the standardized PF-10 score between baseline and week 6. Adoption of this threshold score of a 10-point change in minimal important difference (MID) was based on a distribution-based method by Cohen.Citation17 Based on two one-year studies conducted in COPD patients,Citation4 the baseline standard deviation (SD) for Physical Functioning scores was 22. The MID ranged between 4.4 and 11.
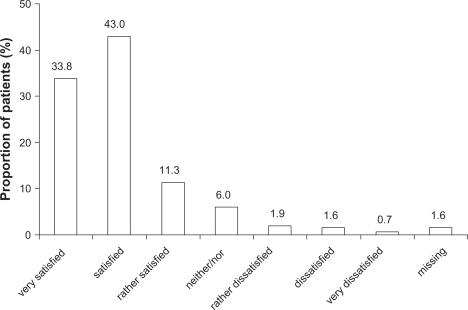
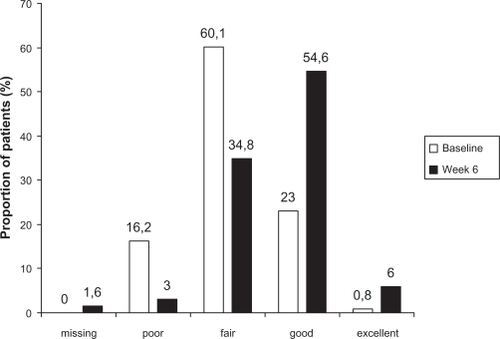
Three secondary efficacy endpoints were also defined, ie, the absolute change in standardized PF-10 score from baseline to week 6, the change from baseline to week 6 in the Physician’s Global Evaluation (PGE) score, ie, the physician’s assessment of the patient’s general condition using an eight-point scale, and, finally, patient satisfaction with tiotropium Respimat, measured at week 6 only. Satisfaction was measured using a seven-point ordinal scale from “very dissatisfied” to “very satisfied”.
Tolerability was assessed by investigators at the week 6 visit, by documenting all adverse events that had occurred during the study treatment period.
Statistical analysis
All patients in the treated set (any patients who received at least one dose of tiotropium Respimat) were analyzed for tolerability. Patients in the treated set who had a diagnosis of COPD constituted the full analysis set, which was used for analysis of PGE and satisfaction results. For the primary efficacy endpoint, results were analyzed for the efficacy set, ie, all those in the full analysis set for whom PF-10 values at baseline and week 6 were available. For all efficacy measures, results were also analyzed in two patient subgroups, ie, smokers and nonsmokers. The group of nonsmokers comprised exsmokers and never-smokers.
Descriptive statistics including differences from baseline were prepared for the standardized PF-10 score, for all patients and for the smoker and nonsmoker subgroups.
Differences between the subgroups in therapeutic success rates (measured by PF-10 scores) were analyzed using the Cochran–Mantel–Haenszel test, and the Wilcoxon signed rank test was used to analyze the difference between the absolute PF-10 scores at baseline and those at week 6. A least squares mean analysis was performed for the changes from baseline in PF-10 scores and comparison of these scores between subgroups (by smoking status); this analysis was adjusted for baseline PF-10 score and for region (federal state). For estimated therapeutic success rates, 95% confidence intervals (CI) were calculated. P values of < 0.05 were considered statistically significant.
Results
Patient disposition
A total of 1280 patients were enrolled from 230 centers. These formed the treated set that was used for the safety analysis. Because all patients in the treated set had a diagnosis of COPD, the full analysis set (used for all efficacy analyses other than the PF-10 endpoint) was also 1280 patients. Data on PF-10 were missing for 50 of the patients in the full analysis set, so the efficacy set consisted of 1230 patients. Fifty-seven of 1280 patients withdrew from the study prematurely, 36 because of adverse events and 21 for other reasons.
The baseline characteristics and demographic profiles of the full analysis set and efficacy set are shown in . More men than women were enrolled, the mean age of the sample was 65.5 years, and the mean duration of COPD was 7.5 years. Just over one-third of the patients (35.5%) were current smokers; 59.9% of smokers and 61.5% of nonsmokers were male patients. Mean age of smokers and nonsmokers was 61.0 (± 10.4) years and 68.0 (±9.8) years, respectively.
Table 1 Patient demographics and characteristics at baseline
In addition to COPD, 71.8% (919) of patients had other diseases, most commonly cardiac in nature (44.5%, n = 569). Vascular disorders and metabolic or endocrine disorders were also prevalent, affecting 22.3% (285) and 18.8% (241) of patients, respectively, and 11.8% (151) of patients had additional pulmonary disorders. Pulmonary comedications were being taken by 83.5% of patients in the full analysis set and 84.1% of the efficacy set. The most common of these were short-acting beta-agonists (54.9%), long-acting beta-agonists either alone (32.0%) or combined with inhaled corticosteroids (26.5%), inhaled corticosteroids alone (21.8%), and theophylline (17.4%).
Efficacy
Physical function
For the primary efficacy endpoint, ie, improvement in the PF-10 score, 61.5% of the patients in the efficacy set achieved “therapeutic success” (95% CI: 58.8%–64.3%). There was no statistically significant difference in the success rate between smokers (61.4%; 95% CI: 56.6%–66.0%) and nonsmokers (61.6%; 95% CI: 58.2%–65.0%; P = 0.93 for difference).
Absolute changes in PF-10 subdomain scores after six weeks are shown in . Mean scores for all patients improved from 49.0 points (SD, 24.5) at baseline to 62.3 (23.5) points at week 6, giving a mean difference of 13.4 points (15.9) that was statistically significant (P < 0.001) and exceeded the minimal important difference of 10 points. Mean score improvements from baseline were also statistically significant in both smoking subgroups (P < 0.001), despite a significantly higher mean baseline PF-10 score in smokers (52.4; 95% CI: 50.0–54.7) than in nonsmokers (47.1; 95% CI: 45.4–48.8). The mean score improvements in both subgroups, from 52.4 to 65.0 in smokers and from 47.1 to 60.8 in nonsmokers, exceeded the MID of 10 points, and least squares mean analysis showed no significant difference between subgroups in the change from baseline to week 6.
Table 2 Absolute values of standardized PF-10 score at baseline and week 6 in efficacy set (n = 1230)
Other efficacy endpoints
The change in PGE scores from baseline to week 6 showed an improvement in patients’ general condition during the study (). The proportion of patients rated as poor (score of 1 or 2) fell from 16.2% (207) to 3.0% (39) and the proportion with a rating of good (score of 5 or 6) more than doubled, from 23.0% (294) to 54.6% (699). The pattern of results in smokers was very similar to that in nonsmokers (data not shown).
Figure 1 Distribution of Physicians’ Global Evaluation scores for all patients in the full analysis set (n = 1280) at baseline and week 6.
At week 6, 76.9% (984) of patients rated their level of satisfaction with the inhalation device as “satisfied” or “very satisfied” (). The ratings in smokers were very similar to those in nonsmokers (data not shown).
Safety
Patients were exposed to tiotropium for a mean of 47.6 days (SD, 15.2 days), the median exposure being 44 days. Fifty-one of 1280 patients in the treated set (4.0%) reported adverse events during the study and 36 patients (2.8%) discontinued study treatment because of adverse events. Twenty-seven patients (2.1%) experienced adverse events that were judged to be related to study medication. On the whole, the events fell into one of two groups, either continuing symptoms and signs of COPD, such as cough, dyspnea, and chest infections, or typical anticholinergic side effects, such as dry mouth (the most common single event, observed in 11 patients) and tachycardia (observed in 3 patients). Five patients each reported dizziness and headache. All reported events are listed by system organ class in .
Table 3 Number of patients in the treated set (n = 1280) reporting adverse events, by system organ classTable Footnote*
In all, 16 serious adverse events were reported by six (0.5%) patients. Six of these events occurred in one patient (benign prostatic hypertrophy, urinary tract infection with urinary retention, constipation, azotemia, and transient collapse). At the time of the last recorded contact with the patient, the patient had recovered from the collapse except for the urinary tract events. No deaths occurred during the study.
Discussion
The present study is the first observational study that investigates the effect of tiotropium Respimat on physical activity in a large patient population in primary care. We showed that PF-10 represents a valid and feasible measure of physical activity in daily living. This is in contrast with other more complex instruments, such as SGRQ, which are of limited use in day-to-day clinical practice.
The majority (61.5%) of patients who received a once-daily dose of tiotropium Respimat for six weeks achieved therapeutic success, as measured by an increase in the standardized PF-10 score of at least 10 points. Results for the other efficacy measures supported the primary endpoint findings. Physical function improved overall during the six-week study period, as shown by a significant increase in the mean standardized PF-10 score of 13.4 points. In addition, the improvement in the distribution of PGE scores at week 6 for all participants showed that patients were generally in a healthier condition than at baseline. The improvement in PF-10 scores that we recorded is similar to the results of two other observational studies of tiotropium in the HandiHaler® at a daily dose of 18 μg;Citation18,Citation19 PF-10 increased by 13.3 and 15.8 points, respectively, and PGE scores improved from baseline.
Patient-reported outcomes have been studied in previous clinical trials of tiotropium, usually with the SGRQ, a specific measure of health status for patients with obstructive lung disease.Citation9 The SF-36 questionnaire, of which the PF-10 is the largest individual subdomain, is a general health status measure which is used less frequently than SGRQ in studies of patients with COPD. Nevertheless, a pooled analysis of two one-year studies of tiotropium by Casaburi et al used both SGRQ and SF-36 to assess health status and found that mean scores for the physical health subdomains, including physical function, were significantly better for tiotropium than placebo throughout the study.Citation4 Another similar combined analysis of two studies reported by Vincken et al found that after one year’s treatment, tiotropium was associated with significantly higher scores than ipratropium for some physical health subdomains of the SF-36 but the authors did not state how much SF-36 scores had improved between baseline and the end of the trial.Citation5
The safety profile of tiotropium in this observational study was consistent with accumulated clinical trial experience.Citation20,Citation21 In all, only 4% of patients reported adverse events, although this result reflects the less stringent reporting procedures for adverse events in observational studies compared with a randomized clinical trial.Citation4,Citation22 Because it was a short-term six-week observational study, no conclusions can be made on any long-term adverse events.
When analyzed by subgroup, our results showed that both smokers and nonsmokers experienced physical function improvements with tiotropium therapy, with no significant differences in treatment responses between these two subgroups either in the proportion who achieved therapeutic success or the absolute change in PF-10 scores from baseline. This result was remarkable, given that mean baseline PF-10 scores were significantly higher in smokers than nonsmokers. This difference in subgroups at baseline could have been due to a difference in COPD severity, ie, GOLD (Global Initiative for Chronic Obstructive Lung Disease) stage between the two treatment groups. In addition, smokers were of younger age compared with nonsmokers (median ages 61 and 68 years, respectively).
Another outcome assessed in this study was satisfaction with the inhalation device. Approximately 77% of the participants were either satisfied or very satisfied with the Respimat inhaler, and 4.2% in total reported some level of dissatisfaction (from “rather dissatisfied” to “very dissatisfied”). These findings are consistent with results of controlled clinical trials that have measured patient satisfaction with the Respimat inhaler using a validated questionnaire specific for inhalation devices.Citation23,Citation24
Our study had some limitations. The patients in this noninterventional, open-label, nonrandomized, observational study were relatively unselected compared with those typically enrolled into randomized controlled trials; entry to the study required a diagnosis of COPD, the need for treatment with a long-acting bronchodilator, and no treatment with tiotropium in the preceding six weeks. Although the benefits in physical functioning were not confirmed by assessing exercise capacity with, for example, the six-minute walk test, the lack of patient selection allows our findings to be more readily generalized to a real-life setting, in which COPD patients have a wide range of comorbidities. Another possible limitation was the short duration of our study. Whether improvement of physical functioning can be maintained over a longer period of time needs further clarification. In addition, this study was neither designed nor powered to study the potential impact of comedication on the results of this study.
Strengths of this noninterventional study include the large patient sample size, the high number of participating physicians and inclusion of patients with coexisting diseases and a wide spectrum of disease severity and treatment tailored to the individual patient. By contrast, randomized clinical trials usually have a distinct group of patients as a result of specific exclusion and inclusion criteria regarding concomitant diseases and therapy, and the study protocol may not be representative of clinical practice.Citation25 Accordingly, this observational study includes typical COPD patients from a real life primary care setting and reflects current treatment approaches, thus complementing the findings of randomized controlled trials. In summary, this observational study showed that treatment with inhaled tiotropium administered via Respimat inhaler was associated with rapid improvements in physical functioning in COPD patients in a real-life setting, irrespective of smoking status.
Disclosure
Medical writing support was provided by Lexeme, UK, and funding for this support was from Boehringer Ingelheim.
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD)Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease Updated 2009. Available from: http://www.goldcopd.org. Accessed 2010 Sep 16.
- WoutersEFCreutzbergECScholsAMSystemic effects in COPDChest2002121Suppl 5S127130
- DecramerMRennardSTroostersTCOPD as a lung disease with systemic consequences – clinical impact, mechanisms, and potential for early interventionCOPD2008523525618671149
- CasaburiRMahlerDAJonesPWA long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary diseaseEur Respir J20021921722411866001
- VinckenWvan NoordJAGreefhorstAPMImproved health outcomes in patients with COPD during 1 yr’s treatment with tiotropiumEur Respir J20021920921611871363
- DonohueJFvan NoordJABatemanEDA 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterolChest2002122475512114338
- BrusascoVHodderRMiravitllesMKorduckiLTowseLKestenSHealth outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPDThorax20035839940412728159
- TashkinDPCelliBSennSA 4-year trial of tiotropium in chronic obstructive pulmonary diseaseN Engl J Med20083591543155418836213
- JonesPWQuirkFHBaveystockCMLittlejohnsPA self-complete measure of health status for chronic airflow limitationAm Rev Respir Dis1992145132113271595997
- BrazierJEHarperRJonesNMBValidating the SF-36 health survey questionnaire: New outcome measure for primary careBMJ19923051601641285753
- WareJEJrSherbourneCDThe MOS 36-item Short-form Health Survey (SF-36). 1: Conceptual framework and item selectionMed Care1992304734831593914
- WareJEJrGandekBOverview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) ProjectJ Clin Epidemiol1998519039129817107
- GummessonCAtroshiAEkdahlCPerformance of health-status scales when used selectively or within multi-scale questionnaireBMC Med Res Methodol20033312585967
- GlaabTVogelmeierCBuhlROutcome measures in chronic obstructive pulmonary disease (COPD): Strengths and limitationsRespir Res2010117920565728
- MichelMGoepelMTreatment satisfaction of patients with lower urinary tract symptoms: Randomised controlled trials vs real life practiceEur Urol200038Suppl 1404711111207
- VoshaarTLapidusRMaleki-YazdiRA randomized study of tiotropium Respimat® Soft Mist inhaler vs ipratropium pMDI in COPDRespir Med2008102324117996436
- CohenJStatistical Power Analysis for the Behavioral Sciences1st revised edHillsdale, NJLawrence Erlbaum Associates Inc1977
- GlaabTRau-BergerHWolfKFreytagFTiotropium improves quality of life and physical function in COPDAm J Respir Crit Care Med2008177 Abstr 646.
- GlaabTRau-BergerHImprovement in physical functioning in COPD patients with tiotropiumAm J Respir Crit Care Med2009179 Abstr 4559.
- KestenSJaraMWentworthCLanesSPooled clinical trial analysis of tiotropium safetyChest20061301695170317166984
- KestenSCelliBDecramerMLeimerITashkinDTiotropium HandiHaler® in the treatment of COPD: A safety reviewInt J Chron Obstruct Pulmon Dis2009439740920037679
- van NoordJACornelissenPJGAumannJ-LPlatzJMuellerAFogartyCThe efficacy of tiotropium administered via Respimat® Soft Mist Inhaler or HandiHaler® in COPD patientsRespir Med2009103222919022642
- SchürmannWSchmidtmannSMoroniPMasseyDQidanMRespimat® Soft Mist™ inhaler versus hydrofluoroalkane metered dose inhaler: Patient preference and satisfactionTreat Respir Med20054536115725050
- HodderRReesePRSlatonTAsthma patients prefer Respimat® Soft Mist™ Inhaler to Turbuhaler®Int J Chron Obstruct Pulmon Dis2009422523219554196
- ConcatoJShahNHorwitzRRandomized, controlled trials, observational studies, and the hierarchy of research designsN Engl J Med20003421887189210861325