Abstract
Obesity is a major risk factor for type 2 diabetes mellitus (T2DM), which is a significant health problem worldwide. Active disease is associated with low-grade chronic inflammation resulting in part from the activation of the innate immune system. In obesity, this activation leads to the release of pro-inflammatory cytokines such as tumor necrosis factor-α, interleukin-1β and interleukin-6 that block major anabolic cascades downstream of insulin signaling and thus disrupt insulin homeostasis and action. Cytokines also trigger the production of acute-phase reactants such as C-reactive protein, plasminogen activator inhibitor-1, serum amyloid-A, and haptoglobin. The elevated synthesis of pro-inflammatory cytokines and acute-phase proteins (inflammatory network) characterizes the early (or pre-clinical) stages of T2DM and exhibits a graded increase with the disease progression. Current evidence suggests that understanding inflammatory networks can point to new biomarkers that may permit capturing the interaction between genetic and environmental risk factors in the pathogenesis of T2DM. Such biomarkers have a significant public health potential in the prediction of disease occurrence beyond risk factors presently monitored, such as family history, lifestyle assessment and standard clinical chemistry profiles. Furthermore, inflammatory markers may assist in the evaluation of novel strategies for prevention, particularly in relation to micronutrients. This review discusses the current knowledge linking T2DM risk to inflammatory signaling pathways interacting with the innate immunity system and the prospect of inflammatory markers serving as molecular targets for prevention and/or biomarkers for early risk prediction of T2DM. The potential of micronutrients replenishment to improve insulin action by attenuating inflammation is also evaluated in the context of the public health relevance of this approach.
Introduction
Type 2 diabetes mellitus (T2DM) represents a significant global health problem. It is estimated that six people die every minute from the disease worldwide, a figure that will soon make T2DM one of the world’s most prevalent causes of preventable mortality.Citation1 The incidence of disease increases with age, obesity, physical inactivity, unhealthy diet, and ethnicity (Hispanics, Africans, and Aboriginals) and the rates are increasing among children.Citation2,Citation3 T2DM is caused by impaired glucose tolerance (IGT) as a result of insulin resistance and consequential islet β-cell exhaustion, with ensuing insulin deficiency impacting skeletal muscle, liver and adipose tissues.Citation4 In individuals with IGT, numerous genetic, host-related, and environmental factors contribute to the progression of insulin resistance to T2DM.Citation5–Citation9 Obesity, however, is a major cause of insulin resistanceCitation7 and can be complicated by metabolic dysregulation including hypertension and dyslipidemia (known collectively as the metabolic syndrome) which is a precursor of T2DM. The dyslipidemia involves high levels of triacylglycerides and circulating fatty acids originating from the diet or accelerated lipolysis in adipocytes. Direct exposure of muscle cells to these fatty acids impairs insulin-mediated glucose uptake and, therefore, may contribute to insulin resistance.Citation10,Citation11
Within the last decade, a hypothesis was proposed to explain the pathogenesis of T2DM that connects the disease to a state of subclinical chronic inflammation.Citation12,Citation13 Inflammation is a short-term adaptive response of the body elicited as a principle component of tissue repair to deal with injuries and microbial infections (eg, cold, flu, etc.). It can be also elevated in chronic conditions such as peripheral neuropathy, chronic kidney disease and fatty liver. While the influence of fats is well known (see below), current thinking suggests that abnormal levels of chemokines released by the expanding adipose tissue in obesity activate monocytes and increase the secretion of pro-inflammatory adipokines. Such cytokines in turn enhance insulin resistance in adipose and other tissues, thereby increasing the risk for T2DM.Citation14,Citation15 Together, lipid toxicity and low-grade inflammation appear to be major assaults on insulin sensitivity in insulin-responding tissues.Citation11,Citation16,Citation17
Activation of innate immunity promotes various inflammatory reactions that provide the first line of defense the body invokes against microbial, chemical, and physical injury, leading to repair of damage, isolation of microbial infectious threats and restoration of tissue homeostasis.Citation18,Citation19 Inherited variations in the degree of innate immune response may determine the lifetime risk of diseases upon exposure to adverse environmental stimuli.Citation20 Therefore, innate immune responses can be viewed as the outcome of interaction between genetic endowment and the environment.Citation21 The systemic reaction of innate immunity, known as the ‘acutephase response’, follows exposure to an exogenous insult (such as a pathogen or dietary factor) which triggers the release of pro-inflammatory cytokines like tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6.Citation22 These cytokines are derived primarily from macrophages and can directly enhance insulin resistance in adipocytes, muscle and liver cells.Citation11,Citation17 Macrophages infiltrate the expanding adipose tissue in obesity to remove dying cells and contribute to tissue angiogenesis.Citation23 The adipose tissue of obese rodents and humans is indeed excessively infiltrated by macrophages and dendritic cells.Citation11 Subsequent activation of macrophages towards an inflammatory phenotype results in cytokine synthesis and release.Citation24,Citation25 Cytokines then downregulate major anabolic cascades involved in insulin signaling and mediate adipocyte insulin resistance.Citation24,Citation25 Ultimately, this contributes to disruption of whole-body insulin sensitivity and impairs glucose homeostasis.Citation26
In addition to their effect on insulin resistance, cytokines also act on the liver to increase the production of very-low density lipoproteins (VLDL), leading to the characteristic diabetic dyslipidemia,Citation27 and to stimulate the hepatic production of fibrinogen, an atherosclerotic risk factor.Citation12 Furthermore, cytokines deactivate the liver X receptors (LXR), resulting in an increased rate of cholesterol accumulation,Citation28 and ultimately trigger the hepatic production and secretion of acute-phase proteins such as C-reactive protein (CRP), plasminogen activator inhibitor-1 (PAI-1), serum amyloid-A, α1-acid glycoprotein, and haptoglobin (collectively known as inflammatory factors or markers) into the circulation. The synthesis of acute-phase reactants following the cytokine release characterizes the pre-clinical (or early) stages of T2DMCitation21 and exhibits graded increases as the disease progresses and clinical complications ensueCitation12 (see below).
Extensive experimental, clinical and epidemiological studies have linked obesity causally to the activation of inflammatory signaling pathways and to the subsequent manifestations of T2DM.Citation7,Citation29,Citation30 This relationship may facilitate the development of preventive measures for the disease at the early stages, as well as its main antecedent, obesity. For example, pharmaceutical and nutritional factors that reduce inflammation and the ensuing acute-phase reactant responses could be employed to improve insulin sensitivity and delay disease onset.Citation31–Citation33 Furthermore, a subset of the pro-inflammatory cytokines and inflammatory factors may provide a phenotypic profile that can be utilized as a sensitive and specific composite biomarker for early detection of risk to T2DM. Taken together, these approaches may aid in reducing the rising rates of disease incidence and better address issues related to prevention and treatment.
This review was undertaken to examine current knowledge linking inflammatory signaling pathways within the innate immune system to the risk of T2DM. The possibility that inflammatory markers could serve as molecular targets for prevention and/or biomarkers for early risk prediction of T2DM is evaluated in the context of enhancing public health strategies for diabetes prevention and control.
Obesity, T2DM, and inflammation: Molecular mechanism(s) of association
In obese people, insulin resistance is linked to the increased release of adipocyte-derived bioactive metabolites (ADBMs) such as lipids, free fatty acids, monocyte chemoattractant protein- 1 (MCP-1), and pro-inflammatory cytokines.Citation30 It should be emphasized, however, that although obesity is viewed as a predisposing factor to insulin resistance, other factors may also contribute. A study of young, insulin- resistant, lean offspring of patients with T2DM and insulin-sensitive controls of similar body mass index (BMI) showed similar plasma concentrations of TNF-α, IL-6, and adiponectin between the insulin-resistant and insulin-sensitive groups.Citation34 This suggests that in lean people, systemic inflammation may not play a significant role in the development of insulin resistance. In this case, proposed mechanisms for insulin resistance might then be attributed to a dysregulation of intramyocellular fatty acid metabolism.Citation14 In the liver this would also include an altered expression of transcription factor 6-α (ATF6) which controls expression of gluconeogenic genes.Citation35 Genetic predisposition also may contribute to the development of T2DM. Genome-wide association (GWA) and candidate gene studies over the past few years have so far uncovered 19 genes associated with T2DM.Citation36 The disease-related genetic variants identified have high frequencies in the populations assessed although their individual contributions to increases in risk of T2DM are modest. Ongoing GWAs that target lowfrequency genetic variants and assess copy number variants (CNVs) in addition to single nucleotide polymorphisms (SNPs) are likely to identify additional loci associated with T2DM risk, and some of these may play a significant role in the risk of disease development.Citation36 In lean subjects with T2DM, the dysregulation of fatty acid metabolism, the abnormal expression of gluconeogenic genes and the genetic predisposition necessitate the development of an additional set of biomarkers that target this subpopulation and relate to these risk factors.
In the obesity-related insulin resistance, once adipocytes are activated (ie, accumulate fat and exhibit caloric excess), they release abnormal levels of ADBMs, leading to the recruitment of monocytes within adipose tissues.Citation30 Monocyte differentiation into the macrophages lineage results in additional increases in the release of inflammatory factors and chemokines, propagating inflammatory responses both locally within adipose tissue and systemically elsewhere.Citation14,Citation17,Citation30 In both humanCitation37 and animalCitation16 models, TNF-α gene expression is upregulated in adipose tissues during obesity, linking pro-inflammatory substances released from adipose tissues to insulin resistance in T2DM.
The role for pro-inflammatory cytokines in regulating insulin action and glucose homeostasis and their function in T2DM has been suggested by several lines of evidence. For example, compared to healthy individuals, subjects with T2DM risk factors such as obesity, hyper-triglyceridemia, or low high-density lipoprotein (HDL)-cholesterol exhibit higher serum levels of pro-inflammatory cytokines and acute-phase reactants than those who are not so predisposed.Citation13 Cytokines also show a gradual increase as the disease progresses to its complications.Citation13 Additionally, numerous prospective trials demonstrate that initial high levels of pro-inflammatory factors are associated with the manifestations of the disease.Citation38–Citation43 Baseline circulating concentrations of IL-6, PAI-1, CRP, and fibrinogen were significantly higher in healthy subjects who became diabetic later in life (ie, after 4–10 years) compared to those who did not develop the disease.Citation38–Citation43 Furthermore, exogenous administration of TNF-α or IL-6 results in insulin resistance,Citation16,Citation17,Citation37 whereas low serum levels of cytokine in knock-out miceCitation17,Citation44 or treatment with anti-TNF-α agents,Citation31 improved insulin sensitivity and glucose homeostasis. However, TNF-α is unlikely to be the only culprit for inducing insulin resistance and indeed many cytokines may act in synchrony to elicit the condition. Taken together, these observations suggest that inflammation is a feature when IGT develops at the pre-clinical stages of T2DM, perhaps at the overweight or obesity (see below).
The mechanisms that govern the association between the increased synthesis of inflammatory factors and T2DM are still being elucidated. In macrophages, adipocytes, antigen-presenting B-cells, dendritic cells, and Kupffer cells in the liver, a number of germline-encoded pattern recognition receptors (PRRs), such as the toll-like receptors (TLR), are activated upon ligand binding with conserved structural motifs that are either specific patterns of microbial components (eg, bacterial lipopolysaccharide [LPS])Citation45 or nutritional factors (eg, free fatty acids [FFAs]).Citation11,Citation18,Citation46–Citation48 Binding to PRRs gives rise to inflammatory responses by mediating downstream transcriptional events that activate nuclear factor-κB (NFκB) and activator protein-1 (AP-1) and their pathways (see ).Citation19 Upon activation, these intracytoplasmic molecular cascades upregulate the transcription of pro-inflammatory cytokine genesCitation22 and, consequently, the synthesis of acute-phase inflammatory mediatorsCitation49–Citation51 and activation of c-Jun N-terminal kinase (JNK) and inhibitor of NFκB kinase-β (IKK). In liver and adipose tissue, these two molecules can inactivate the first target of the insulin receptor (INSR), IRS-1, thereby reducing downstream signaling towards metabolic outcomes.Citation52,Citation53 The interrelationship between these molecular targets suggests the expression and action of PRRs are the primary effector molecules in a downstream cascade that initiate dysregulation of insulin homeostasis. Therefore, altered expression/action, eg, as a result of SNP, in these PRRs may be employed to predict the possible development of T2DM (or earlier predisposing conditions) upon exposure to high levels of their ligands.
Figure 1 The interaction between insulin signaling and fatty acids in the synthesis of pro-inflammatory cytokines and inflammatory markers.
Abbreviations: AKT, protein kinases B (PKB); AP-1, activator protein-1; APPs, acute phase proteins; G, glucose; GLUT4, glucose tansporter-4; I, insulin; IKK, inhibitor of NFκB kinase-β; IL-1β and -6, interleukin-1 β and -6; INSR, insulin receptor; IRAK1 and 4, interleukin-1 receptor-associated kinase 1 and 4; IRS, insulin substrate; JNK, c-Jun amino-terminal kinase; MyD88, myeloid differentiation primary response gene-88; NFκB, nuclear factor κ B; PI3K, phosphoinositide-3 kinase. PI(3,4,5)P3, Phosphatidylinositol 3,4,5-trisphosphate. PKC, protein kinase C. TAB, TAK binding protein. TAK1, mitogen-activated protein kinase kinase kinase (MAPKKK). TLR4, toll-like receptor-4; TNFα, tumor necrosis factor-α; TRAF6, TNF receptor-associated factor 6.
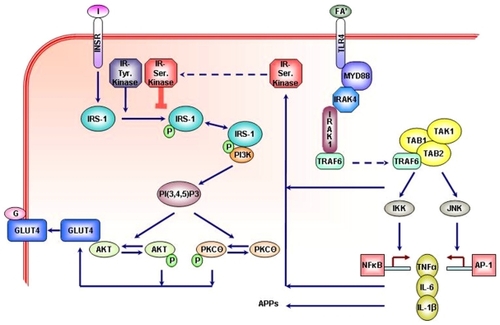
In addition to the activation of inflammation signals, strong evidence supports a direct role of FFAs in promoting insulin resistance. This has been ascribed to actions of their intracellular metabolites, primarily ceramide and diacylglycerol, on proteins that impinge on insulin-derived signals. Mixtures of FFAs arterially injected in vivo induced the activation of protein kinase C (PKC)-Θ or JNK in skeletal muscle.Citation11,Citation17,Citation52 PKC-Θ, like JNK, can inactivate IRS-1 and its downstream signaling towards metabolic outcomes.Citation52 This is achieved by distinct serine phosphorylation of IRS-1 that reduces the signal-bearing tyrosine phosphorylation.Citation29 In contrast, saturated FFAs act largely to produce ceramides that spare IRS-1 but still inhibit insulin action downstream, at the level of the serine/threonine kinase Akt to also inhibit metabolic outcomes.Citation54,Citation55 Interestingly, direct exposure of insulin-target cells to cytokines such as TNF-α and IL-1β also activates JNK and IKK,Citation56 making these factors a common link in the molecular mechanism of lipotoxicity and inflammation leading to insulin resistance and T2DM via post-transcriptional actions.Citation53 Indeed, heterozygous deletion in IKKβ (ie, in IKKβ+/− mice) improved insulin sensitivity in obese mice target cells.Citation57 In addition, TNF-α upregulates the expression of suppressor of cytokine signaling (SOCS) proteins, which bind to IRS-1 to mediate its degradation.Citation58 Furthermore, cytokine-mediated transcriptional up-regulation of JNK and IKK increases the expression of AP-1 and NFκBCitation59 that, in turn, activate TNF-α to further propagate local and systemic inflammatory responses.Citation60 Hence, JNK–AP-1 and IKK–NFκB are the major inflammatory pathways that disrupt insulin signaling via a series of transcriptional events and can be potentially modulated to improve insulin sensitivity and glucose homeostasis.Citation17
An additional and highly relevant interaction between lipids and inflammation is the recent discovery that FFAs can directly activate macrophages to produce cytokines that render muscle cells insulin resistant.Citation11,Citation61 Such studies in cell culture are proof of principle that elevated FFAs can activate cells of the innate immune system to provoke muscle cell insulin resistance. In this case, both the macrophages and muscle cells showed activated JNK and IKK.
Inflammatory network: Biomarkers of T2DM
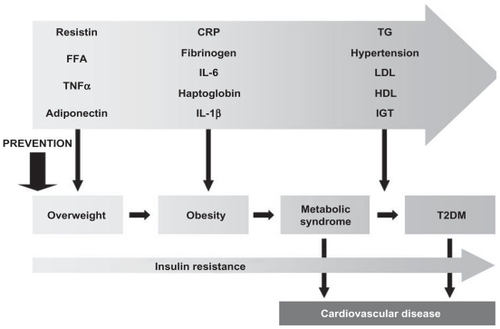
It is estimated that up to 25% of patients with newly diagnosed T2DM already present evidence of systemic inflammation at the time of diagnosis,Citation62 suggesting that the disease (or the hypertriglyceridemia) has been present already for years since the development of status that predisposes to the disease (eg, overweight; see ).Citation63–Citation65 Current approaches to diabetes screening include determination of an elevated fasting plasma glucose level (≥126 mg/dL, ie, 7.0 mmol/L) on two occasions, or an oral glucose tolerance test (OGTT) yielding ≥200 mg/dL (11.1 mmol/L) after 2 h, or symptoms of uncontrolled diabetes with a random plasma glucose level ≥200 mg/dL (11.1 mmol/L). In addition, a score of glycosylated hemoglobin levels above 7% (HbA1c ≥0.07) has been suggested as evidence of disease.Citation4 None of these techniques has been found to detect all incident cases of T2DM and there is still a need to establish more sensitive and specific predictors of early risk.Citation66 Although inherited dysfunction in insulin signaling plays a role in the risk of T2DM, it is likely that any genetic component will be modified by environmental factors. Capturing the contribution of these genetic factors and their interaction with environmental covariates (eg, diet, physical activity, etc.) in the development of T2DM may provide an effective approaches for early detection and prevention.Citation66,Citation67
Figure 2 The relationship of inflammatory markers and disease factors to specific stages pathologic continuum from overweight to T2DM and cardiovascular diseases.Citation63,Citation64
Abbreviations: CRP, C-reactive protein; FFA, free fatty acids; IGT, impaired glucose tolerance; IL, interleukin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglycerides; T2DM, type 2 diabetes mellitus.
Inherited variations such as SNPs or CNVs in genes encoding for innate immunity-related inflammation may play a pivotal role in the susceptibility to T2DM and an array of chronic diseases.Citation68–Citation72 Genetic polymorphisms, in conjunction with various environmental factors, may affect serum levels of cytokines and inflammatory markers and the subsequent risk of T2DMCitation68–Citation71 (see ). Identification of SNPs or CNVs of inflammation-related genes, their frequencies and their association with risk of T2DM will assist in defining the genetic basis of the disease and early detection of risk.
Table 1 Selected pro-inflammatory cytokine gene polymorphisms positively associated with T2DM and related risk factors and clinical complicationsCitation68–Citation72 Table Footnotea
The feasibility of utilizing inflammatory markers in screening T2DM risk can be substantiated from numerous experimental, clinical and epidemiological observations demonstrating: a) the ability of inflammatory factors to predict the disease independently from established risk factors, Citation73–Citation76 b) their link to one another by virtue of their common action, and c) the availability of quantitative, lessinvasive methods of assay, that would allow for repeated measurements. These characteristics render inflammatory mediators as potential candidates for a panel of powerful biomarkers for predicting T2DM incidence. As indicated in , overweight sets the stage for low-grade chronic inflammation, with adiponectin levels decreasing while resistin, FFAs and TNF-α increase. As overweight progresses to obesity, continued inflammation further leads to elevated CRP, fibrinogen, IL-6, IL-1β and haptoglobin. Obesity can be complicated by metabolic dysregulation (metabolic syndrome) to develop frank T2DM where LDL-cholesterol and triglyceride levels increase, HDL-cholesterol levels deceases and hypertension and IGT manifest.Citation9,Citation29,Citation30,Citation65,Citation66,Citation77 Throughout the pathologic continuum from overweight to T2DM, insulin resistance increases progressively and the risk of cardiovascular disease (CVD) elevates. Metabolic syndrome is associated with about twofold increased susceptibility to CVD whereas T2DM is linked to fourfold higher risk.Citation63,Citation64 Public health initiatives aimed at preventing and controlling T2DM should be targeted towards the early stages of the disease, to prevent obesity and the cascade of inflammatory events that eventually leads to the clinical manifestation of T2DM. We next discuss the characteristics of cytokines and chemokines with potential to become biomarkers of inflammation and insulin resistance associated with obesity, ie, early T2DM risk detection.
TNF-#α
TNF-α is a primary mediator of many of the systemic acute responses related to severe infections with gramnegative bacteria.Citation78 The major cellular source of TNF-α is activated mononuclear phagocytes, antigen-stimulated T-cells, natural killer (NK) cells, and mast cells. The TNF-αgene is constitutively expressed in adipose tissue, where it originates principally from macrophage infiltration rather than from the adipocytes themselves.Citation16 In adipose tissue from obese subjects, TNF-α mRNA expression is 2.5-fold higher than in lean subjects and is strongly correlated with hyperinsulinemia.Citation26 Body weight reduction in obese peopleCitation26 and in vivo TNF-α inhibitionCitation16 significantly reduced serum TNF-α and improved insulin sensitivity. High TNF-α is related to the pathophysiology of insulin resistance and T2DM,Citation45 possibly through its impact on IRS-1 as discussed above,Citation16,Citation26,Citation58,Citation79 or by enhancing the apoptosis of pancreatic β-cells.Citation80
The association of the TNF-α gene polymorphisms with T2DM has been extensively investigated but with conflicting results. Some studies demonstrate a strong association between TNF-α SNPs and T2DMCitation81–Citation83 () whereas others show no relationship.Citation84,Citation85 Although a number of variants have been identified in the TNF-α gene, the functional SNPs rs361525 (−238 G>A) and rs1800629 (−308 G>A) were identified in the promoter region. The functionality of the variant −308A allele is related to a twofold increase in transcriptional activity compared to the wild-type allele.Citation86 Interestingly, this variant was suggested to play a critical role in the genetic predisposition to excessive fat accumulation in women in early stages of obesity,Citation82 and to predict the conversion from IGT to T2DM.Citation81 The rs1800629 SNP exhibited a gene–gene interaction effect with the IL-6 rs1800795 (−174 C>G) in the early risk prediction of T2DM.Citation81 On the other hand, the rs361525 is located in a repressor site of the promoter, a 10 bp sequence with homology to the binding site of the activator protein AP-2 regulating TNF-α function. Citation87 Another SNP in TNF-α which was recently reported to be associated with increased risk of T2DM, is rs1800610 (IVS1+123 G>A).Citation83 The functional relevance of this SNP pertains to the YY1 transcription factor that binds specifically to the intron 1 region where the rs1800610 resides and evokes increased TNF-α expression.Citation83
IL-6
IL-6 is produced by many cell types including fibroblasts, endothelial cells, and monocytes-macrophages.Citation88,Citation89 However, a significant proportion of the circulating IL-6 (15%–30%) rep., repeats; TNFα, tumor necrosis factor-α; T2DM, type 2 diabetes mellitus. derives from adipose tissue production in the absence of acute inflammationCitation90 to modulate adipocyte glucose and lipid metabolism.Citation91,Citation92 In obesity, IL-6 synthesis is upregulated in adipocytesCitation88,Citation93 and correlates with circulating IL-6Citation94 and with the extent of insulin resistance.Citation77,Citation93,Citation95 IL-6 also triggers the hepatic synthesis of CRP and correlates with its serum levels. Citation94,Citation95 Additionally, IL-6 promotes hepatic VLDL secretion and hypertriglyceridemia.Citation27,Citation96 These observations suggest a link between IL-6 levels, obesity and inflammation in the pathogenesis of T2DMCitation97 and demonstrate that IL-6 can be considered as a candidate biomarker for early T2DM risk detection.
Several mechanisms have been proposed to explain how IL-6 can mediate insulin resistance.Citation17,Citation56,Citation77,Citation93,Citation95,Citation98 The IL-6 receptor (IL6R) belongs to the cytokine class I receptor family that includes the JNK signal transduction pathway.Citation53,Citation99 JNK activation by IL-6 induces STAT (signal transducers and activators of transcription) phosphorylation, dimerization, and translocation to the nucleus to regulate the transcription and function of an array of target genes including IRS, AP-1, and NFκB Citation53,Citation59 in hepatocytes and adipocytes.Citation100 Transcriptional upregulation of these genes promotes both localized and systemic inflammatory responsesCitation60 to mediate fatty liver and insulin resistance.Citation101 The interaction between IL-6 and the insulin pathway also involves mediating the interaction between SOCS proteins and the INSR.Citation102–Citation104 Therefore, IL-6 antagonists and factors that target JNK and IKK may offer promising preventive and/or therapeutic approaches to improve insulin sensitivity.Citation32,Citation105
In humans, numerous studies have demonstrated that elevated levels of IL-6 are associated with an increased incidence of T2DM independent of obesityCitation73–Citation76 or fasting insulin levels.Citation74 The relative risk of the disease for individuals with IL-6 levels in the highest tertile (vs lowest tertile) was 2.02 (95% confidence interval [CI]: 1.14–3.58), after adjustment for BMI, lifestyle factors, and pre-existing CVD.Citation73 Furthermore, chronically elevated IL-6 was related to a range of metabolic abnormalities typical of an insulin resistant state.Citation97 Several polymorphisms exist in the IL-6 and IL6R (). Two common SNPs in the promoter region of IL-6, rs1800795 (−174 G>C) and rs1800796 (−572 G>C) were examined for their role in predisposition to T2DM. These SNPs were investigated in a large meta-analysis of >20,000 participants from 21 published and unpublished studies for their association with T2DM.Citation106 The allele −174C was associated with reducing the risk of T2DM by nearly 10% (odds ratio [OR] = 0.91; P < 0.037) and exhibited a gene–gene interaction with the TNF-α rs1800629 in predicting disease risk.Citation81 No evidence for association was found, however, between rs1800796 and T2DM.Citation106 Overall, these findings, although indicative of a role for IL-6 polymorphisms in T2DM, suggest that additional studies are needed to confirm this association, particularly in relation to other genes along the innate immunity and inflammatory pathways. Moreover, studies that examine gene–environment interactions may yield more meaningful results and provide important insights into the role of IL-6 in the pathogenesis of T2DM as well as its value as a biomarker of early disease risk detection.
CRP
CRP is an acute-phase reactant produced primarily in the liver under the stimulation of adipocyte-derived IL-6 and TNF-α. It exhibits several characteristics that imply a fundamental immunoregulatory function. Specifically, CRP is a member of the pentraxin family of oligomeric proteins involved in PRRs activation. CRP also enhances leukocyte reactivity, complement fixation, modulation of platelet activation, and clearance of cellular debris from sites of active inflammation. Citation50,Citation107 The magnitude and rapidity of its induction and its cooperative role in innate immune response, as well as its ease of measurement, all make CRP a common marker for inflammation.Citation108 Furthermore, CRP is invariably correlated with various parameters relevant to diabetes, including obesity, lipogenesis, and adiponectin.Citation66 In this respect, CRP was shown to be consistently associated with the incidence of T2DM in populations of otherwise healthy persons.Citation66,Citation109 A recent study estimated that one-third of T2DM cases can be associated with elevated serum CRP.Citation109 Although the relationship between CRP and T2DM was thought to be stronger in women than men,Citation42 subjects of both sexes with high serum CRP (>2.6 mg/L) had more than double the overall T2DM risk (OR = 2.37; 95% CI: 1.57–3.58) compared to those with levels of <0.5 mg/L.Citation109 Together, these findings substantiate a role for CRP as a possible candidate biomarker for early T2DM risk detection.
Numerous prospective studies demonstrate an independent positive association of CRP with the presence of T2DMCitation38,Citation75,Citation76,Citation109–Citation114 while others show no association after adjustment for adiposity and insulin resistance.Citation39,Citation41,Citation74,Citation115 A meta-analysis of available data from 10 prospective studies showed a positive association between serum CRP and T2DM independent of obesity.Citation109 Another meta-analysis of 16 published studies with 3,920 T2DM cases and 24,914 controls demonstrated a relative risk (RR) of T2DM of 1.72 (95% CI: 1.54–1.92) for subjects with high CRP levels.Citation116 A major mechanism by which CRP plays a critical role in T2DM is primarily by its action on pancreatic β-cell.Citation117,Citation118 For example, CRP significantly inhibits cell proliferation and increases the rates of apoptotic cell death.Citation119 This effect was connected to the CRP-mediated modulation of protein kinase B (PKB), a key factor for cell survival pathways and to its ability to induce the production of TNF-α, IL-1β and matrix metalloproteinase-9 (MMP-9) in a concentration-dependent manner.Citation119
Several SNPs were identified in the CRP gene and were linked to elevated levels of serum CRP,Citation120 low insulin sensitivity,Citation121 and incidence of T2DM.Citation122–Citation124 Among these SNPs, the variants rs3093059 (−757 A>G)Citation120,Citation121,Citation123 and rs2794521 (−717 A>G)Citation120–Citation123 were extensively studied and were reported to be associated with an increased risk of T2DM. Furthermore, CRP SNPs at the 3’ end–rs1800947 (1059 G>C) and rs1130864 (1444 C>T)–were associated with decreased and increased concentrations of CRP, respectively.Citation125 Several studies demonstrate that serum CRP can be additively influenced by the IL-6–CRP gene–gene interaction.Citation126–Citation129 Increased serum CRP in obese individuals was correlated with elevated secretion of IL-6 and TNF-α in adipocytes and predisposes to the chronic inflammatory state associated with T2DM.Citation21
Overall, evaluating the effect of inflammatory factors in T2DM may provide new public health approaches for disease prevention and a novel strategy for early detection. These markers are evidently related to risk of T2DM and can be employed in early detection of disease risk and as a predictive measure of response to preventive intervention. If the extent of interaction between the genetic (eg, polymorphisms in TNF-α, IL-6, and CRP genes), biochemical (eg, serum levels of TNF-α, IL-6, and CRP), and environmental (eg, micronutrient supplementation) factors and the risk of T2DM can be established, a selected set of innate immunity-related inflammatory markers could become a powerful panel of integrated multiplex biomarkers for the early risk detection of T2DM. This integration may permit maximizing the sensitivity, specificity, and predictability of biomarkers to T2DM in the general population. A set of inflammatory markers could be utilized to develop effective and novel strategies for disease intervention based on supplementation with micronutrients that modify (ie, lower) their gene expression or serum levels (see below).
Attenuating inflammatory response in T2DM by micronutrients
Factors that attenuate inflammation (and the inflammatory markers proposed above, such as TNFα, IL-6, and CRP) could provide an important public health tool to reduce the burden of diseases related to this pathway, such as obesity, T2DM and cardiovascular diseases, in the general population. The feasibility of modulating innate immunity-related inflammation as an approach for the prevention of T2DM is based on reports that evaluated the efficacy of anti- inflammatory pharmaceutical agents on disease manifestation and outcome. Citation14,Citation32,Citation105
The major current therapeutic agents to treat T2DM, sulfonylureas, metformin, and insulin-sensitizing glitazones, all improve metabolic control and lead to normalization of circulating inflammation markers through, at least partly, interaction with the innate immunity-related pathway. Sulfonylureas increase insulin release from pancreatic β-cells,Citation130 whereas metformin suppresses glucose production in the liver and increases insulin sensitivity in peripheral tissues.Citation131 Glitazones bind to peroxisome proliferator-activated receptors (PPARs), initiating transcriptional activity that leads to improved insulin action.Citation132 While not necessarily acting primarily to reduce inflammation, it is likely that these drugs’ correction of glucose and insulin levels impinges on the innate immune system, attenuates inflammation and further improves insulin action. Accordingly, glitazones reduced serum levels of CRP (30% reduction in 1 week), PAI-1, TNF-α and other inflammatory markers.Citation7,Citation133 Statins, metformin and sulfonylureas have comparable anti-inflammatory activity.Citation8,Citation9,Citation134
A therapeutic strategy for T2DM that would act primary on the inflammatory system has been proposed in the form of salicylates, an anti-inflammatory agent that inhibits IKKCitation135 and is long known to have a hypoglycemic effect.Citation136,Citation137 Nonsteroidal anti-inflammatory drugs (NSAIDs) and cyclooxygenase inhibitors are able to enhance glucose-induced insulin release, improve glucose tolerance, and increase the effect of insulin in patients with T2DM.Citation17,Citation29,Citation105 In humans, treatment with NSAIDs improved various biochemical indices associated with T2DM (). Although these observations support the notion that inflammation plays a pivotal role in T2DM, attenuating inflammation as a strategy for disease prevention in a public health setting will necessitate a substantially different perspective. In this case, a strategy that can be introduced into the general population with the least (if any) side effects and the maximal preventive outcome should be adopted. In this context, a nutritional intervention approach would be a desirable option.
Table 2 Effect of attenuating inflammation by anti-inflammatory factors on biochemical measures of predisposition to T2DMCitation105 Table Footnotea
In general, studies in animal models and humans have demonstrated that disrupting the IKKβ-NFκB pathway improves obesity-related insulin sensitivity.Citation14 Furthermore, blocking JNK activity in animal models of diabetes enhanced systemic glucose homeostasis and insulin sensitivity,Citation32 whereas inhibition of IKK by salicylates led to an overall improved insulin action.Citation105
Micronutrient, certain fatty acids (eg, omega-3 fatty acid) and/or trace element supplementation can modify innate immune-related responses and, subsequently, modify the risk of a range of chronic conditions. With respect to T2DM, the consensus of available information suggests that micronutrient intake modulates the innate immune systemCitation138 and can subsequently influence the predisposition to (and prevention of) disease.Citation80,Citation138,Citation139 By virtue of this observation, the hope is that the outcome of nutritional supplementation can be simply monitored via its modifying action on the levels of inflammatory biomarkers. Many micronutrients exhibit well-characterized anti-inflammatory or immunomodulatory functions (see below). Vitamins (eg, D, E, and C), certain fatty acids (eg, omega-3 fatty acid) and trace elements (eg, selenium, zinc, copper and iron) are known to improve the overall function of the immune system, prevent excessive expression and synthesis of inflammatory cytokines, and increase the ‘oxidative burst’ potential of macrophages.Citation138 Natural health products (NHPs) that contain pertinent micronutrients (eg, so-called adaptogenic medicinal plants) or modulate the innate immune system on their own (eg, omega-3 fatty acids and probiotics) also represent an interesting avenue to study, although the evidence base for their beneficial action is highly variable.Citation140 Exploring the possibility that supplementation with selected micronutrients, trace elements and/or other NHPs can attenuate obesityrelated inflammation in order to delay the development of T2DM should be considered alongside existing public health practices to reduce the rising incidence of the disease.
Vitamin D
Vitamin D insufficiency has been associated with a wide range of chronic diseases, including autoimmune diseases, atherosclerosis, cancer, obesity, cardiovascular diseases, and diabetes.Citation141 In T2DM, the role of vitamin D was suggested from the presence of vitamin D receptors (VDR) in the pancreatic β-islet cells.Citation142 In these cells, the biologically active metabolite of vitamin D (ie, 1,25-dihydroxy-vitamin D; 1,25(OH)D)Citation138 enhances insulin production and secretion via its action on the VDR.Citation142 Indeed, the presence of vitamin D-binding protein (DBP), the principal predictor of serum levels of 25(OH)D and response to vitamin D supplementation, Citation143 and VDR initiated several studies demonstrating a relationship between SNPs in the genes regulating VDR and DBP with glucose intolerance and insulin secretion.Citation144–Citation146 This further supports a role of vitamin D in T2DM and may explain the reduced overall risk of T2DM in subjects who ingest >800 IU/d of vitamin D.Citation80,Citation139 However, an alternative, and perhaps related, explanation was recently proposed based on the potent immunomodulatory functions of vitamin D.Citation147–Citation149 VDRs are present in most types of immune cellsCitation150 and 1,25(OH)D modulates the production of the immunostimulatory IL-12 and the immunosuppressive IL-10.Citation151 In this respect, supplementation with vitamin DCitation33 or its bioactive form 1,25(OH)DCitation138 improved insulin sensitivity by preventing the excessive synthesis of inflammatory cytokines. This effect on cytokine synthesis is due to its interaction with vitamin D response elements (VDRE) present in the promoter region of cytokine-encoding genes. This interaction downregulates the transcriptional activities of cytokine genes and attenuates the synthesis of the corresponding proteins.Citation33 Vitamin D also deactivates NFκB that transcriptionally regulates the pro-inflammatory cytokineencoding genes.Citation152 Downregulating the expression of NFκB and downstream cytokine genes inhibits β-cell apoptosis and promotes their survival.Citation33 Numerous intervention trials, clinical studies, and epidemiological findings demonstrated that supplementation with vitamin D can be beneficial in optimizing processes linked to T2DM such as insulin response, and hence, may markedly improve T2DM prevention strategies. Citation80,Citation142 For example, increasing intake of vitamin D to >800 IU daily, along with 1200 mg of calcium, reduced the risk of developing T2DM by 33%.Citation80 In agreement, healthy older adults with impaired fasting glucose showed significant improvement in glycemic and insulin responses when they increased their intake of vitamin D by 700 IU/day and calcium by 500 mg/day for three years.Citation139 Therefore, exploring the possibility that vitamin D can modify the action of innate immunity genes to attenuate the T2DM-associated lowgrade chronic inflammatory response, and prevent disease development and/or progression, may represent an attractive approach for public health intervention, both in the general population as well as in vulnerable subpopulations. It should however, be considered that vitamin D serum levels, and subsequently extent of supplementation, will vary considerably depending on sunlight exposure; an observation that should be taken into account when employing it in public health intervention of T2DM.
Vitamin E
Vitamin E is known to have a significant impact on improving a variety of immune functions.Citation153 Supplementation with vitamin E increases the rate of lymphocyte proliferation by enhancing the ability of T cells to undergo cell division cycles.Citation154 The effective anti-inflammatory action of vitamin E was substantiated from observations such as the increased expression of the IL-2 gene and IL-1 receptor antagonist and the decreased expression of IL-4 following vitamin E supplementation in animal models.Citation153 Furthermore, vitamin E reduced the serum levels of IL-1β, IL-6, TNF-α, PAI-1, and CRP in T2DM patients.Citation155 These anti-inflammatory actions are thought to result from its inhibitory effect on the 5-lipoxygenase (5-LOX) at the post-transcriptional level.Citation156 5-LOX is a member of the lipoxygenase family of enzymes involved in the synthesis of the inflammatory prostaglandins. Additional anti-inflammatory functions of vitamin E include its downregulation of NFκBCitation155 and its potent lipophilic antioxidant effect on internal and external cell membranes as well as plasma lipoproteins, notably LDL. Based on this latter characteristic, studies in both animal models and humans have demonstrated that vitamin E intake blocks LDL lipid peroxidation, prevents the oxidative stress linked to T2DMassociated abnormal metabolic patterns (hyperglycemia, dyslipidemia, and elevated levels of FFAs), and, subsequently, attenuates cytokine gene expression.Citation153,Citation155,Citation157,Citation158 Despite these findings, a recent study evaluated the effects of a combination of vitamin C (1000 mg/day) and vitamin E (400 IU/day) for four weeks on insulin sensitivity in untrained and trained healthy young men and concluded that such supplementation may preclude the exercise-induced amelioration of insulin resistance in humans.Citation159 This may relate to the source of vitamin E used, ie, α-, β- γ-, or δ-tocopherol.Citation160 Nevertheless, in general, the immunomodulatory, anti-inflammatory and anti-oxidative functions of vitamin E strongly support its possible application in designing effective prevention and/or treatment protocols for T2DM.Citation157
Current practices for diabetes prevention in the general population include lifestyle change, dietary intervention and exercise. As suggested above, micronutrient supplementation may aid in T2DM prevention and control through the anti-oxidant, anti-inflammatory and immunomodulatory properties of various vitamins and trace elements. It seems reasonable, therefore, to suggest that the two preventive approaches for T2DM (ie, micronutrient supplementation and lifestyle change) may be combined into a single program to enhance the success and effectiveness of intervention. This strategy could be more efficient in reducing the low-grade inflammation associated with pre-clinical T2DM and, subsequently the disease burden, than when a single approach is considered. Moreover, such a combined strategy can be introduced in general practice settings and in a population-based fashion with low expenditure and minimal side effects.
Conclusions
The current state of knowledge warrants further study into the extent of association between inflammatory markers and early stages of T2DM, on one hand, and the effect of micronutrients in modifying this relationship, on the other. Such an approach is critical to comprehensively evaluate the prospect of applying inflammatory network assessment in disease surveillance. A significant public health potential of developing this set of evidence-based biomarkers can be conceptualized from their application as precise genomic-based measures of risk prediction for T2DM beyond the simple risk factors presently employed, such as family history or physical examination. In this respect, inflammatory biomarkers may permit capturing the etiological function of (and interaction between) genetic constitution and environmental risk modifiers in the T2DM pathogenesis. Furthermore, a major benefit of introducing inflammatory markers into public health settings stems from their potential to facilitate developing a novel class of agents which attenuate low-grade inflammation prior to the clinical onset of T2DM and to be employed in disease prevention. However, before applying these biomarkers in public health, protocols for their assessment should be standardized and laboratory reference intervals need to be used in decision-making processes.
Employing this set of biomarkers in evaluating responses to prevention may necessitate examining the contribution of dynamic interaction between genetic and dietary modifiers in the etiology of T2DM, as well as the influence of these factors on inflammation at the early disease stages. It seems likely that micronutrient supplementation can modify the genotype–phenotype association within the innate immune response. This proposition may elucidate the mechanisms by which nutritional factors prevent or delay disease development and can be introduced into the general population and susceptible subpopulations. The efficacy of micronutrient supplementation to attenuate or balance the innate immune response and the ensuing inflammation first needs to be further explored and optimized; in some cases, this includes developing tools to accurately and reproducibly measure the circulating/tissue levels of potential biomarkers in order to relate it to clinical outcome. The impact of micronutrients on T2DM incidence may then be assessed through a series of pilot population-based studies: firstly, to determine the feasibility and effectiveness of this protocol; second, to validate and evaluate the strategy and ensure replication of results; and, third, to monitor the outcome to quantify the overall preventive response in comparison (and combination) with the current preventive approaches for T2DM such as lifestyle changes, exercise, and dietary intervention.
Acknowledgments
The authors would like to acknowledge the suggestions and input of Dr Linda Greene-Finestone and Dr Margaret de Groh of the Public Health Agency of Canada. The authors report no conflicts of interest in this work.
References
- Wild S Rolic C Green A Global prevalence of diabetes: Estimates for the year 2000 and projection for 2030 Diabetes Care 2004 37 1047 1053 15111519
- Mokdad AH Bowman BA Ford ES Vinicoor F Marks JS Koplan JP The continuing epidemics of obesity and diabetes in the United States JAMA 2001 286 1195 1200 11559264
- Venkataraman R Nanda NC Baweja G Parikh N Bhatia V Prevalence of diabetes mellitus and related conditions in Asian Indians living in the United States Am J Cardiol 2004 94 977 980 15464696
- Stumvoll M Goldstein B van Haeften T Type 2 diabetes: principles of pathogenesis and therapy Lancet 2005 365 1333 1346 15823385
- Zimmet P Alberti KG Shaw J Global and societal implications of the diabetes epidemic Nature 2001 414 782 787 11742409
- Alberti KG Treating type 2 diabetes–today’s targets, tomorrow’s goals Diabetes Obes Metab 2001 3 Suppl 1 S3 S10 11685827
- Dandona P Aljada A Bandyopadhyay A Inflammation: the link between insulin resistance, obesity and diabetes Trends Immunol 2004 25 4 7 14698276
- Dandona P Aljada A A rational approach to pathogenesis and treatment of type 2 diabetes mellitus, insulin resistance, inflammation, and atherosclerosis Am J Cardiol 2002 90 27G 33G
- Dandona P Aljada A Chaudhuri A Bandyopadhyay A The potential influence of inflammation and insulin resistance on the pathogenesis and treatment of atherosclerosis-related complications in type 2 diabetes J Clin Endocrinol Metab 2003 88 2422 2429 12788837
- Dimopoulos N Watson M Sakamoto K Hundal HS Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in raty L6 skeletal muscle cells Biochem J 2006 399 473 481 16822230
- Bilan PJ Samokhvalov V Koshkina A Schertzer JD Samaan MC Klip A Direct and macrophage-mediated actions of fatty acids causing insulin resistance in muscle cells Arch Physiol Biochem 2009 115 176 190 19671019
- Pickup JC Crook MA Is type II diabetes mellitus a disease of the innate immune system? Diabetologia 1998 41 1241 1248 9794114
- Pickup JC Matttock MB Chusney GD Burt D NIDDM as a disease of the innate immune system: association of acute phase reactants and interleukin-6 with metabolic syndrome X Diabetologia 1997 40 1286 1292 9389420
- King GL The role of inflammatory cytokines in diabetes and its complications J Periodontol 2008 79 1527 1534 18673007
- Larsen GL Henson PM Mediators of inflammation Annu Rev Immunol 1983 1 335 359 6399978
- Hotamisligil GS Shargill NS Spiegelman BM Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance Science 1993 259 87 91 7678183
- Hotamisligil GS Inflammation and metabolic disorders Nature 2006 444 860 867 17167474
- Beutler B Innate immunity: an overview Mol Immunol 2004 40 845 859 14698223
- Takeda K Akira S TLR signaling pathways Seminars Immunol 2004 16 3 9
- Le Souëf PN Gene-environmental interaction in the development of atopic asthma: new developments Curr Opin Allergy Clin Immunol 2009 9 123 127 19295429
- Fernandez-Real JM Pickup JC Innate immunity, insulin resistance and type 2 diabetes Trends Endocrin Metabol 2007 19 10 16
- Medzhitov R Janeway C Innate immunity N Engl J Med 2000 343 338 344 10922424
- Pang C Goa Z Yin J Zhang J Jia W Ye J Macrophage infiltration into adipose tissue may promote angigenesis for adipose tissue remodeling in obesity Am J Physiol Endocrinol Metab 2008 295 E313 E322 18492768
- Lumeng CN Bodzin JL Saltiel AR Obesity induces a phenotypic switch of adipose tissue macrophage polarization J Clin Invest 2007 117 175 184 17200717
- Lumeng CN Deyoung SM Bodzin JL Saltiel AR Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity Diabetes 2007 56 16 23 17192460
- Hotamisligil GS Arner P Caro JF Atkinson RL Spiegelman BM Increased adipose tissue expression of tumor necrosis factor-a in human obesity and insulin resistance J Clin Invest 1995 95 2409 2415 7738205
- Sjoholm A Nystrom T Inflammation and the etiology of type 2 diabetes Diabetes Metab Res Rev 2006 22 4 10 15991254
- Castrillo A Joseph SB Vaidya SA Crosstalk between LXR and Toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism Mol Cell 2003 12 805 816 14580333
- Wellen KE Hotamisligil GS Inflammation, stress, and diabetes J Clin Invest 2005 115 1111 1119 15864338
- Shoelson SE Lee J Goldfine AB Inflammation and insulin resistance J Clin Invest 2006 116 1793 1801 16823477
- Kiortsis DN Mavridis AK Vasakos S Nikas SN Drosos AA Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis Ann Rheum Dis 2005 64 765 766 15458960
- Liu G Rondinone CM JNK: bridging the insulin signaling and inflammatory pathway Curr Opin Investig Drugs 2005 6 979 987
- Riachy R Vandewalle B Kerr CJ 1,25-dihydroxyvitamin D3 protects RINm5F and human islet cells against cytokine-induced apoptosis: implication of the antiapoptotic protein A20 Endocrinology 2002 143 4809 4819 12446608
- Petersen KF Dufour S Befroy D Garcia R Shulman GI Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes N Engl J Med 2004 350 664 671 14960743
- Wang W Vera L Fischer WH Montminy M The CREB coactivator CRCT2 links hepatic ER stress and fasting gluconeogenesis Nature 2009 460 534 537 19543265
- McCarthy MI Zeggini E Genome-wide association studies in type 2 diabetes Curr Diabetes Rep 2009 9 164 171
- Krogh-Madsen R Plomgaard P Moller K Mittendorfer B Pedersen BK Influence of TNF-α and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans Am J Physiol Endocrinol Metab 2006 291 E108 E114 16464907
- Pradhan AD Manson JE Rifai N Buring JE Ridker PM C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus JAMA 2001 286 327 334 11466099
- Festa A D’Agostino RJr Tracy RP Haffner SM Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study Diabetes 2002 51 1131 1137 11916936
- Meigs JB Hu FB Rifai N Manson JE Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus JAMA 2004 291 1978 1986 15113816
- Thorand B Lowel H Schneider A C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984–1998 Arch Intern Med 2003 163 93 99 12523922
- Thorand B Baumert J Kolb H Sex differences in the prediction of type 2 diabetes by inflammatory markers: results from the MONICA/KORA Augsburg case-cohort study, 1984–2002 Diabetes Care 2007 30 854 860 17392546
- Pradhan AD Obesity, metabolic syndrome, and type 2 diabetes: Inflammatory basis of glucose metabolic disorders Nutr Rev 2007 65 S152 S156 18240540
- Uysal KT Wiesbrock SM Marino MW Hotamisligil GS Protection from obesityinduced insulin resistance in mice lacking TNF-alpha function Nature 1997 389 610 614 9335502
- Pickup JC Inflammation and activate innate immunity in the pathogenesis of type 2 diabetes Diabetes Care 2004 27 813 823 14988310
- Song MJ Kim KH Yoon JM Kim JB Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes Biochem Biophys Res Commun 2006 346 739 745 16781673
- Sondergaard L Homology between the mammalian liver and the Drosophila fat body Trends Genet 1993 9 193 8337758
- Shi H Kokoeva MV Inouye K Tazmeli I Yin H Flier JS TLR4 links innate immunity and fatty acid-induced insulin resistance J Clin Invest 2006 116 3015 3025 17053832
- Baumann H Gauldie J The acute phase response Immunol Today 1994 15 74 80 7512342
- Steel DM Whitehead AS The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein Immunol Today 1994 15 81 87 8155266
- Gabay C Kushner I Acute-phase proteins and other systemic responses to inflammation N Engl J Med 1999 340 448 454 9971870
- Montecucco F Steffens S Mach F Insulin resistance: A proinflammatory state mediated by lipid-induced signaling dysfunction and involved in atherosclerotic plaque instability Mediators Inflamm 2008 2008 767623 18604303
- Aguirre V Uchida T Yenush L Davis R White MF The c-Jun NH2- terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307 J Biol Chem 2000 275 9047 9054 10722755
- Summers SA Ceramides in insulin resistance and lipotoxicity Prog Lipid Res 2006 45 42 72 16445986
- JeBailey L Wanono O Niu W Roessler J Rudich A Klip A Ceramideand oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells Diabetes 2007 56 394 403 17259384
- Hotamisligil GS Peraldi P Budavari A Ellis R White ME Spiegelman BM IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α- and obesity-induced insulin resistance Science 1996 271 665 668 8571133
- Yuan M Konstantopoulos N Lee J Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of IKKβ Science 2001 293 1673 1677 11533494
- Morris MF Insulin receptor signalling and regulation Pickup JC Williams G Textbook of Diabetes 3rd edn Oxford, UK Blackwell 2003141 14 17
- Baud V Karin M Signal transduction by tumor necrosis factor and its relatives Trends Cell Biol 2001 11 372 377 11514191
- Hu PHZ Couvillon AD Kaufman RJ Exton JH Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression Mol Cell Biol 2006 26 3071 3084 16581782
- Nguyen MT Favelyukis S Nguyen AK A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways J Biol Chem 2007 282 35279 35292 17916553
- Zahng Y Dall TM Mann SE The economic costs of undiagnosed diabetes Popul Health Manage 2009 12 95 101
- Reilly MP Rader DJ The metabolic syndrome: more than the sum of its parts? Circulation 2003 108 1546 1551 14517150
- Luscher TF Creager MA Beckman JA Cosentino F Diabetes and vascular disease: Pathophysiology, clinical consequences, and medical therapy: Part II Circulation 2003 108 1655 1661 14517152
- LeRoith D Dyslipidemia and glucose dysregulation in overweight and obese patients Clin Cornerstone 2007 8 38 52 18452841
- Sattar N Wannamethee SG Forouhi NG Novel biochemical risk factors for type 2 diabetes: pathogenic insights or prediction possibilities? Diabetologia 2008 51 926 940 18392804
- Haffner SM Insulin resistance, inflammation, and the prediabetic state Am J Cardiol 2003 92 18J 26J
- Bidwell J Keen L Gallagher G Cytokine gene polymorphisms in human disease on-line database Gene Immun 1999 1 3 19
- Bidwell J Keen L Gallagher G Cytokine gene polymorphisms in human disease on-line database, supplement 1 Gene Immun 2001 2 61 70
- Haukim N Bidwell J Smith AJP Cytokine gene polymorphisms in human disease on-line database, supplement 2 Gene Immun 2002 3 313 330
- Hollegaard MV Bidwell LJ Cytokine gene polymorphisms in human disease on-line database, supplement 3 Gene Immun 2006 2006 6364301
- Ollier WER Cytokine genes and disease susceptibility Cytokine 2004 28 174 178 15588692
- Wannamethee SG Lowe GD Rumley A Cherry L Whincup PH Sattar N Adipokines and risk of type 2 diabetes in older men Diabetes Care 2007 30 1200 1205 17322479
- Duncan BB Schmidt MI Pankow JS Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study Diabetes 2003 52 1799 1805 12829649
- Hu FB Meigs JB Li TY Rifai N Manson JE Inflammatory markers and risk of developing type 2 diabetes in women Diabetes 2004 53 693 700 14988254
- Spranger J Kroke A Mohlig M Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective populationbased European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study Diabetes 2003 52 812 817 12606524
- Bastard JP Maachi M Lagathu C Recent advances in the relationship between obesity, inflammation and insulin resistance Eur Cytokine Network 2006 17 4 12
- Vassalli P The pathophysiology of tumor necrosis factors Annu Rev Immunol 1992 10 411 452 1590993
- Moller DE Potential role of TNF-a in the pathogenesis of insulin resistance and type 2 diabetes Trends Endocrinol Metab 2000 11 212 217 10878750
- Pittas AG Lau J Hu FB Dawson-Hughes B Review: The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis J Clin Endocrinol Metabol 2007 92 2017 2029
- Kubaszek SA Pihlajamaki J Komarovski V Finnish diabetes prevention study: promoter polymorphisms of the TNF-α (G-308A) and IL-6 (C-174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish diabetes prevention study Diabetes 2003 52 1872 1876 12829659
- Hoffstedt J Eriksson PHL Rossner S Ryden PAM Excessive fat accumulation is associated with the TNFα-308 G/A promoter polymorphism in women but not in men Diabetologia 2000 43 117 120 10672452
- Susa S Daimon M Sakabe J A functional polymorphism of the TNF-alpha gene that is associated with type 2 DM Biochem Biophys Res Commun 2008 369 943 947 18328809
- Rasmussen SK Urhammer SA Jensen JN Hansen T Borch- Johnsen K Pedersen O The −238 and −308 G>A polymorphisms of the tumor necrosis factor a gene promoter are not associated with features of the insulin resistance syndrome or altered birth weight in Danish Caucasians J Clin Endocrinol Metab 2000 85 1731 1734 10770222
- Zeggini E Groves CJ Parkinson JR Large-scale studies of the association between variation at the TNF/LTA locus and susceptibility to type 2 diabetes Diabetologia 2005 48 2013 2017 16132956
- Kroeger KM Carville KS Abraham LJ The −308 tumor necrosis factor-α promoter polymorphism effects transcription Mol Immunol 1997 34 391 399 9293772
- Fong CL Siddiqui AH Mark DF Identification and characterization of a novel repressor site in the human tumor necrosis factor alpha gene Nucleic Acids Res 1994 22 1108 1114 8152914
- Fried SK Bunkin DA Greenberg AS Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid J Clin Endocrinol Metab 1998 83 847 850 9506738
- Fain JN Madan AK Hiler ML Cheema P Bahouth SW Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans Endocrinology 2004 145 2273 2282 14726444
- Mohamed-Ali V Goodrick S Rawesh A Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo J Clin Endocrinol Metab 1997 82 4196 4200 9398739
- Orban Z Remaley AT Sampson M Trajanoski Z Chrousos GP The differential effect of food intake and beta-adrenergic stimulation on adipose-derived hormones and cytokines in man J Clin Endocrinol Metab 1999 84 2126 2133 10372721
- Sandler S Bendtzen K Eizirik DL Interleukin-6 affects insulin secretion and glucose metabolism of rat pancreatic islets in vitro Endocrinology 1990 126 1288 1294 2404746
- Bastard JP Maachi M Van Nhieu JT Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro J Clin Endocrinol Metab 2002 87 2084 2089 11994345
- Maachi M Pieroni L Bruckert E Systemic low-grade inflammation is related to both circulating and adipose tissue TNFα, leptin and IL-6 levels in obese women Int J Obes Relat Metab Disord 2004 28 993 997 15211360
- Bastard JP Jardel C Bruckert E Elevated levels of interleukin -6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss J Clin Endocrinol Metab 2000 85 3338 3342 10999830
- Nonogaki K Fuller GM Fuentes NL Interleukin-6 stimulates hepatic triglyceride secretion in rats Endocrinology 1995 136 2143 2149 7720663
- Yudkin JS Kumari M Humphries SE Mohamed-Ali V Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis 2000 148 209 214 10657556
- Grimble RF Inflammatory status and insulin resistance Curr Opin Clin Nutr Metab Care 2002 5 551 559 12172480
- Ihle JN Witthuhn BA Quelle FW Yamamoto K Silvennoinen O Signaling through the hematopoietic cytokine receptors Annu Rev Immunol 1995 13 369 398 7612228
- Kristiansen OP Mandrup-Poulsen T Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes 2005 54 Suppl 2 S114 S124 16306329
- Hirosumi J Tuncman G Chang L A central role for JNK in obesity and insulin resistance Nature 2002 420 333 336 12447443
- Mooney RA Senn J Cameron S Suppressors of cytokine signaling-1 and -6 associate with and inhibit the insulin receptor. A potential mechanism for cytokine-mediated insulin resistance J Biol Chem 2001 276 25889 25893 11342531
- Lagathu C Bastard JP Auclair M Maachi M Capeau J Caron M Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone Biochem Biophys Res Commun 2003 311 372 379 14592424
- Rieusset J Bouzakri K Chevillotte E Suppressor of cytokine signaling 3 expression and insulin resistance in skeletal muscle of obese and type 2 diabetic patients Diabetes 2004 53 2232 2241 15331532
- Hundal RS Peterson KF Mayerson AB Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes J Clin Invest 2002 109 1321 1326 12021247
- Huth C Heid IM Vollmert C IL6 gene promoter polymorphisms and type 2 diabetes: joint analysis of individual participants’ data from 21 studies Diabetes 2006 55 2915 2921 17003362
- Gewurz H Zhang XH Lint TF Structure and function of the pentraxins Curr Opin Immunol 1995 7 54 64 7772283
- Pearson TA Mensah GA Alexander RW Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association Circulation 2003 107 499 511 12551878
- Dehghan A Kardys I de Maat MP Genetic variation, C-reactive protein levels, and incidence of diabetes Diabetes 2007 56 872 878 17327459
- Doi Y Kiyohara Y Kubo M Elevated C-reactive protein is a predictor of the development of diabetes in a general Japanese population: the Hisayama Study Diabetes Care 2005 28 2497 2500 16186286
- Laaksonen DE Niskanen L Nyyssonen K C-reactive protein and the development of the metabolic syndrome and diabetes in middleaged men Diabetologia 2004 47 1403 1410 15309290
- Nakanishi S Yamane K Kamei N Okubo M Kohno N Elevated C-reactive protein is a risk factor for the development of type 2 diabetes in Japanese Americans Diabetes Care 2003 26 2754 2757 14514575
- Freeman DJ Norrie J Caslake MJ C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study Diabetes 2002 51 1596 1600 11978661
- Barzilay JI Abraham L Heckbert SR The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study Diabetes 2001 50 2384 2389 11574423
- Krakoff J Funahashi T Stehouwer CD Inflammatory markers, adiponectin, and risk of type 2 diabetes in the Pima Indian Diabetes Care 2003 26 1745 1751 12766104
- Lee CC Adler AI Sandhu MS Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis Diabetologia 2009 52 1040 1047 19326095
- Anan F Takahashi N Nakagawa M Ooie T Saikawa T Yoshimatsu H High-sensitivity C-reactive protein is associated with insulin resistance and cardiovascular autonomic dysfunction in type 2 diabetic patients Metabolism 2005 54 552 558 15798966
- Pfutzner A Standl E Strotmann HJ Association of high-sensitive C-reactive protein with advanced stage beta-cell dysfunction and insulin resistance in patients with type 2 diabetes mellitus Clin Chem Lab Med 2006 44 556 560 16681424
- Nabata A Kuroki M Ueba H C-reactive protein induces endothelial cell apoptosis and matrix metalloproteinase-9 production in human mononuclear cells: Implications for the destabilization of atherosclerotic plaque Atherosclerosis 2008 196 129 135 17531242
- Hage FG Szalai AJ C-reactive protein gene polymorphisms, C-reactive protein blood levels, and cardiovascular disease risk J Am Coll Cardiol 2007 50 1115 1122 17868801
- Obisesan TO Leeuwenburgh C Ferrell RE C-reactive protein genotype affects exercise training-induced changes in insulin sensitivity Metabolism 2006 55 453 460 16546475
- Wolford JK Gruber JD Ossowski VM A C-reactive protein promoter polymorphism is associated with type 2 diabetes mellitus in Pima Indians Mol Genet Metab 2003 78 136 144 12618085
- Zee RY Germer S Thomas A C-reactive protein gene variation and type 2 diabetes mellitus: a case-control study Atherosclerosis 2008 197 931 936 17900590
- Lange LA Burdon K Langefeld CD Heritability and expression of C-reactive protein in type 2 diabetes in the Diabetes Heart Study Ann Hum Genet 2006 70 717 725 17044846
- Grammer TB Marz W Renenr W Bohm BO Hoffmann MM C-reactive protein genotypes associated with circulating C-reactive protein but not with angiographic coronary artery disease: the LURIC study Eur Heart J 2009 30 170 182 18499652
- Kushner I Jiang SL Zhang D Lozanski G Samols D Do posttranscriptional mechanisms participate in induction of C-reactive protein and serum amyloid A by IL-6 and IL-1? Ann N Y Acad Sci 1995 762 102 107 7668521
- Vickers MA Green FR Terry C Genotype at a promoter polymorphism of the interleukin-6 gene is associated with baseline levels of plasma C-reactive protein Cardiovasc Res 2002 53 1029 1034 11922913
- Libra M Signorelli SS Bevelacqua Y Analysis of G(-174)C IL-6 polymorphism and plasma concentrations of inflammatory markers in patients with type 2 diabetes and peripheral arterial disease J Clin Pathol 2006 59 211 215 16443741
- Paik JK Kim OY Koh SJ Additive effect of interleukin-6 and C-reactive protein (CRP) single nucleotide polymorphism on serum CRP concentration and other cardiovascular risk factors Clin Chim Acta 2007 380 68 74 17335789
- Rendell M The role of sulphonylureas in the management of type 2 diabetes mellitus Drugs 2004 64 1339 1358 15200348
- Kirpichnikov D McFarlane SI Sowers JR Metformin: An update Ann Intern Med 2002 137 25 33 12093242
- Krentz AJ Bailey CJ Oral antidiabetic agents: Current role in type 2 diabetes mellitus Drugs 2005 65 385 411 15669880
- Delerive P Fruchart JC Staels B Peroxisome proliferator-activated receptors in inflammation control J Endocrinol 2001 169 453 459 11375115
- Fukuzawa M Satoh J Qiang X Inhibition of tumor necrosis factor-alpha with anti-diabetic agents Diabetes Res Clin Pract 1999 43 147 154 10369423
- Shoelson SE Lee M Yuan M Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance Int J Obes Related Metab Disord 2003 27 Suppl 3 S49 S52
- Robertson RP Prostaglandins as modulators of pancreatic islet function Diabetes 1979 28 942 948 383557
- Robertson RP Arachidonic acid metabolism, the endocrine pancreas, and diabetes mellitus Pharmacol Ther 1984 24 91 106 6427797
- Maggini S Wintergerst ES Beveridge S Hornig DH Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses Br J Nutr 2007 98 Suppl 1 S29 S35 17922955
- Pittas AG Dawson-Hughes B Li T Vitamin D and calcium intake in relation to type 2 diabetes in women Diabetes Care 2006 29 650 656 16505521
- Haddad PS Azar GA Groom S Boivin M Natural health products, modulation of immune function and prevention of chronic diseases. Evid Based Complement Alternat Med 2005 2 513 520
- Holick MF Vitamin D: a D-Lightful health perspective Nutr Rev 2008 66 Suppl 2 S182 S194 18844847
- Holick MF Diabetes and the vitamin D connection Curr Diabetes Rep 2008 8 393 398
- Fu L Yun F Oczak M Wong BYL Veith R Cole DEC Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation Clin Biochem 2009 42 1174 1177 19302999
- Hirai M Suzuki S Hinokio Y Variation in vitamin D-binding protein (group-specific component protein) are associated with fasting plasma insulin levels in Japanese with normal glucose tolerance J Clin Endocrinol Metab 2000 85 1951 1953 10843180
- Bair LJ Dobberfuhl AM Pratley RE Hanson RL Bogardus C Variation in the vitamin D-binding protein (Gc locus) are associated with oral glucose tolerance in nondiabetic Pima Indians J Clin Endocrinol Metab 1998 83 2993 2996 9709981
- Szathmary EJ The effect of Gc genotype on fasting insulin level in Dogrib Indians Hum Genet 1987 75 368 372 3552957
- Hayes CE Nashold FE Spach KM Pedersen LB The immunological functions of the vitamin D endocrine system Cell Mol Biol 2003 49 277 300 12887108
- Griffin MD Xing N Kumar R Vitamin D and its analogs as regulators of immune activities and antigen presentation Annu Rev Nutr 2003 23 117 145 12651965
- Cantorna MT Zhu Y Froicu M Wittke A Vitamin D status, 1,25-dihydroxy-vitamin D3, and the immune system Am J Clin Nutr 2004 80 1717S 1720S 15585793
- Veldman CM Cantorna MT DeLuca HF Expression of 1,25- dihydroxyvitamin D3 receptor in the immune system Arch Biochem Biophys 2000 374 334 338 10666315
- DeLuca HF Cantorna MT Vitamin D: its role and uses in immunology FASEB J 2001 15 2579 2585 11726533
- van Etten E Mathieu C Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts J Steroid Biochem Mol Biol 2005 97 93 101 16046118
- Han SN Adolfsson O Lee CK Prolla TA Ordovas J Meydani SN Vitamin E and gene expression in immune cells Ann N Y Acad Sci 2004 1031 96 101 15753137
- Adolfsson O Huber BT Meydani SN Vitamin E-enhanced IL-2 production in old mice: naive but not memory T cells show increased cell division cycling and IL-2 -producing capacity J Immunol 2001 167 3809 3817 11564798
- Singh U Jialal I Anti-inflammatory effects of α-tocopherol Ann N Y Acad Sci 2004 1031 195 203 15753145
- Devaraj JS Jialal I Alpha-tocopherol decreases interleukin-1 beta release from activated human monocytes by inhibition of 5-lipoxygenase Arterioscler Thromb Vasc Biol 1999 19 1125 1133 10195945
- Scott JA King GL Oxidative stress and antioxidant treatment in diabetes Ann N Y Acad Sci 2004 1031 204 213 15753146
- Thomas SR Stocker R Molecular action of vitamin E in lipoprotein oxidation: Implications for athrosclerosis Free Radic Biol Med 2000 28 1795 1805 10946221
- Ristow M Zarse K Oberbach A Antioxidants prevent healthpromoting effects of physical exercise in humans Proc Natl Acad Sci U S A 2009 106 8665 8670 19433800
- Buijsee B Feskens EJM Kwape L Kok FJ Kormhout D Both alphaand beta-carotene, but not tocopherols and vitamin C, are inversely related to 15-year cardiovascular mortality in Dutch elderly men J Nutr 2008 138 344 350 18203902
