Abstract
Intrauterine devices (IUDs) provide highly effective, long-term, safe, reversible contraception, and are the most widely used reversible contraceptive method worldwide. The levonorgestrel-releasing intrauterine system (LNG-IUS) is a T-shaped IUD with a steroid reservoir containing 52 mg of levonorgestrel that is released at an initial rate of 20 μg daily. It is highly effective, with a typical-use first year pregnancy rate of 0.1% – similar to surgical tubal occlusion. It is approved for 5 years of contraceptive use, and there is evidence that it can be effective for up to 7 years of continuous use. After removal, there is rapid return to fertility, with 1-year life-table pregnancy rates of 89 per 100 for women less than 30 years of age. Most users experience a dramatic reduction in menstrual bleeding, and about 15% to 20% of women become amenorrheic 1 year after insertion. The device’s strong local effects on the endometrium benefit women with various benign gynecological conditions such as menorrhagia, dysmenorrhea, leiomyomata, adenomyosis, and endometriosis. There is also evidence to support its role in endometrial protection during postmenopausal estrogen replacement therapy, and in the treatment of endometrial hyperplasia.
Introduction
Intrauterine devices (IUDs) provide highly effective, long-term, safe, reversible contraception and are the most widely used reversible contraceptive method worldwide.Citation1 The levonorgestrel intrauterine system (LNG-IUS) is marketed under the brand name Mirena® (Bayer HealthCare), and in some European countries as Levonova®. It was first introduced in Finland in 1990, and has since been approved for use in over 100 countriesCitation2 and has over 10 million users.Citation3
In 2007, IUDs were used by 16% of women worldwide aged 15 to 49 who are married or in a union.Citation4 They were most commonly used in Asia, with over 40% of women using an IUD in China, the Democratic People’s Republic of Korea, Kazakhstan and Uzbekistan. Between 30% to 39% of women used IUDs in Israel, Krygyzstan, Mongolia, Turkmenistan and Vietnam. This is in contrast to 14% overall use in Europe, with 21% in Eastern Europe, 10% in Northern Europe and only 6% in Southern Europe. Estimated use is considerably lower in the United States, at only 1.8% of women.
This wide variation in IUD use reflects different patterns of availability, clinician and patient perceptions, and cultural influences. Concerns about pelvic inflammatory disease (PID) and possible infertility were related to complications with the Dalkon Shield from the 1970s, and have been disproved in regard to modern IUDs, including the LNG-IUS and copper T380A IUD.Citation6 Because of the growing evidence of their safety and efficacy,Citation5–Citation8 and the non-contraceptive therapeutic benefits of the LNG-IUS, IUDs are undergoing a renaissance in Europe, the United States and elsewhere. In addition, liberalization of previously over restrictive labeling and medical protocols, including the United States Food and Drug Administration (FDA) product labeling, has helped to encourage more widespread use of IUDs in recent years.Citation9 This article provides an overview of the wide range of contraceptive and non-contraceptive benefits of the LNG-IUS.
The device
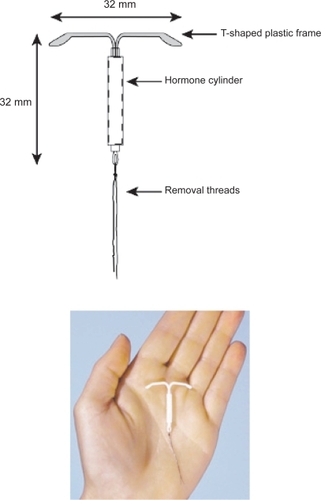
The LNG-IUS is a T-shaped polyethylene device that is 32 mm long and 32 mm wide () – slightly smaller than the copper T380A device which is 36 mm long and 32 mm wide. It is sterilely packaged with a single use inserter. A monofilament polyethylene removal thread attached to a loop at the base of the stem, allows for identification of the device and facilitates removal. The T-body contains barium sulfate, which makes it easily visible on X-ray.
The active ingredient, levonorgestrel (LNG), is dispersed in a silicone (polydimethylsiloxane) reservoir on the stem. This reservoir contains 52 mg of LNG, and is covered by a polydimethylsiloxane membrane which allows for a controlled release of the hormone over time. The initial release rate of approximately 20 μg per day occurs after insertion, and gradually decreases to approximately 10 μg per day after 5 years of use.Citation10
Pharmacokinetics
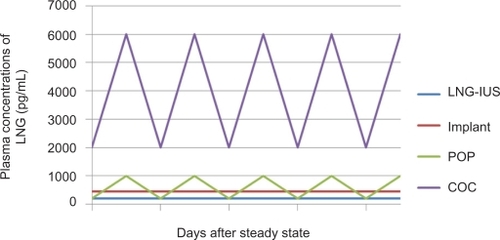
Although the mechanism of action of the LNG-IUS is primarily local, the LNG that is released within the uterus is swiftly absorbed into the systemic circulation. Maximum plasma levels are reached within a few hours after LNG-IUS insertion and plateau at 150 to 200 pg/mL (0.4 to 0.6 nmol/L) within the first few weeks.Citation10 This is in contrast to the much higher plasma hormone levels of combined oral contraceptives, progesterone only pills and Norplant® ().Citation11 Plasma LNG levels from the LNG-IUS remain quite stable over time, but there is marked variation between individuals.Citation12,Citation13
Figure 2 Comparison of plasma concentrations of different contraceptives containing levonorgestrel (LNG). From data of Nilsson et alCitation11 and Diaz et al.Citation12
Changes in endometrial morphology
In contrast to the relatively low plasma levels, the LNG-IUS achieves high concentrations of LNG in the endometrium and adjacent tissues. A small study that examined tissue concentrations found the average level of LNG in the endometrium to be approximately 808 ng/g in users of a prototype LNG-IUS that released 30 μg /day compared with 3.5 ng/g in a group of women taking oral contraceptive pills containing 250 μg LNG.Citation14 This allows the LNG-IUS to have profound endometrial effects that lead to the suppression of endometrial growth. Within 2 to 3 weeks after insertion, local LNG concentrations lead to decidualization of the stroma, mucosal thinning and an inactive endometrium.Citation15 A foreign body reaction is characterized by an increase in inflammatory cells including neutrophils, lymphocytes, plasma cells and macrophages.Citation16 These endometrial changes are finalized within 3 months after insertion of the LNG-IUS,Citation16 and no further microscopic changes are identified within the endometrium thereafter.Citation17 The sequence of these endometrial changes influences and explains the why the initial irregular bleeding pattern improves with time in most users.
Effects on the ovary and pituitary
The ovarian response to the LNG-IUS is directly dependent upon serum LNG levels. More than 50 μg must be released daily for complete suppression of ovulation, while the LNG-IUS releases a maximum of 20 μg per day.Citation18 Serum LNG levels are highly variable between individuals, so ovulation is inhibited only in some women. After LNG-IUS insertion, anovulatory cycles have been seen in 5% to 15% of cycles, and correlate with higher circulating levels of LNG.Citation19 These effects occur mostly during the first year of use, after which, the majority of cycles are ovulatory.Citation20 Plasma estradiol (E2) and progesterone measurements are comparable to those of normally ovulating women.Citation19
LNG-IUS for contraception
Contraceptive mechanisms of action
The contraceptive ability of the LNG-IUS comes from the local effects of LNG within the uterine cavity that primarily prevent fertilization. A common misconception, however, is that the primary mechanism of action of IUDs is abortifacient. As this concern represents a barrier to the use or recommendation of IUDs by some women and health care providers, the issue deserves thorough exploration.
Although interference with implantation plays a role in the postcoital effectiveness of copper IUDs, the available evidence suggests that this is not the mechanism in women using IUDs continuously.Citation21 All IUDs produce a sterile foreign body reaction within the uterine cavity, which creates a hostile environment for sperm.Citation22 The LNG-IUS thickens the cervical mucus (interfering with sperm passage),Citation23 and inhibits sperm motility and function inside the uterus and the fallopian tubes (preventing fertilization).Citation24 In addition, the LNG-IUS induces expression of glycodelin A (anendometrial glycoprotein that inhibits sperm–egg binding) during the otherwise fertile midcycle, when this protein is normally absent.Citation25 Studies on the recovery of eggs from women using IUDs compared with women using no method of contraception showed that normal fertilization did not occur in IUD users.Citation21
These data show that contraceptive efficacy is related to preconceptual mechanisms, not implantation disruption. It can be concluded that when the rare normal fertilization does occur in a LNG-IUS user, it is associated with method failure.
Efficacy of LNG-IUS for contraception
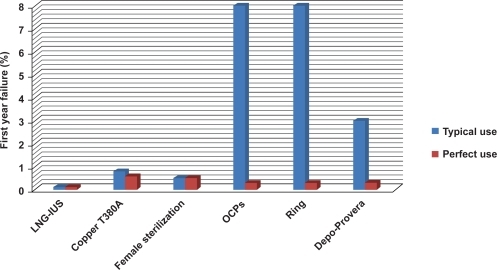
Compared to other reversible methods, the LNG-IUS is among the most effective with a failure rate of 0.1% in the first year – similar to or even better than female sterilization ().Citation26 It is approved for 5 years of contraceptive use, and there is evidence that it can be effective for up to 7 years of continuous use.
Figure 3 Typical use versus perfect use (first year failure rates). LNG-IUS provides highly effective contraception that is not user-dependent. From data of Trussell.Citation124
In 2004 in a large retrospective study that was conducted in 17,360 Finnish women who used the LNG-IUS, all reported pregnancies were analyzed over 5 years (giving a total exposure of 58,600 woman-years). Sixty-four pregnancies occurred, which provided a cumulative pregnancy rate of 0.1% at 1 year and of 0.5% at 5 years.Citation27 A Cochrane Review that evaluated 21 randomized controlled trials concluded that the effectiveness of the LNG-IUS is similar to copper IUDs with copper surface >250 mm2, and significantly higher than IUDs with a copper surface of <250 mm2.Citation28 There is also the suggestion that the LNG-IUS may be more effective than the copper T380A; however, no randomized controlled trials have directly compared these IUDs long term.
The endometrial suppression has been observed up to 7 years after insertion,Citation29 however the effect on cervical mucus may diminish after 5 years of use.Citation30 Nonetheless, contraceptive efficacy appears to remain intact with up to 7 years of continuous use. In 1991, in a randomized multicenter study that was conducted in 2244 women, the cumulative pregnancy rate after 7 years of use was 1.1% for the levonorgestrel IUD and 1.4% for the copper T380A device.Citation31 Two other studies that prospectively followed women using a LNG-IUS for 7 years reported no pregnancies, one among 293 women in Brazil,Citation32 and the other among 82 women in Sweden.Citation33 While a 1.1% pregnancy rate at 7 years is low compared with other reversible methods, this rate is double the efficacy achieved at 5 years. Therefore, replacement of the LNG-IUS at 5 years is recommended for women desiring continuing optimal contraception.
In addition, efficacy does not appear to be related to patient age. Other contraceptives, including other IUDs, have reported higher failure rates in younger women, mostly related to higher fertility rates with younger age. However, data indicate that this is not the case with the LNG-IUS – women can enjoy the same high contraceptive efficacy throughout their reproductive years. Citation34
Return to fertility
The contraceptive actions of the LNG-IUS reverse soon after removal of the device. One-year life-table pregnancy rates after removal are 89 per 100 for women less than 30 years of age – rates are similar to women who had not been using any form of birth control.Citation35–Citation37
Acceptability
IUD users report higher satisfaction than users of other methods. Overall, 99% of IUD users who continue with the method beyond 1 year report being “very satisfied” or “somewhat satisfied” with the method.Citation38
Because most women using the LNG-IUS experience changes in menstrual bleeding patterns, long-term satisfaction requires that users find these menstrual changes acceptable or desirable. Removal rates attributed to bleeding irregularities or amenorrhea have varied greatly among study populations, with differing counseling quality as one contributing factor.Citation33 Thorough counseling about expected changes in bleeding patterns before IUD insertion correlates with satisfaction and continuation rates after 1 year of use.Citation39
A Finnish survey of over 16,000 women using the LNG-IUS found high continuation rates at 1 to 5 years: 93%, 87%, 81%, 75% and 65%, respectively. Rates were lowest for the 18- to 32-year age group and highest for the 39- to 48-year age group.Citation40 Risk of premature removal was most strongly associated with excessive bleeding and spotting. It was also significantly related to symptoms of pelvic infection, depression, abdominal pain, and recurrent vaginal infection. In contrast, there was a significantly lower risk of removal among those who had occasional or no menstruation.
Studies in Brazil report a 90% satisfaction with the LNG-IUS,Citation41 and 78% continuation at 1 year.Citation42 In a survey of approximately 500 US women, satisfaction with clinician counseling and written product information was directly related to the length of LNG-IUS continuation at 12 months.Citation43 This study also evaluated overall satisfaction with the LNG-IUS on a 7 point scale. The majority of women, 84.5%, indicated a level or satisfaction with a value of 6 or 7, while only 3.8% indicated low satisfaction, with a value of 1 or 2. The most frequently reported reasons for liking the LNG-IUS were ease of use, reliability for birth control and increasingly lighter menstrual periods (reported by 29.9%, 29.2% and 21.8% of subjects, respectively). The most frequently reported reasons for disliking the LNG-IUS were “nothing,” “other” and the “increase in spotting between periods;” these were reported by 46.2%, 12.7% and 12.1% of the subjects, respectively. Specific reasons included in the “other” category were cramping, spotting or bleeding, felt during intercourse by partner or subject, insertion procedure, and shortening or absence of menstrual periods.
Potential barriers to use of LNG-IUS
Pelvic inflammatory disease (PID) and infertility
One of the most persistent questions about IUDs is whether they increase the risk of PID. Overall, the rates of PID in the few available randomized control trials of IUDs do not exceed estimates for the general population.Citation44 Combined data from 13 WHO clinical trials conducted in Africa, the Americas, Asia, and Europe found that the risk of developing PID was 6.3 times greater during the first 20 days after IUD insertion than at any later time.Citation45 After the first 20 days from insertion, the number of new PID cases occurring each year remained at a fairly constant low level, approximately 1.4 per 1000 woman-years, throughout 8 years of use. This low level is similar to or even lower than that among women in developed countries who do not use IUDs.Citation46,Citation47 In addition, some studies have suggested that the LNG-IUS may actually protect against upper genital tract infection,Citation34,Citation48 perhaps by thickening cervical mucus. A comparative study of the LNG-IUS versus the Nova-T copper IUD noted significantly lower rates of PID among LNG-IUS users at 3 and 5 years of follow-up.Citation48 A second trial comparing the LNG-IUS and the copper T 380A IUD showed low terminations rates for PID/endometritis (0.7 per 100 years) with both devices.Citation49
The polymicrobial nature of these early infections suggests that most occur due to contamination of the uterine cavity with endogenous flora during insertion. In addition, women with pre-existing asymptomatic chlamydia or gonorrhea have a higher risk of PID immediately after IUD insertion.Citation50 Recommended steps to lower the risk of insertion related infection include careful screening and the use of multi-year devices (fewer insertions). Randomized trials of women at low risk for sexually transmitted infections have found no benefit of empiric antibiotic prophylaxis in reducing this insertion-associated risk.Citation51 The role of sexually transmitted pathogens in later infections is independent of the device. In most cases of PID, treatment can be administered without removal of the device.Citation52,Citation53
Further reassurance of safety comes from a landmark 2001 case control study in nulliparous women who were seeking treatment for primary infertility that found no association between tubal infertility and past IUD use.Citation54 In this study, 358 women with primary infertility who had documented tubal occlusion were compared with two control groups: 953 nulliparous women with primary infertility and no tubal occlusion (infertile controls), and 584 primigravid women (pregnant controls). Women with tubal occlusion reported prior IUD use at the same rate as infertile women without tubal occlusion or primigravid controls. Tubal infertility was associated with past chlamydia infection (as evidenced by chlamydia antibodies) and not with IUD use. This study supports the association between PID and infertility, and not IUD use and infertility.
Ectopic pregnancy
Considerable misunderstanding exists over the relationship between IUDs and ectopic pregnancy. Although the predominant mechanism for medicated IUDs is preconceptual, when fertilization does occur intrauterine effects or factors related to tubal transport may discourage normal implantation. For this reason, a disproportionate number of failures resulting in clinical pregnancies will be ectopics. However, the absolute number of ectopic pregnancies is lower among IUD users compared to non-contraceptors, because the overall pregnancy rate in IUD users is lower. In an evaluation of 17,360 women using the LNG-IUS (total exposure 58,600 woman-years), 108 reported a pregnancy, with 44 of these pregnancies found to be ectopic.Citation40 An analysis of 42 randomized trials estimated ectopic rates per 1000 woman-years to be 0.5 for copper IUDs and 0.2 for the LNG-IUS,Citation55 compared with 1.2 to 1.6 per 100 woman-years for the control population of sexually active women not using contraception.Citation34 Importantly, past or prolonged use of an IUD does not increase risk of ectopic pregnancy.Citation56
Since the evidence shows that both copper T380A and LNG-IUS users enjoy a substantial reduction in the overall risk of both ectopic and intrauterine pregnancy, they both should be considered appropriate choices for contraception in women with a history of a prior ectopic. Keeping in line with this information, restrictive labeling listing a history of ectopic pregnancy as a contraindication to use of the device was removed from the US package insert of the LNG-IUS in 2008.Citation10
Perforation
Perforation is a rare, but recognized potential complication of IUD use. It is estimated to occur at a frequency of 0 to 1.3 per 1000 insertions.Citation57 If a perforation does occur and the device is within the peritoneal cavity, the manufacturer of the LNG-IUS recommends removal.Citation10 If perforation is suspected at the time of insertion (difficult insertion, unusual heavy bleeding), the device should be immediately retrieved using the removal threads; most cases are asymptomatic and surgical exploration or hospitalization is not required. If the removal threads are not accessible, or if the perforation is recognized later, laparoscopy is the preferred surgical approach to removal. Perforation should always be considered in the differential for a patient that presents with a history of IUD placement, but no device present in the uterus on ultrasound exam. An abdominal X-ray can confirm the diagnosis. Surgical removal is not an emergency in the asymptomatic patient, but should be performed when convenient, as a benign natural history for an intrabdominal IUD cannot be assumed. A history of a perforation does not preclude later use of an IUD, but clinicians should allow 4 to 6 weeks for healing of the uterine wound, and ultrasound should be used to verify correct placement.
There have been 36 reports of fetal exposure to the LNG-IUS, 34 with intrauterine exposure and 2 with intraperitoneal exposure.Citation58 Two of the 36 infants had congenital anomalies – one had a hypoplastic pulmonary artery, and the second had cystic hypoplastic kidneys. There is no indication that these two anomalies were related to exposure to levonorgestrel or to the physical device within the uterine cavity.
Patient selection
WHO Medical Eligibility Criteria (MEC) guidelines report few contraindications to IUD use, and suggest that most women can safely use the LNG-IUS ().Citation5 Anyone at high risk for sexually transmitted infections (STIs) should be screened prior to or at the time of IUD insertion. Anyone not in a mutually monogamous relationship should utilize condoms for STI prevention in addition to the IUD for contraception.
Table 1 WHO medical eligibility criteria contraindications to levonorgestrel-releasing intrauterine system insertion
A past history of PID in a woman with no current risk factors for STIs is not a contraindication to use. It is important to point out, however, that a prior episode of PID is associated with a 15% incidence of infertility.Citation47 Future difficulty with pregnancy should not be attributed to the device. IUD insertion should be postponed for at least 3 months after an episode of PID or cervicitis, and negative cervical screening tests for gonorrhea and chlamydia should be obtained in that situation prior to insertion.
Although nulliparity was specifically removed as a contraindication from the US FDA labeling of the copper T380A in 2005, confusion exists around the language in the LNG-IUS package insert. The US LNG-IUS labeling was revised in 2008.Citation10 Under indications and usage, it states that “Mirena is recommended for women who have had at least one child.” However, nulliparity is not listed as a contraindication to use of the LNG-IUS in the package insert. Clinicians should be guided by the opinion of most experts and the WHO MEC that nulliparity is not a contraindication to use of either device.Citation5,Citation59 Nulliparous women may have higher rates of expulsion, bleeding and pain – probably related to uterine size.Citation60 Small uterine size and a tight cervical canal may also make insertion more difficult. Still, the advantages of IUD use make it an appropriate choice for many nulliparous women.
Undiagnosed genital bleeding should be fully worked up prior to IUD insertion. Anatomic features that distort the uterine cavity, such as leiomyomata or Mullerian anomalies can increase expulsion or bleeding or make placement difficult.
Women who have illnesses that result in a serious immunocompromised state should be carefully monitored for infection if they choose to use an IUD. The risk of pregnancy should be weighed against the theoretical risk of infection. Data indicate that the IUD can safely be used in HIV-infected women who have access to medical care. IUD use is a WHO level 2 recommendation (benefit generally outweighs risks) for those at high risk for HIV, and for HIV-infected or AIDs patients clinically stable on antiretroviral therapy.Citation5 LNG-IUS use provides the same general benefits as for immunocompetent women,Citation61 and does not appear to have any drug interactions with antiretroviral therapies.Citation62 In addition, IUD use in treated HIV patients does not appear to result in an increased risk in overall complications or infections beyond the insertion interval.Citation63 It is a WHO level 3 recommendation (risk generally outweighs benefits) to insert an IUD in a patient with untreated AIDS.Citation5
Women with diabetes also may safely use an IUD.Citation5 No increased risk of infection or other complications have been observed. The LNG-IUS provides an attractive alternative to systemic hormonal methods, particularly in diabetics with vascular disease, smokers, and women with a history of thrombosis.
Women with thrombophilia or coagulopathies represent other important groups that can benefit from use of the LNG-IUS.Citation5 In general, the risk of pregnancy due to less effective contraception represents a much greater risk of thrombosis than use of LNG alone.Citation64 Women with coagulopathies, including those on warfarin, experience a reduction in bleeding with the LNG-IUS.Citation65
Insertion timing
In non-pregnant women, IUDs can be inserted during any phase of the menstrual cycle.Citation66 Traditionally, physicians felt that it was best to insert an IUD either during or just after menstruation to ensure that the woman was not pregnant. However, limiting insertion to menses creates a barrier to IUD use. To maximize flexibility in scheduling and reduce the risk of luteal phase pregnancy that may not be detected by a sensitive urine pregnancy test, insertion of a LNG-IUS can occur anytime during the first half of the menstrual cycle in women not currently using contraception. Another option is that the patient may abstain from sex for at least 2 weeks prior to insertion to ensure that a luteal phase pregnancy will not be missed. There is no evidence that the LNG-IUS can be effective as emergency contraception, and it should not be used in this capacity.
Interestingly, insertion of a T-shaped IUD during the middle of the menstrual cycle has been shown to result in higher IUD continuation than menstrual insertion. A CDC review of more than 9000 T-200 IUD insertions showed that insertion from day 12 through 17 of the menstrual cycle (mid-cycle) resulted in fewer removals for expulsion, pain, bleeding, or unintended pregnancy during the first 2 months after IUD insertion.Citation67 Since it takes a few days for the LNG-IUS contraceptive effects to become established, patients should be cautioned to use a back-up method or remain abstinent for at least 7 days after midcycle placement.
Immediate insertion after vaginal delivery, cesarean section, or abortion also can be a safe option for women. With an experienced provider, immediate insertion following first trimester abortion has a reported expulsion rate of 5% to 8%Citation68,Citation69 compared to interval insertion expulsion rates of 4% to 5%.Citation70,Citation71 There are less data about insertion immediately after second trimester abortion, but there appears to be a somewhat higher expulsion rate than with first trimester abortion insertion.Citation72 Studies are ongoing to evaluate the use of the LNG-IUS in this setting. After term delivery or cesarean section, the expulsion rate is approximately 12% to 15% when the IUD is placed within 10 to 30 minutes after delivery of the placenta.Citation73,Citation74 An IUD can be inserted at any time after a first trimester abortion, but should be delayed until after full uterine involution following a second trimester procedure, approximately 2 to 3 weeks.Citation5 Postpartum insertion may be performed anytime after 4 weeks.Citation5
Insertion technique
All prospective users should be thoroughly counseled regarding expected changes in bleeding patterns, and the insertion process. Most patients require no premedication for IUD insertion. Although the administration of a nonsteroidal anti-inflammatory (NSAID) agent such as ibuprofen 400 to 800 mg 30 to 60 minutes prior to the procedure is common, a large, well-designed randomized controlled trial found no benefit.Citation75 Paracervical block may improve comfort, particularly with a closed cervix in a young, nulliparous woman or if dilation is needed. Recognizing that the paracervical block provides no uterine anesthesia, the discomfort of the block may outweigh the benefits, especially in parous women. For this reason, we discourage the routine use of paracervical block. Some women may have a vasovagal reaction to the passage of an instrument through the cervical canal. Paracervical block may be considered in patients with a history of a prior vasovagal reaction to cervical manipulation.
A Swedish study of 80 nulliparous women showed that providers found IUD insertion to be easier with the use of 400 μg misoprostol sublingually 1 hour prior to IUD insertion.Citation76 However, the majority of insertions in this study were rated as “easy,” regardless of misoprostol use. Misoprostol may help with difficult IUD insertions, but there is no evidence to support its routine use, particularly since many women experience side effects with use.Citation76
The package insert provides detailed information about insertion technique, and it is recommended that all providers undergo insertion training.Citation10 The LNG-IUS has an inserter system that draws the arms into the insertion tube when the threads are pulled. The product packaging facilitates meticulous sterile technique during preparation of the device. Thereafter, a no-touch technique should be adopted to ensure that the device remains sterile.
The cervix is prepped and the anterior lip is then grasped with a tenaculum to straighten the cervical canal. With a retroverted uterus, grasping the posterior lip may facilitate this step. While most women tolerate the gentle application of the tenaculum, if severe pain occurs, the tenaculum is removed and local anesthetic can be administered. Sound the uterine cavity to determine the correct depth for insertion. The LNG-IUS has a small movable guide (flange) to mark correct depth along the insertion tube. While applying firm downward traction on the tenaculum to straighten the canal, pass the insertion tube into the uterine cavity. Take care to position the device so that the arms will lie in the horizontal plan of the uterus with the tips pointing into the cornual regions of the cavity when released. The insertion tube of the LNG-IUS is positioned until the flange is about 1.5 to 2 cm from the external os. At this point, the arms of the device are released from the insertion tube by pulling the slider back to a marked position on the insertion tube. After a slight delay to allow expansion of the arms, the inserter tube is gently pushed into the uterine cavity until the previously set flange guide touches the cervix. The LNG-IUS should now be at the fundal position, and can be released by pulling the slider down all the way. Confirm that the threads are released (this should occur automatically) and slowly withdraw the insertion tube.
Trim the threads to a length of approximately 3 cm.Citation10 Err on the side of extra tail length as the threads may always be trimmed later if they are too long. Long threads usually curl around the cervix into the fornices. Short threads tend to stick out from the os along the long axis of the cervix and may result in dyspareunia for the male partner.
Expected changes in bleeding and warning signs of infection or expulsion should be reviewed with all patients after insertion. Follow-up within the next month after insertion is not necessary for all patients – this can be individualized as needed. A follow-up appointment can allow for identification of expulsion or infection. It also can provide an opportunity for additional counseling about changes in bleeding, or other concerns which might result in premature removal of the device. While not recommended for all insertions, selective use of ultrasound following difficult insertions or in training settings can confirm correct placement of the device ().
Non-contraceptive uses of LNG-IUS
While millions of women worldwide enjoy the contraceptive protection of the LNG-IUS, the non-contraceptive benefits of the system present unique opportunities for treatment of a variety of gynecologic problems in symptomatic patients with or without contraceptive needs. The health benefits of systemic hormonal contraception are well established.Citation77 However, some women have contraindications to the use of estrogen, and not all women tolerate systemic progestin therapy. The LNG-IUS offers many of the same health benefits seen in users of systemic hormonal contraception – reduction in menstrual flow, dysmenorrhea, and pelvic pain symptoms. With the unique local delivery system of the LNG-IUS, LNG is released directly into the uterine cavity with low systemic levels. For most patients, this translates into fewer hormone-related symptoms.Citation34 Currently, all non-contraceptive use of LNG-IUS is off-label in the US, but several of these uses are common approved indications in many other countries.
Changes in bleeding patterns
Use of the LNG-IUS results in endometrial thinning, glandular atrophy, stromal decidualization.Citation17 During this process, spotting occurs frequently during the initial 4 to 6 cycles. However, after this transition, most women with normal menstrual cycles enjoy a reduction in both the number of menstrual bleeding days and the amount of objectively measured menstrual blood loss.Citation78 Amenorrhea occurs in 15% to 20% of LNG-IUS users during the first year of use, and increases to 30% to 40% with longer durations of use.Citation49,Citation79 This is a desirable attribute for many women worldwide, even if eliminating their menses is more for convenience than to treat a gynecologic disorder.Citation80
Menorrhagia
The LNG-IUS is approved in over 100 countries for the treatment of menorrhagia.Citation81 It has been shown to outperform other hormonal and non-hormonal medical treatment options.Citation82,Citation83 A study of 34 Chinese women who failed conventional therapy for menorrhagia showed a 98% reduction in average menstrual blood loss 2 years after insertion of a LNG-IUS. Significantly, this improvement occurred early, with approximately one third of the women reporting amenorrhea by 6 months.Citation84
In addition, a number of studies support LNG-IUS use as an effective, conservative treatment option that preserves reproductive function and avoids surgical risks and costs. A Finnish 5-year study comparing women with menorrhagia randomized to LNG-IUS or hysterectomy found similar measures of satisfaction and quality of life between treatment groups, and significantly lower costs in the LNG-IUS group.Citation85 Comparisons of the LNG-IUS with endometrial resection or thermal balloon ablation have shown that both treatment options provide significant reduction in bleeding and similar patient satisfaction.Citation86,Citation87 A recent Cochrane review included five randomized trials directly comparing ablation to LNG-IUS for management of menorrhagia.Citation87 Of these, two showed better controlled bleeding at 1 year with ablation,Citation87,Citation88 and three showed no difference in bleeding control at 1 to 3 years between these two options. A subsequent randomized trial of 83 women in New Zealand showed a better bleeding profile with LNG-IUS after 2 years compared to balloon ablation.Citation89 All studies that have evaluated cost of surgical treatments compared to LNG-IUS have shown that the LNG-IUS is more cost-effective.Citation87
Anemia
Since excessive menstrual bleeding is a common cause of anemia in fertile women, the profound reduction in bleeding associated with LNG-IUS use not surprisingly results in sustained increases in hemoglobin, hematocrit, and serum ferritin.Citation33 Long-term follow-up studies of LNG-IUS in healthy women have demonstrated favorable sustained increased from baseline in hemoglobin concentrations with a mean increase of 1.6 g/dL after 5 years of useCitation90 and 1.44 g/dL after 7 years of use.Citation31
Endometrial protection during hormone replacement
Use of the LNG-IUS provides endometrial protection in women receiving estrogen replacement therapy, and is an approved indication of the LNG-IUS in over 90 countries.Citation81 In a randomized study of 40 perimenopausal women who received oral estradiol 2 mg and either a LNG-IUS or levonorgestrel 250 μg orally for the last 10 days of the cycle,Citation91 bleeding disturbances gradually diminished in the LNG-IUS group, and 83% of women were amenorrheic by 12 months. No endometrial proliferation or atypia was apparent in any of the biopsy samples from either group. These favorable findings were corroborated by studies using the LNG-IUS in conjunction with sustained-release subdermalCitation92,Citation93 transdermal,Citation94 and oralCitation91 estrogen delivery systems with 1 to 3 years of follow-up. Three further trials have reported the sustained endometrial protection effects of the LNG-IUS over 5 years.Citation95–Citation97
Uterine myoma
The LNG-IUS has been shown to reduce blood loss associated with fibroids, however there is less consistent data about its ability to reduce the size of fibroids or overall uterine dimensions.Citation98 In a Russian study of 67 women with myomas, uterine size of ≤12 weeks, and a normal uterine cavity, the LNG-IUS substantially reduced blood loss at 12 months, and decreased uterine and leimyomata size.Citation99 Forty percent of these women were amenorrheic by 12 months, and all but 1 woman had hemoglobin concentrations of >12 g/dL by 12 months. A Turkish study enrolled 32 women with menorrhagia, at least one submucosal fibroid <50% into the uterine cavity, and overall uterine volume 380 mL to receive a LNG-IUS.Citation100 These subjects were followed for 12 months, and bleeding and hemoglobin levels were compared to 32 historical controls who underwent thermal balloon ablation. There was significant and similar improvement in bleeding and hemoglobin levels in both groups, and no changes in uterine volume or fibroid sizes.
There is also evidence of a potential preventive effect. Among women randomized to treatment with either the LNG-IUS or copper T380A, the incidence over 7 years of a new diagnosis of uterine myoma or myoma-related surgery was significantly lower in the LNG-IUS group.Citation49
Treatment of pelvic pain associated endometriosis and adenomyosis
Several small pilot studies suggest that the LNG-IUS can be useful in the treatment of pelvic pain associated with endometriosis and adenomyosis.Citation101–Citation108 Two studies that evaluated postoperative insertion of a LNG-IUS resulted in a significant reduction in the symptoms of dysmenorrhea in women treated with laparoscopy for endometriosis.Citation102,Citation103 Another randomized study found that the improvement in pain and quality of life achieved in women with endometriosis with the LNG-IUS is comparable to that achieved with a GnRH agonist, even with Stage III/IV disease.Citation104
Successful management of pain and abnormal bleeding associated with adenomyosis has also been reported.Citation105,Citation106 A Brazilian study of 29 women with adenomyosis showed a reduction in junctional zone thickness between endometrium and myometrium by magnetic resonance imaging (MRI), but no decrease in uterine volume.Citation107 A recent Chinese study showed effectiveness in treating adenomyosis with 3 years of continuous use.Citation108
Treatment of endometrial hyperplasia and cancer
A number of studies consistently have shown a decreased risk of endometrial cancer in IUD users compared to nonusers, however most studies did not specify the IUD types being investigated.Citation109 A recent meta-analysis found a protective association between IUD use and endometrial cancer, with a pooled odds ratio of 0.54 (95% CI 0.47 to 0.63).Citation110 The mechanism by which copper and inert IUDs provide this protection is unclear. However, there is biologic plausibility that locally delivered LNG by the LNG-IUS would oppose estrogen related endometrial changes that lead to hyperplasia and cancer.Citation111 Since systemic progestins are poorly tolerated by some patients, local progestin delivery can be an attractive alternative.
There is evidence of complete histological regression of endometrial hyperplasia with LNG-IUS treatment.Citation112–Citation115 A recent, non-randomized, Norwegian study compared LNG-IUS, oral progestins and observation among 370 women with simple and atypical hyperplasia. In this study, the LNG-IUS showed superior resolution over oral progestins or observation, and there were no cases that progressed to cancer when followed to a maximum of 106 months.Citation116
In women with proven early endometrial cancer, three case series have reported treatment with the LNG-IUS. These studies, which collectively include a total of 27 women with grade 1 endometrial carcinoma, have shown a regression rate of 25% to 75% after LNG-IUS exposure.Citation117–Citation119 Further studies are needed to determine the utility and safety of LNG-IUS for the treatment of endometrial cancer.
Adjuvant therapy with tamoxifen
Tamoxifen stimulates the uterus, and increases the risk of endometrial polyps, fibroids, hyperplasiaCitation120 and endometrial cancer.Citation121 Results from a randomized trial determined that the LNG-IUS prevented the development of endometrial polyps or hyperplasia in patients receiving tamoxifen over 1 year of use.Citation122 However, women who received the LNG-IUS also experienced more bleeding. Given the unproven safety of the LNG-IUS in breast cancer patients, longer-term randomized studies are needed to determine the benefit to risk balance in these patients. It is reassuring to note that population-based studies have not shown an increase in the risk of breast cancer among LNG-IUS users.Citation123
Summary
The wide range of benefits of the LNG-IUS, in both contraception and treatment of gynecological disorders, will continue to make it an attractive option for women worldwide. Its contraceptive efficacy is excellent – comparable to, if not higher than, surgical sterilization, and it is reversible. In addition, it can offer women improvement in anemia, menorrhagia, endometriosis, control of uterine fibroids and adenomyosis, and protection against and treatment of endometrial hyperplasia and possibly early endometrial cancer. Because of its effectiveness, high patient acceptability and demonstrated safety, the LNG-IUS will continue to provide important benefits in women’s reproductive health.
Disclosures
Dr Bednarek and Dr Jensen serve as consultants for Bayer HealthCare.
References
- PetersonHBCurtisKMLong-acting methods of contraceptionN Engl J Med20053532169217516291986
- SalemRNew Attention to the IUD: Expanding women’s contraceptive options to meet their needsPop Rep, Series B, No. 7BaltimoreJohns Hopkins Bloomberg School of Public Health, The INFO Project2006
- http://www.mirena-us.com/press_room/index.jsp Accessed February 8, 2009.
- United NationsDepartment of Economic and Social Affairs. Population DivisionWorld Contraceptive use in 20072008United Nations publication (ST/ESA/SER.A/273).
- World Health OrganizationImproving Access to Quality Care in Family Planning Medical Eligibility Criteria for Contraceptive Use3rd edGenevaWHO2004
- GrimesDAIntrauterine devices and upper-genital tract infectionLancet20003561013101911041414
- HubacherDLara-RicaldeRTaylorDJUse of copper intrauterine devices and the risk of tubal infertility among nulligravid womenN Engl J Med200134556156711529209
- SheltonJDRisk of clinical pelvic inflammatory disease attributable to an intrauterine deviceLancet200135744311273068
- NelsonALGrimesDAriasRShulmanLMooreAIntrauterine copper contraceptive: update and opportunitiesOBG Management2006S1S8
- Mirena [package insert]Wayne, NJBayer HealthCare Pharmaceuticals Inc2008 Available at: http://berlex.bayerhealthcare.com/html/products/pi/Mirena_PI.pdf
- NilssonCGLahteenmakiPRobertsonDNLuukkainenTPlasma concentrations of levonorgestrel as a function of the release rate of levonorgestrel from medicated intra-uterine devicesActa Endocrinol1980933803847376797
- DiazSPavezMMirandaPJohanssonEDBCroxattoHBLong-term follow-up of women treated with Norplant implantsContraception1987355515673117490
- NilssonCGLahteenmakiPLALuukkainenTRobertsonDNSustained intrauterine release of levonorgestrel over five yearsFertil Steril1986458058073086130
- NilssonCGHaukkamaaMVierolaHTissue concentrations of levonorgestrel in women using a levonorgestrel-releasing IUDClin Endocrinol198217529536
- PerinoAQuartararoPCatinellaEGenovaGCittadiniETreatment of endometrial hyperplasia with levonorgesterel releasing intrauterine devicesActa Eur Fertil1987181371403115027
- ZhuPLuoHXuRThe effect of intrauterine devices, the stainless steel ring, the Copper T220, and releasing levonorgestrel, on the bleeding profile and the morphological structure of the human endometrium – a comparative study of three IUDs: a morphometric study of 96 casesContraception1989404254382510968
- SilverbergSGHaukkamaaMArkoHNilssonCGLuukkainenTEndometrial morphology during long-term use of levonorgestrel-releasing intrauterine devicesInt J Gynecol Pathol198652352413093395
- LuukkainenTLahteenmakiPToivonenJLevonorgestrel-releasing intrauterine deviceAnn Med19902285902113816
- XiaoBZhouLZhangXJiaMLuukkainenTAllonenHPharmacokinetic and pharmacological studies of levnorgestrel-releasing intrauterine deviceContraception1990413533622335100
- NilssonCGLahteenmakiPLALuukkainenTOvarian function in amenorrheic and menstruating users of a levonorgestrel-releasing intrauterine deviceFertil Steril19844152556420203
- OrtizMECroxattoHBBardinCWMechanism of action of intrauterine devicesObstet Gynecol Survey199651S42S53
- SivinIIUDs are contraceptives, not abortifacient: A comment on research and beliefStud Fam Plan198920355359
- BarbosaIBakosOOlssonS-EOdlindVJohanssonEDBOvarian function during use of a levonorgestrel-releasing IUDContraception19904251662117516
- MunuceMJNascimentoJAARosanoGFaundesABahamondesLDoses of levonorgestrel comparable to that delivered by the levonorgestrel-releasing intrauterine system can modify the in vitro expression of zona binding sites of human spermatozoaContraception2006739710116371304
- MandelinEKoistinenHKoistinenRLevonorgestrel-releasing intrauterine device-wearing women express contraceptive glycodelin A in endometrium during midcycle: another contraceptive mechanism?Hum Reprod199712267126759455833
- TrusselJContraceptive failure in the United StatesContraception200470899615288211
- BackmanTRauramoIHuhtalaSKoskenvuoMPregnancy during the use of levonorgestrel intrauterine systemAm J Obstet Gynecol2004190505414749634
- FrenchRVan VlietHCowanFHormonally impregnated intrauterine systems (IUSs) versus other forms of reversible contraceptives as effective methods of preventing pregnancyCochrane Database Syst Rev2004CD00177615266453
- SilverbergSGHaukkamaaMArkoHEndometrial morphology during long-term use of levonorgestrel-releasing intrauterine devicesInt J Gynecol Pathol198652352413093395
- BarbosaIOlssonSEOdlindVOvarian function after seven year’s use of a levnorgestrel IUDAdv Contracept19951185957491859
- SivinISternJCoutinhoEProlonged intrauterine contraception: a seven-year randomized study of the levonorgestrel 20 mcg/day (LNg 20) and the Copper T380 Ag IUDSContraception1991444734801797462
- DiazJFaundesADiazMMarchiNEvaluation of the clinical performance of a levonorgestrel-releasing IUD, up to seven years of use, in Campinas, BrazilContraception1993471691758449017
- RonnerdagMOdlindVHealth effects of long-term use of the intrauterine levonorgestrel-releasing system: a follow-up study over 12 years of continuous useActa Obstet Gynecol Scand19997871672110468065
- AnderssonKOdlindVRyboGLevonorgestrel-releasing and copperreleasing (Nova T) IUDs during five years of use: a randomized comparative trialContraception19944956728137626
- BelhadjHSivinIDiazSRecovery of fertility after use of the levonorgestrel 20 mcg/d or copper T 380Ag intrauterine deviceContraception1986342612673098498
- SivinISternJDiazSRates and outcomes of planned pregnancy after use of Norplant capsules, Norplant II rods, or levonorgestrel-releasing or copper Tcu 380Ag intrauterine contraceptive devicesAm J Obstet Gynecol1992166120812131566771
- AnderssonKBatarIRyboGReturn to fertility after removal of a levonorgestrel-releasing intrauterine device and Nova-TContraception1992465755841493717
- ForrestJDUS women’s perceptions of and attitudes about the IUDObstet Gynecol Surv199651S30S348972500
- CostalesAJensenJNelsonAKornerPUddinMA US multicenter, open-label trial with the levonorgestrel-releasing intrauterine system (LNG-IUS) – clinical and device-related experienceContraception200674178
- BackmanTHuhtalaSBlomTLength of use and symptoms associated with premature removal of the levonorgestrel intrauterine system: a nation-wide study of 17,360 usersBJOG200010733533910740329
- NascimentoRBahamondesLHidalgoMPerrottiMEspejo-ArceXPettaCAUsers’ perspectives on bleeding patterns after two years of levonorgestrel-releasing intrauterine system useDrugs R D20023638739112516941
- DiazJBahamondesLMonteiroIPettaCHildalgoMMArceXEAcceptability and performance of the levonorgestrel-releasing intrauterine system (Mirena) in Campinas, BrazilContraception200062596111102588
- JensenJTNelsonALCostalesACSubject and clinician experience with the levonorgestrel-releasing intrauterine systemContraception200877222918082662
- MumfordSDKesselEWas the Dalkon Shield a safe and effective intrauterine device? The conflict between case-control and clinical trial study findingsFertil Steril199257115111761601137
- FarleyTMRosenbergMJRowePJChenJMeirikOIntrauterine devices and pelvic inflammatory disease: an international perspectiveLancet19923397857881347812
- SimmsIRogersPCharlettAThe rate of diagnosis and demography of pelvic inflammatory disease in general practice: England and WalesInt J of STD and AIDS199910744845110454179
- WestromLIncidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countriesAm J Obstet Gynecol19801388808927008604
- ToivonenJLuukkainenTAllonenHProtective effect of intrauterine release of levonorgestrel on pelvic infection: three years’ comparative experience of levonorgestrel- and copper-releasing intrauterine devicesObstet Gynecol1991772612641899136
- SivinISternJHealth during prolonged use of levonorgestrel 20 micrograms/d and the copper TCu 380Ag intrauterine contraceptive devices: a multicenter study. International Committee for Contraception Research (ICCR)Fertil Steril19946170778293847
- World Health Organization Task Force on the Prevention and Management of InfertilityTubal infertility: Serologic relationship to past chlamydial and gonococcal infectionSex Trans Dis1995227177
- GrimesDASchulzKFProphylactic antibiotics for intrauterine device insertion: a metaanalysis of the randomized controlled trialsContraception199960576310592851
- GrimesDAIntrauterine device and upper-genital tract infectionLancet20003561013101911041414
- SoderbergGLindgrenSInfluence of an intrauterine device on the course of an acute salpingitisContraception1981241371437297065
- HubacherDLara-RicaldeRTaylorDJGuerra-InfanteFGuzman-RodriguezRUse of copper intrauterine devices and the risk of tubal infertility among nulligravid womenN Engl J Med200134556156711529209
- SivinIDose- and age-dependent ectopic pregnancy risks with intrauterine contraceptionObstet Gynecol1991782912982067778
- MarchbanksPAAnnegersJECoulamCBStrathyJHKurlandLTRisk factors for ectopic pregnancy. A population based studyJAMA1988259182318273343790
- AnderssonKRyde-BlomqvistELindellKOdlindVMilsomIPerforations with intrauterine devices: report from a Swedish surveyContraception1998572512559649917
- HopkinsMRAgudelo-SuarezPEl-NasharSCreedonDJRoseCHFamuyideAOTerm pregnancy with intraperitoneal levonorgestrel intrauterine system: a case report and review of the literatureContraception20097932332719272503
- Intrauterine Device and AdolescentsACOG Committee Opinion no. 392. American College of Obstetricians and GynecologistsObstet Gynecol20071101493149518055754
- Otero-FloresJBGuerrero-CarrenoFJVazquez-EstradaLAA comparative randomized study of three different IUDs in nulliparous Mexican womenContraception20036727327612684147
- LehtovirtaPPaavonenJHeikinheimoOExperience with the levonorgestrel-releasing intrauterine system among HIV-infected womenContraception200775373917161122
- CastanoPMUse of intrauterine devices and systems by HIV-infected womenContraception200775S51S5417531617
- MorrisionCSSekadde-KigonduCSineiSKWeinerDHKwokCKokonyaDIs the intrauterine device appropriate contraception for HIV-infected women?BJOG2001108878479011510700
- GomesMPVDeitcherSRRisk of venous thromboembolic disease associated with hormonal contraceptives and hormone replacement therapy: a clinical reviewArch Intern Med20041641965197615477430
- PisoniCNCuadradoMJKhamashtaMAHuntBJTreatment of menorrhagia associated with oral anticoagulation: efficacy and safety of the levonorgestrel releasing intrauterine device (Mirena coil)Lupus20061587788017211994
- TriemanKLiskinLKolsAIUDs: an updatePop RepSeries B, No. 6BaltimoreJohns Hopkins School of Public Health Population Information Program1995 Available at: http://www.infoforhealth.org/pr/online.shtml#b
- WhiteMKOryHWRooksJBIntrauterine device termination rates and the menstrual cycle day of insertionObstet Gynecol1980552202247352085
- OrtayliNBulutASahinTSivinIImmediate postabortal contraception with the levonorgestrel intrauterine device, Norplant, and traditional methodsContraception20016330931411672552
- World Health OrganizationIUD insertion following termination of pregnancy: a clinical trial of the TCu 220C, Lippes loop D, and copper 7Stud Fam Plann198314991086351364
- JensenJTNelsonALCostalesACSubject and clinician experience with the levonorgestrel-releasing intrauterine systemContraception200877222918082662
- LuukkainenTAllonenHHaukkamaaMHolmaPPyoralaTTerhoJEffective contraception with the levonorgestrel-releasing intrauterine device: 12-month report of a European multicenter studyContraception1987361691793123132
- World Health OrganizationIUD insertion following termination of pregnancy: a clinical trial of the TCu 220C, Lippes loop D, and copper 7Stud Fam Plann198314991086351364
- CelenSMoroyPSucakAAktulayADanismanNClinical outcomes of early postplacental insertion of intrauterine contraceptive devicesContraception20046927928215033401
- HayesJLCwiakCGoedkenPZiemanMA pilot clinical trial of ultrasound-guided post-placental insertion of a levonorgestrel intrauterine deviceContraception20077629229617900440
- HubacherDReyesVLilloSZepedaAChenPLCroxattoHPain from copper intrauterine device insertion: randomized trial of prophylactic ibuprofenContraception200619512721277
- SaavIAronssonAMarionsLStephanssonOGemzell-DanielssonKCervical priming with sublingual misoprostol prior to insertion of an intrauterine device in nulliparous women: a randomized controlled trialHum Reprod2007222647265217652452
- BurkmanRSchlesselmanJJZiemanMSafety concerns and health benefits associated with oral contraceptionAm J Obstet Gynecol2004190S5S2215105794
- SuvisaariJLahteenmakiPDetailed analysis of menstrual bleeding patterns after postmenstrual and postabortal insertion of a copper IUD or a levonorgestrel-releasing intrauterine systemContraception1996542012088922872
- HidalgoMBahamondesLPerrottiMDiazJDantas-MonteiroCPettaCBleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two yearsContraception20026512913211927115
- GlasierAFSmithKBvan der SpuyZMAmenorrhea associated with contraception-an international study on acceptabilityContraception2003671812521650
- MacIsaacLEspeyEIntrauterine Contraception: The Pendulum Swings BackObstet Gynecol Clin N Am20073491111
- IrvineGACampbell-BrownMBLumsdenMAHeikkilaAWalkerJJCameronITRandomised comparative trial of the levonorgestrel intrauterine system and norethisterone for treatment of idiopathic menorrhagiaBJOG1998105592598
- MilsomIAnderssonKAnderschBRyboGA comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagiaAm J Obstet Gynecol19911648798831900665
- XiaoBWuSCChongJZengTHanLHLuukkainenTTherapeutic effects of the levonorgestrel-releasing intrauterine system in the treatment of idiopathic menorrhagiaFertil Steril20037996396912749438
- HurskainenRTeperiJRissanenPClinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-upJAMA20042911456146315039412
- CleggJPGuestJFHurskainenRCost-utility of levonorgestrel intrauterine system compared with hysterectomy and second generation endometrial ablative techniques in managing patients with menorrhagia in the UKCurr Med Res Opin2007231637164817559758
- MarjoribanksJLethabyAFarquharCSurgery versus medical therapy for heavy menstrual bleedingCochrane Database of Syst Rev2006CD00385516625593
- SoysalMSoysalSOzerSA randomized controlled trial of levonorgestrel releasing IUD and thermal balloon ablation in the treatment of menorrhagiaZentral Gynakol2002124213219
- BusfieldRAFarquharCMSowterMCA randomised trial comparing the levonorgestrel intrauterine system and thermal balloon ablation for heavy menstrual bleedingBJOG200611325726316487195
- AnderssonKOdlindVRyboGLevonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: a randomized comparative trialContraception19944956728137626
- AnderssonKStadbergEMattssonLARyboGSamsioeGIntrauterine or oral administration of levonorgestrel in combination with estradiol to perimenopausal women – effects on lipid metabolism during 12 months of treatmentInt J Fertil Menopausal Stud1996414764838934257
- SuhonenSPAllonenHOLahteenmakiPSustained-release estradiol implants and a levonorgestrel-releasing intrauterine device in hormone replacement therapyAm J Obstet Gynecol19951725625677856686
- SuhonenSHolmstromTLahteenmakiPThree-year follow-up of the use of a levonorgestrel-releasing intrauterine system in hormone replacement therapyActa Obstet Gynecol Scand1997761451509049288
- RaudaskoskiTHLahtiEIKauppilaAJApaja-SarkkinenMALaatikainenTJTransdermal estrogen with a levonorgestrel-releasing intrauterine device for climacteric complaints: clinical and endometrial responsesAm J Obstet Gynecol19951721141197847516
- HamptonNRReesMCLoweDGRauramoIBarlowDGuillebaudJLevonorgestrel intrauterine system (LNG-IUS) with conjugated oral equine estrogen: a successful regimen for HRT in perimenopausal womenHum Reprod2005202653266015905289
- VarilaEWahlstromTRauramoIA 5-year follow-up study on the use of a levonorgestrel intrauterine system in women receiving hormone replacement therapyFertil Steril20017696997311704119
- Suvanto-LuukkonenEKauppilaAThe levonorgestrel intrauterine system in menopausal hormone replacement therapy: five-year experienceFertil Steril19997216116310428167
- KaunitzAProgestin-releasing intrauterine systems and leiomyomaContraception200775S130S13317531604
- GrigorievaVChen-MokMTarasovaMMikhailovAUse of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomasFertil Steril2003791194119812738516
- SoysalSSoysalMThe efficacy of levnorgestrel-releasing intrauterine device in selected cases of myoma-related menorrhagia: a prospective controlled trialGynecol Obstet Invest200559293515377823
- FedeleLBianchiSZanconatoGPortueseARaffaelliRUse of a levonorgestrel-releasing intrauterine device in the treatment of rectovaginal endometriosisFertil Steril20017548548811239528
- VercelliniPFrontinoGDe GiorgiOAimiGZainaBCrosignaniPGComparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot studyFertil Steril20038030530912909492
- LockhatFBEmemboluJOKonjeJCThe efficacy, side effects and continuation rates in women with symptomatic endometriosis undergoing treatment with an intrauterine administered progestogen (levonorgestrel): a 3 year follow-upHum Reprod20052078979315608040
- PettaCAFerrianiRAAbraoMSRandomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosisHum Reprod2005201993199815790607
- MaiaHJrMaltezACoelhoGAthaydeCCoutinhoEMInsertion of mirena after endometrial resection in patients with adenomyosisJ Am Assoc Gynecol Laparosc20031051251614738640
- FongYFSinghKMedical treatment of a grossly enlarged adenomyotic uterus with the levonorgestrel-releasing intrauterine systemContraception19996017317510640162
- BraghetoAMCasertaNBahamondesLPettaCAEffectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imagingContraception20077619519917707716
- ShengJZhangWYShangJPLuDThe LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrheal associated with adenomyosisContraception20097918919319185671
- HubacherDGrimesDANoncontraceptive health benefits of intrauterine devices: a systematic reviewObstet Gynecol Surv20025712012811832788
- BeiningRMDennisLKSmithEMDokrasAMeta-analysis of intrauterine device use and risk of endometrial cancerAnn Epidemiol20081849249918261926
- AkhmedkhanovAZeleniuch-JacquotteATonioloPRole of exogenous and endogenous hormones in endometrial cancer: review of the evidence and research perspectivesAnn N Y Acad Sci200194329631511594550
- VereideABKainoTSagerGArnesMOrboAEffect of levonorgestrel IUD and oral medroxyprogesterone acetate on glandular and stromal progesterone receptors (PRA and PRB), and estrogen receptors (ER-alpha and ER-beta) in human endometrial hyperplasiaGynecol Oncol2005
- WildemeerschDDhontMTreatment of nonatypical and atypical endometrial hyperplasia with a levonorgestrel-releasing intrauterine systemAm J Obstet Gynecol20031881297129812748501
- VarmaRSonejaHBhatiaKThe effectiveness of a levonorgestrel-releasing intrauterine system (LNG-IUS) in the treatment of endometrial hyperplasia – A long-term follow-up studyEur J Obstet Gynecol2008139169175
- BahamondesLRibeiro-HuguetPde AndradeKCLeon-MartinsOPettaCALevonorgestrel-releasing intrauterine system (Mirena) as a therapy for endometrial hyperplasia and carcinomaActa Obstet Gynecol Scand20038258058212780432
- OrboAArnesMHanckeCVereideABPettersenILarsenKTreatment results of endometrial hyperplasia after prospective D-score classification: A follow-up study comparing effect of LNG-IUD and oral progestins versus observation onlyGynecol Oncol2008111687318684496
- DharKKNeedhiRajanTKoslowskiMWoolasRPIs levonorgestrel intrauterine system effective for treatment of early endometrial cancer? Report of four cases and review of the literatureGynecol Oncol20059792492715943993
- MontzFJBristowREBovicelliATomacruzRKurmanRJIntrauterine progesterone treatment of early endometrial cancerAm J Obstet Gynecol200218665165711967486
- SignorelliMCaspaniGBonazziCChiappaVPeregoPMangioniCFertility-sparing treatment in young women with endometrial cancer or atypical complex hyperplasia: a prospective single-institution experience of 21 casesBJOG200911611411819087082
- KedarRPBourneTHPowlesTJEffects of tamoxifen on uterus and ovaries of postmenopausal women in a randomised breast cancer prevention trialLancet1994343131813217910323
- van LeeuwenFEBenraadtJCoeberghJWRisk of endometrial cancer after tamoxifen treatment of breast cancerLancet19943434484527905955
- GardnerFJKonjeJCAbramsKREndometrial protection from tamoxifen stimulated changes by levonorgestrel-releasing intrauterine system: a randomised controlled trialLancet20003561711171711095258
- BackmanTRauramoIJaakkolaKUse of the levonorgestrel-releasing intrauterine system and breast cancerObstet Gynecol200510681381716199640
- TrussellJContraceptive failure in the United StatesContraception200470899615288211