Abstract
Glioblastoma multiforme (GBM) is the most common malignant primary brain tumor in adults and carries the poorest prognosis. Despite recent progress in molecular biology, neuro-imaging and neuro-surgical care, the management of patients with GBM continues to harbor significant challenges. Survival after diagnosis is poor even with the most aggressive approach using multimodality therapy. Although the etiology of malignant gliomas is not known, the dependency of tumor growth on angiogenesis has identified this pathway as a promising therapeutic target. Bevacizumab was the first antiangiogenic therapy approved for use in cancer and received accelerated Food and Drug Administration approval for the treatment of recurrent GBM in 2009, the first new drug for this disease in over a decade. This review describes the rationale behind the treatment of GBM with bevacizumab. The pharmacology, efficacy, safety and tolerability of bevacizumab will also be reviewed.
Introduction
Cancers of the brain and nervous system are relatively rare. Glioblastoma multiforme (GBM) continues to be the most common and lethal malignant primary brain tumor in adults.Citation1 The exact pathogenesis thus far has remained elusive, and most occur in a sporadic fashion.Citation2–Citation7 Rarely they occur in the setting of hereditary syndromes.Citation8 Despite an aggressive multimodal approach, the median survival time after diagnosis is approximately a year with population-based studies demonstrating even lower median survival rates.Citation9,Citation10 Surgery allows histological diagnosis and can provide relief for neurological deficits related to mass effect. Surgery, however, is not curative due to the infiltrative nature of the disease. While only retrospective data are available to evaluate survival benefit, extent of resection correlates with better prognosis.Citation11–Citation13 Radiation therapy has been the mainstay treatment for GBM for decades extending median survival to about 9 months versus a median survival of 3 months with no therapy.Citation14–Citation18 The role of chemotherapy in gliomas has historically been disappointing, with adjuvant therapy extending longer-term survival in the minority of GBM patients.Citation16,Citation17,Citation19 This is in contrast to the more chemosensitive oligodendrogliomas harboring 1p/19q deletions.Citation20 Chemotherapy as standard of care for GBM was only recently established in 2005, when Stupp et al demonstrated that daily temozolomide (TMZ) combined with radiation followed by 6 months of adjuvant monthly cycle TMZ increased median survival by 3 months when compared to radiotherapy alone, and increased 2-year survival from 10% to 26%.Citation21 Once disease progression occurs, available salvage chemotherapies are usually unsuccessful, demonstrating a 6-month progression-free survival (PFS-6) of only 15%.Citation22
Because of its poor prognosis with current multimodality treatment, a concerted effort is underway to develop new and novel therapeutic strategies that will increase survival and quality of life in patients with GBM. There has been progress in elucidating the molecular changes that underlie the pathogenesis of GBM. GBMs are hypervascular in nature and growth has been shown to be angiogenesis-dependent.Citation23,Citation24 Bevacizumab is a humanized monoclonal antibody against vascular endothelial growth factor (VEGF) and has been shown to be an extremely potent inhibitor of angiogenesis. Based on an improved response rate compared to that of historical controls, bevacizumab (Avastin®; Genentech, San Francisco, CA, USA) received accelerated approval by the Food and Drug Administration (FDA) for recurrent GBM in May 2009, thereby becoming the first new drug labeled for gliomas in over a decade. We will review the role of VEGF pathways in glioma angiogenesis and the rationale for bevacizumab in this disease.
Angiogenesis in brain tumors
For tumors to attain a size beyond a few millimeters requires a process known as angiogenesis.Citation25,Citation26 Angiogenesis is a physiological process that depends on a well orchestrated balance of angiogenic factors and inhibitors that control the growth of microvessel sprouts via migration and proliferation of endothelial cells.Citation27 When dysregulation of this process occurs, it may provide a suitable milieu for the initiation and maintenance of certain chronic disease states such psoriasis,Citation28 ocular neovascularizationCitation29 and atherosclerosis.Citation30 In addition, pathological angiogenesis has been shown to be a hallmark of certain tumor types such as GBM, colorectal carcinoma, breast and renal cell carcinomas.Citation31 Tumor-associated neo-vascularization differs from physiological angiogenesis, characterized by a substantial increase in the proliferation activity of endothelial cells that are structurally “leaky”.Citation32 Tipping the scales in favor of a proangiogenic state requires upregulation of factors such as VEGF-A, platelet-derived growth factor (PDGF) and basic fibroblast growth factor (bFGF2).Citation33–Citation35 Additional pathways implicated in tumor angiogenesis are angiopoietin and Notch.Citation36 Enzymes such as metalloproteinase and serine proteinase have been shown to be involved in the induction and suppression of angiogenesis by degrading the extracellular matrix.Citation37 These have all been identified as possible substrates for therapeutic intervention.
Tissue hypoxia resulting from a tumor’s fast exponential growth has been a well-defined trigger of angiogenesis in solid tumors and manifests downstream by a number of inducible proangiogenic molecular changes. One such example involves hypoxia-inducible factor-1, a transcription factor that regulates the expression of many angiogenesis- and glucose metabolism-related genes and in addition activates the transcription of VEGF in malignant gliomas.Citation38,Citation39 VEGF mRNA expression, as well as VEGF receptor expression, are well documented hypoxic induced changes in malignant gliomas.Citation40
Various chemokines and mitogens that promote angiogenesis have been shown to be produced by both primary and recurrent gliomas and include basic fibroblast growth factor,Citation41 interleukin-8 (CXCL8),Citation42,Citation43 CXCL12,Citation44 and hepatocyte growth factor/scatter factor.Citation43 Neurotrophins and their corresponding receptors, which primarily mediate their effects via the receptor kinases TrkA-C, have been shown to support endothelial cell survival and proliferation as well as neuronal proliferation, differentiation and synaptogenesis.Citation45–Citation48 Other mechanisms include endothelial-cell spread and migration in response to certain growth factors mediated by certain integrins,Citation49 as well as signaling via stem cell factor and its receptor c-Kit pathway, which is thought to be central in inducing tumor-based angiogenesis.Citation50
VEGF-mediated angiogenesis
The first observations of the increased vascular nature of brain tumors were made by Rudolf Virchow during the nineteenth century.Citation26 Later, based upon the concept that tumor angiogenesis was mediated by diffusible factors produced by tumor cells, Folkman proposed that inhibition of angiogenesis would be a reasonable strategy to treat cancer and initiated the isolation of tumor angiogenesis factors.Citation26 In 1983, Senger et al reported the partial purification of vascular permeability factor (VPF), a protein that induced vascular leakage in the skin.Citation51 In 1989, Ferrara et al isolated VEGF, an endothelial-cell-specific mitogen.Citation52 The proteins VEGF and VPF were shown to be one and the same molecule by the work of Connolly et al.Citation53 VEGF and its signaling are important mediators of glioma-induced angiogenesis.
The human VEGF gene has been located to chromosome 6p21.3.Citation52,Citation54,Citation55 VEGF has been described as a basic, heparin-binding, homodimeric glycoprotein. There are at least five VEGF glycoproteins (VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E) and placental growth-factors PIGF-1 and PlGF-2 that belong within the same family. VEGF-A binding is the dominant mediator of pro-angiogenic signaling in human cancers and VEGF-A has different isoforms that are generated by alternate splicing. The VEGF glycoprotein acts in a number of ways to mediate the above-described effects. It binds two related receptor tyrosine kinases (RTK), named Flt-1 (VEGFR-1) and KDR/Flk-1 (VEGFR-2)Citation56,Citation57 and interacts with a family of co-receptors known as neuropilins. By binding to these receptors, VEGF (and in a similar manner PDGF),Citation58 induces homodimerization of two receptor subunits and thereby induces autophosphorylation of the intracellular tyrosine kinase domains.Citation56,Citation57,Citation59 This phenomenon then leads to downstream signal transduction. VEGFR-3 has been shown to mediate the mitogenic and survival activity of VEGF via the phosphatidylinositol-3 kinase/phosphatase and tensin homologue/Akt/mammalian target of rapamycin (PtdIns3K/PTEN/Akt/mTOR) pathway,Citation60 the PLCγCitation61 and Ras/Raf/mitogen-activated protein kinases MAPK p44/42.Citation62–Citation64
The Notch transmembrane protein and its ligand Jagged/Delta are activated by VEGF signaling and tend to suppress angiogenesis. Blocking the Delta-like ligand 4 has been shown to increase sprouting in a glioma model, paradoxically minimizing tumor growth. Notch signaling seems essential to the negative feedback control of VEGF signaling in brain tumors.Citation65–Citation67 In recent years another molecule known as γ-secretase, a presenilin-dependant protease complex also implicated in the pathogenesis of Alzheimer’s disease, has been identified as a significant player in the induction and maintenance of tumor-based angiogenesis by cleaving the Notch molecule.Citation68,Citation69 VEGFR1 and insulin-like growth factor-1, which are receptors involved in promoting angiogenesis in GBM, have also been shown to be cleaved by γ-secretase.Citation70,Citation71
VEGF has been shown to be a significant regulator of embryonal and physiological and pathological angiogenesis, including that of tumor growth.Citation72 In vitro studies have shown that VEGF can promote the growth of vascular endothelial cells derived from both blood vessels and lymphatic vessels,Citation73 act as a survival factor for endothelial cells,Citation63,Citation74 induce vasodilatation, Citation75 promote inflammation through vascular leakage,Citation51 induce chemotaxis of endothelial cells,Citation76 increase proteolytic enzyme expression and hence promote extracellular matrix degradation,Citation76 promote monocyte activation and chemotaxis,Citation76 and inhibit the maturation of antigen-presenting dendritic cells.Citation77 A number of studies have shown that in addition to endothelial cells VEGF exerts a mitogenic and survival effect on nonendothelial cell types such as nerve cells.Citation78
The efficacy of antiangiogenic agents has been demonstrated in preclinical xenograft brain tumor models.Citation80,Citation81 Calabrese et al demonstrated that self-renewal capacity of brain tumor cells were maintained by endothelial factors and modulation of this “vascular niche” with antiangiogenic agents decreased tumor growth.Citation79 Because VEGF plays such a significant role in the process of angiogenesis, development of therapeutic interventions targeting VEGF and VEGFR signaling is rational. The use of such agents to treat brain tumors has been increasing and to date there are a number of clinical trials in progress dedicated to this approach, including, the identification of agents that bind specifically to VEGF ligands and those that directly target VEGF receptors.
Bevacizumab
Bevacizumab is a recombinant humanized IgG1 monoclonal antibody (MAb) with an approximate molecular weight of 149 kD. It consists of approximately 93% human and 7% murine sequences.Citation82 The antibody itself contains a human IgG1 framework region and the antigen–binding complementarity-determining regions of a murine antibody that binds to VEGF, Mab A.4.6.1. It selectively binds with high affinity (kd = 1.1 nM) and sterically inhibits all biologically active isoforms of human vascular endothelial growth factor to its receptors Flt-1 (VEGFR-1) and KDR (VEGFR-2) on the surface of endothelial cells. By activating these receptors downstream effects include tyrosine phosphorylation and induction of signal transduction pathways involved in mitogenesis and pro-survival activity within vascular endothelial cells.Citation82
Nonhuman safety and toxicology profile
To date there have been no studies assessing carcinogenicity or mutagenicity of bevacizumab. No studies have been conducted to investigate excretion in milk of lactating animals but excretion of IgGs is expected to occur in breast milk. In nonclinical studies using Cynomolgus monkeys it was shown that bevacizumab may impair fertility and that this may be a reversible effect once it has been stopped.Citation83 In addition, when administered at doses of 0.4 to 20 times the weekly human exposure, anatomical pathology revealed several adverse effects on general growth and skeletal development, fertility and wound healing capacity. In rabbits that were treated with bevacizumab there was reduced wound healing capacity.Citation83 Teratogenicity studies performed on pregnant rabbits demonstrated reduced or irregular ossification in the skull, jaw, spine, ribs, tibia and bones of the paws; meningocele; fontanel, rib and hindlimb deformities; corneal opacity; and absent hindlimb phalanges.
Pharmacokinetic profile of bevacizumab
The pharmacokinetic profile of bevacizumab was assessed in humans using an assay that measures total serum bevacizumab concentrations.Citation84 Patients who received 1 to 20 mg/kg of bevacizumab weekly, every 2 weeks, or every 3 weeks, had an estimated half-life of bevacizumab of 20 days, with a range of 11 to 50 days and a predicted time to reach steady state of 100 days. The clearance rate of bevacizumab was influenced by body weight, gender and tumor burden. After correcting for body weight, males had a higher bevacizumab clearance (0.262 L/day vs 0.207 L/day) and a larger Vc (3.25 L vs 2.66 L) than females.Citation84
Human safety profile
In the initial clinical studies that led to the FDA approval of bevacizumab in colon, breast, kidney and lung cancers the short-term toxicity of bevacizumab, alone or in combination with chemotherapy, was found to be acceptable.Citation85–Citation90 Mild hypertension, manageable by medication, was the most common adverse event in addition to fatigue. A low frequency of more serious adverse events did occur, which included arterial thromboembolic events, congestive heart failure, bone marrow suppression, intracranial hemorrhage, impaired wound healing and gastrointestinal perforations. Toxicities may be potentiated by combining chemotherapeutic agents with bevacizumab; examples of such adverse events include asthenia or fatigue, marrow suppression, neuropathy and liver dysfunction. One relatively uncommon adverse event potentiated by such a regimen is the increased risk of congestive heart failure from 0.5% to 2.2% in patients who have received prior or concomitant anthracyclines. As has been demonstrated in rabbit models bevacizumab impairs wound healing. In a controlled clinical trial in patients with metastatic colorectal carcinoma who underwent surgery, the incidence of wound healing complications, including serious and fatal complications during the course of bevacizumab treatment was 15% and in those who did not receive bevacizumab, was 4%.Citation87,Citation88,Citation89 In the clinical trial setting, bevacizumab was administered until at least 28 days after surgery. The appropriate timing of when bevacizumab should be discontinued during the peri-surgical period has not been determined but should take into account the half-life of the drug which is about 21 days with a range of 11 to 50 days. Although there is a potential for immunogenicity no anti-bevacizumab antibodies have been detected thus far.
Bevacizumab and non-CNS solid tumors
Bevacizumab was the first anti-angiogenic inhibitor approved as an anti-tumor therapeutic agent. On February 26, 2004, the FDA approved bevacizumab as first-line treatment for patients with metastatic colorectal cancer. In a randomized-double, blind-clinical trial of more than 800 patients with metastatic colorectal carcinoma, bevacizumab was compared to the standard chemotherapy of irinotecan, leucovorin (folinic acid) and fluorouracil (IFL). Patients who were given bevacizumab in combination with IFL survived about five months longer and the average time to tumor progression was four months longer than patients receiving IFL alone. The overall response rate to the treatment was 45% compared to 35% for the control arm of the trial.Citation88,Citation89 In June 2006, bevacizumab was granted labeling extension for co-administration with 5-fluoruracil-based chemotherapy for the treatment of metastatic colorectal carcinoma based on data from the E3200 trial. This trial was an open-label, randomized, three-arm, active-controlled, multi-center clinical trial in which bevacizumab alone was compared to bevacizumab plus FOLFOX4 (5-flourouracil, leucovorin, and oxaliplatin) and FOLFOX4 alone. There was a statistically significant improvement in overall survival (OS) in patients receiving bevacizumab plus FOLFOX4 compared to those receiving FOLFOX4 alone.Citation87
Bevacizumab received FDA approval in October of 2006 for a labeling extension for patients with unresectable, locally advanced, recurrent or metastatic nonsquamous, nonsmall cell lung carcinoma. This was based on the primary trial E4599, which was a randomized, active controlled, open label, multicenter clinical study evaluating bevacizumab plus carboplatin and paclitaxel versus carboplatin and paclitaxel alone. There was a statistically significant improvement in OS in those receiving bevacizumab with carboplatin and paclitaxel (median OS 12.3 vs 10.3 months; hazard ratio 0.80, P = 0.013 stratified log rank test).Citation85
An accelerated approval was granted in February 2008 for use in conjunction with paclitaxel in patients with metastatic HER-2 negative breast carcinoma in a single, open-label, randomized, multi-centre study E2100. While response rates were improved there was no improvement in disease-related symptoms nor increased OS.Citation90 To date there are no data demonstrating an improvement in disease-related symptoms or increased OS for breast cancer.
Most recently bevacizumab received FDA approval to be used in conjunction with interferon-alfa for patients with metastatic renal cell carcinoma who had undergone nephrectomy. This was largely based on data from the BO17705 trial, a randomized, double-blind, placebo-controlled, multinational clinical trial which demonstrated a median PFS of 10.2 months for the bevacizumab plus interferon arm compared to 5.4 months for the interferon and placebo arm (hazard ratio [HR], 0.60 (95% confidence interval [CI] 0.49 to 0.72), P < 0.0001).Citation86 There was no statistically significant advantage in OS.Citation86
Bevacizumab and recurrent malignant gliomas
A number of retrospective studies have been published documenting institution experiences with bevacizumab in patients with recurrent malignant glioma (MG) (). It is difficult to interpret data from these studies due to their retrospective nature. In addition, there often is no distinction between World Health Organization (WHO) grade III and IV tumors. These studies include a variety of combinations of chemotherapeutic agents such as irinotecan, TMZ and carboplatin. Response rates range between 11% to 79%, median progression-free survival (mPFS) from 4.2 to 7.6 months and median overall survival (mOS) from 4.6 to 12.6 months.Citation91–Citation96 Ali et al reported a case series of 13 patients with recurrent heavily pretreated malignant glioma treated with the combination of bevacizumab and irinotecan. Of the thirteen patients nine were started on bevacizumab at a dose of 5 mg/m2 every 2 weeks while the rest received a dose of 10 mg/m2; irinotecan was given at a dose of 125 mg/m2 every week for 3 weeks. Of the 13 treated patients, 10 (77%) had a radiographic partial response and 3 (23%) had stable disease. The median time to disease progression was 24 weeks while the mOS was 27 weeks.Citation91 Narayana et al reported on 61 patients with recurrent high-grade gliomas treated with bevacizumab at 10 mg/kg every 2 weeks for 4 doses in an 8-week cycle along with either irinotecan or carboplatin. At a median follow-up of 7.5 months (range 1 to 19 months), 50 (82%) patients relapsed and 42 patients (70%) died of the disease. The mPFS and OS were 5 (95% CI 2.3 to 7.7) and 9 (95% CI 7.6 to 10.4) months, respectively. Radiographic responses were noted in 73.6% of cases.Citation92 Norden et al in a retrospective study reviewed 55 consecutive patients with recurrent malignant gliomas who were treated with bevacizumab and chemotherapy (irinotecan, carboplatin, carmustine, temozolomide) to determine efficacy, toxicity, and patterns of recurrence. Only 2.3% of patients had a complete response, 31.8% had a partial response, 29.5% a minimal response, and 29.5% had stable disease. A PFS-6 was 42% for patients with GBM and 32% for patients with anaplastic glioma.Citation97 In a retrospective study by Zuniga et al of bevacizumab plus irinotecan in recurrent GBM, 6 (11.8%) of 51 patients discontinued treatment due to a treatment-emergent adverse event, including one with end-stage renal failure and another with gastric perforation.Citation94
Table 1 Selected trials of bevacizumab treatment in recurrent malignant glioma
There are several prospective trials of bevacizumab in recurrent GBM patients. The first study was published by Vredenburg et al.Citation98 They reported on a phase II trial of 35 patients divided into two treatment cohorts. The first cohort included 23 patients who received bevacizumab at 10 mg/kg plus irinotecan every 2 weeks. The second cohort included 12 patients who were treated with bevacizumab 15 mg/kg every 21 days and irinotecan on days 1, 8, 22, and 29. The group reported 57% of patients achieving a partial response to therapy and a PFS-6 of 46% (95% CI 32% to 66%). However, 11/35 (31%) patients discontinued therapy due to toxicity and an additional four withdrew due to fatigue. The results were an improvement when compared to historical controls which demonstrate a 6 month PFS of only 9% and response rates of 7% to 9%.Citation22,Citation99,Citation100
Two pivotal trials have documented the bevacizumab monotherapy experience. This included the industry sponsored AVF3708g open-label, multi-center trialCitation101 and a separate independent study, NCI 06-C-0064E, conducted at the National Cancer Institute.Citation102 The AVF3708g open-label trial included a sample of 167 patients who were randomly assigned to receive bevacizumab alone or in combination with irinotecan 340 mg/m2 or 125 mg/m2 depending on the use of enzyme-inducing anti-seizure medications. The estimated PFS-6 rates were 42.6% (97.5% CI 29.6% to 55%) for the monotherapy group and 50.3% (97.5% CI 36.8% to 63.9%) for the combined group. While the study was not designed to be comparative, there was no statistically significant difference in survival between the two arms. Objective response rates were 28.2% (97.5% CI 18.5% to 40.3%) for the monotherapy group and 37.8% (97.5% CI 26.5% to 50.8%) for the combined group. The mOS rates were 9.2 months (97.5% CI 8.2 to 10.7 months) for the monotherapy group and 8.2 months (97.5% CI 7.8 to 10.9 months) for the combined group.
In the NCI trial, patients were treated with bevacizumab 10 mg/kg every 2 weeks. Patients who progressed were offered participation in a companion study where irinotecan 125 to 340 mg/m2 was immediately added to biweekly bevacizumab. None of the patients in the NCI trial who were subsequently treated with irinotecan had a response after progression on bevacizumab alone. A response rate of 35% and PFS6 29% (95% CI 18% to 48%) were documented in the first 48 of the 56 patients enrolled into this study. As a result of the data obtained in these trials, bevacizumab received an accelerated FDA approval in May 2009 as monotherapy in patients with GBM who progressed after initial treatment.
The utility of irinotecan in combination with bevacizumab has not been established. This is of little surprise since single agent irinotecan in glioma patients has shown little efficacy in previous studies.Citation103,Citation104 To date, no other standard therapy has proven itself to be superior to other treatments when combined with bevacizumab for the treatment of recurrent GBM. As such, various other combinations of bevacizumab have been attempted. Mohile et al reported on a small group of 12 patients who achieved a 58% response rate and a PFS-6 of 76% with fractionated focal radiotherapy on small volume tumors.Citation105 In an attempt to answer the question of whether the addition of cytotoxic agents may have a synergistic effect, various groups have reviewed their experience with bevacizumab in combination with agents such as TMZ, irinotecan, carboplatin, nitrosureas, etoposide and erlotinib. In a group of 54 patients treated with irinotecan versus 7 patients treated with carboplatin in combination with bevacizumab no significant difference in survival was documented.Citation92
Based on the NCI trial that led to the FDA approval for bevacizumab in recurrent GBM, patients overall tolerated monotherapy well.Citation102 The most frequently observed severe adverse event possibly or probably related to bevacizumab in 48 treated patients was the occurrence of thromboembolic events which occurred in six patients (12.5%). One patient experienced a stroke and the other three experienced a pulmonary embolus. None of the patients experienced an intracranial hemorrhage. Hypertension was the second most frequent drug-related adverse event that was easily treated with antihypertensive medication. Six patients (12.5%) were removed from the study for drug-associated toxicity that included five thromboembolic events, and one bowel perforation. Grade 1 proteinuria was reported in one patient and Grade 3 hepatic dysfunction was reported in one patient as well. Grade 2 and 3 thrombocytopenia was observed in 1 and 2 patients respectively.
In the AVF3708G trial,Citation101 98.8 % of patients in the bevacizumab alone arm experienced adverse events with the most common being fatigue (45.2%), headache (36.9%), hypertension (29.8%), diarrhea (21.4%) and epistaxis (19%). Adverse events took place in all patients in the irinotecan and bevacizumab arm with the most common being fatigue (75.9%), diarrhea (74.7%), nausea (67.1%) and constipation (40.5%). Grade 3 or higher treatment emergent AEs occurred in 46.4% of bevacizumab recipients and 65.8% of bevacizumab plus irinotecan recipients. Selected AEs associated with bevacizumab treatment included arterial thromboembolism (grade ≥ 3; bevacizumab, 2.4%; in the irinotecan group, 2.5%), venous thromboembolism (bevacizumab, 3.6%; combined group, 8.9%), and wound-healing complications. Two patients (2.5%) experienced grade 3 gastrointestinal perforation and one patient (1.3%) experienced serious reversible posterior leukoencephalopathy syndrome in the combined group. A grade 1 intracranial hemorrhage was noted in two patients (2.4%) who received only bevacizumab versus 3 patients in the combined group (3.8%) experiencing a grade 1, 2 and 4 respectively. AEs led to bevacizumab discontinuation for four patients (4.8%) in the bevacizumab arm and for 14 patients (17.7%) in the irinotecan group. Two patients in the bevacizumab arm died secondary to neutropenia and pulmonary embolism while one reportedly died of a seizure within the combined group.
Newly diagnosed malignant gliomas
A number of investigators are conducting upfront studies for newly diagnosed GBM since survival benefit data for recurrent disease with the use of bevacizumab have not been established and will not likely be an end-point in future trials for recurrent disease. Nicholas et al reported a study of bevacizumab added to adjuvant TMZ after concurrent chemoradiation. Citation106 Preliminary results from 42 of 48 enrolled patients demonstrated complete radiographic responses in 5/42 (12%) patients, a partial response in 9 (21%), 13 (31%) were stable and 7 (17%) showed progressive disease.
Lai et al reported a phase II trial of 70 patients treated with focal external beam radiation (60 Gy in 30 fractions), biweekly bevacizumab 10 mg/kg and daily TMZ 75 mg/m2. After a two week post-radiation interval, combination therapy with biweekly bevacizumab and monthly TMZ was given. All but 2 patients had total or partial resections. Median follow-up was 17.2 months and 50% of the group had a Kamovsky Performance Scale of 60% to 80%. Median PFS was 13 months (95% CI 11.3 to 15.9 months) and PFS-6 was 89.1% (95% CI 78.6 to 94.7 months) compared to 8.1 months (95% CI 7.0 to 11.7 months) and 64.4% (95% CI 54.5 to 72.7 months) for an internal control group. Median OS was 25 months (95% CI 16.1 to NA) compared to 21.1 months in the control group. In the treatment group, 6- and 18-month OS was 98.6% (95% CI 90.2 to 99.8 months) and 61.1% (95% CI 45.9 to 73.3 months), respectively, compared to 88.2% (95% CI 80.5 to 93 months) and 60.6% (95% CI 50.8 to 69.1 months) in the control group. Unexpected adverse events included isolated cases of retinal detachment and optic neuropathy.Citation107 The most common treatment related serious adverse effects included thrombotic complications with 12 (17%) patients being diagnosed with deep vein thrombosis. Hypertension was reported in 8 (11%) patients. Four (6%) patients had wound related infections involving their craniotomy sites. Other adverse events included involvement of the gastrointestinal and renal systems. Neurological events included seizures in 5 (7%) patients, transient ischemic attack/stroke in 3 patients and 1 patient with a traumatic hemorrhage.Citation107
A similarly designed study of 15 patients was reported by Narayana et al with 1-year PFS and OS being 59.3% and 86.7% respectively.Citation108 Radiographic responses were noted in 13 of 14 assessable patients (92.8%). Gruber et al utilized a more dose intense schedule of adjuvant TMZ with bevacizumab for newly diagnosed GBM. The PFS-6 survival was 77.5%, the median PFS was 17 months, while the 1- and 2-year OS was 83% and 57%, respectively.Citation109
Two ongoing randomized phase III trials will investigate the efficacy and safety of adding bevacizumab to standard upfront chemoradiation with temozolomide. A phase III, double-blind, placebo controlled trial (Radiation Therapy Oncology Group 0825) has a target accrual of 720 patients. Patients with newly diagnosed GBM will undergo 3 weeks of standard chemoradiation and in addition will undergo analysis for MGMT promoter methylation and molecular profiling. Patients will then be stratified into two arms for the final 3 weeks of chemoradiation. The first arm will receive concurrent placebo while the second arm will receive biweekly bevacizumab. This will be followed by adjuvant treatment in which the first arm will continue with placebo in addition to TMZ and the second arm will receive TMZ with biweekly bevacizumab.Citation110 The other phase III trial is an industry sponsored randomized, double-blind, placebo-controlled trial with an expected target accrual of over 500 patients. In this trial patients will be randomly assigned to either bevacizumab or the placebo arm, in combination with standard radiation therapy plus temozolomide for 6 weeks. After a 4 week hiatus, patients will continue to receive bevacizumab or placebo, plus adjuvant temozolomide for 6 cycles of maintenance therapy. Bevacizumab or placebo monotherapy will then continue until disease progression.Citation111 As yet, the role of bevacizumab in the upfront management of GBM remains to be determined.
Bevacizumab and its clinical benefit
The effects of bevacizumab on vascular permeability are akin to the effects of steroids on cerebral edema.Citation112 We, therefore, have at our disposal another drug other than dexamethasone that can produce improvement of neurological signs and symptoms, which can translate into improved quality of life.Citation112 This was demonstrated in the two trials that led to the FDA approval of bevacizumab in recurrent GBM. In the NCI trial, 50% patients had decreased cerebral edema. Approximately 58% of patients on steroids at the start of treatment were able to achieve an average dose reduction of 59% and 52% had improved neurological symptoms.Citation102 Findings were similar in the industry sponsored trial which also assessed cognitive function. The majority of patients demonstrated stable performance on a variety of tests at the six week follow-up and 18% to 25% had improved performance.Citation113 Bevacizumab clearly has a role in the treatment of patients with GBM, independent of survival benefit, for its steroid-sparing effect
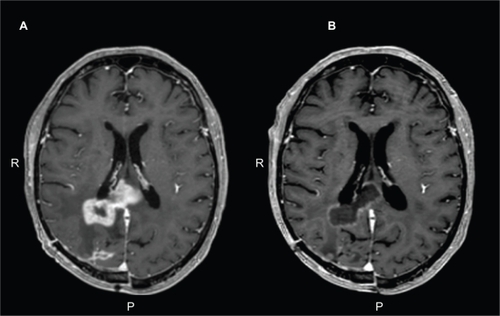
The dramatic effects of bevacizumab on the MRI contrast signal () is at least partially a result of its effect on vascular permeability since reduction in enhancement can be observed with just one dose of the drug; too short of an interval for true tumorcidal activity. Consequently, MRI contrast signal may not be a reliable proxy marker for extent of tumor as it is for evaluating cytotoxic therapy. For this reason alternative imaging methods are being investigated to assess tumor response such as dynamic susceptibility contrast enhanced MRI and apparent diffusion coefficient measures.Citation114–Citation116
Figure 1 A) Axial T1-weighted, post-contrast image of the brain in a patient with glioblastoma multiforme who progressed on temozolomide therapy. B) Response after 2 months of bevacizumab monotherapy.
Despite the beneficial effect of bevacizumab, concern has arisen that it may actually influence the pattern of disease progression and thereby promote tumor invasion. This was observed in both in vitro and in vivo studies which demonstrated the up-regulation of invasion related genes such as MMP 9 or other pro-angiogenic factors.Citation117 The pattern of relapse, prognosis and outcome of further therapy in patients who failed bevacizumab was recently described in an institutional review. Iwamoto et al reported a series of 37 patients with recurrent GBM who progressed on treatment with bevacizumab. The mOS after progression on bevacizumab was 4.5 months. Seventeen patients (46%) had local recurrence and 6 (16%) had multifocal recurrence. Thirteen patients (35%) had non-enhancing disease progression. The patients with non-enhancing tumor did worse with shorter survival, which was thought to be due to a larger disease burden impacting negatively on performance status. Non-enhancing tumor was also thought to be an independent prognostic factor.Citation118 Similar findings were reported by Zuniga et al but others have reported lower rates of infiltrative disease on the order of 20% to 30%.Citation92,Citation120 It is difficult to discern whether anti-VEGF therapy actually accelerates or promotes tumor invasion or whether the natural course of the disease is altered so that patients are alive long enough for us to observe this degree of tumor progression, unmasked by the effect bevacizumab has on gadolinium enhancement. It is clear that clinical trials that are using anti-VEGF therapy will need to measure response using methods that incorporate evaluation of non-enhancing disease.
While bevacizumab appears to be an effective agent for recurrent GBM, the majority of patients do not achieve durable disease control and other salvage regimens are required. Often, adding a cytotoxic agent or switching the companion cytotoxic agent is attempted, but efficacy of this practice is unclear. Quant et al described a retrospective review of 54 patients with recurrent MG who progressed on either bevacizumab mono- or combination therapy who were then subsequently treated with an alternate bevacizumab-containing regimen.Citation119 Tumor progression was determined clinically and radiographically. The median prior chemotherapy regimens including the first bevacizumab-containing regimen was 3 (range, 2 to 5). The mPFS on the first bevacizumab-containing regimen was 124 days (95% CI 87 to 154 days); PFS-6 was 33% and the mPFS on the second bevacizumab-containing regimen was 37.5 days (95% CI 34 to 42 days) with a PFS-6 of 2%. In the review by Iwamoto et al 19 of the 37 patients received salvage chemotherapy after failure with bevacizumab. The mOS in those who received salvage treatment was 5.2 months and the PFS-6 was 0%. It is clear that other therapeutic options need to be considered for such patients.Citation118
Conclusion
Tumor angiogenesis has emerged as a valid therapeutic target in clinical oncology and the VEGF system represents a key mediator in this process. While monotherapy with bevacizumab for recurrent GBM has afforded encouraging results, it by no means approaches a cure or durable disease control for the majority of patients. Continued efforts are needed to improve on this early success. Rational combinations of targeted therapy with bevacizumab are appropriately being studied, as well as bevacizumab’s role in the upfront treatment of glioma patients. The post-bevacizumab era will prove to be a challenging environment for the neuro-oncology community in evaluating new salvage therapy, but one that for the first time may carry real promise.
Disclosures
The authors disclose no potential conflicts of interest.
References
- HayatMJHowladerNReichmanMEEdwardsBKCancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) ProgramOncologist2007121203717227898
- HolickCNGiovannucciELRosnerBStampferMJMichaudDSProspective study of cigarette smoking and adult glioma: dosage, duration, and latencyNeuro Oncol20079332633417504930
- HuncharekMKupelnickBWheelerLDietary cured meat and the risk of adult glioma: a meta-analysis of nine observational studiesJ Environ Pathol Toxicol Oncol200322212913714533876
- KaripidisKKBenkeGSimMROccupational exposure to ionizing and non-ionizing radiation and risk of non-Hodgkin lymphomaInt Arch Occup Environ Health200780866367017334774
- KaripidisKKBenkeGSimMRYostMGilesGOccupational exposure to low frequency magnetic fields and the risk of low grade and high grade gliomaCancer Causes Control200718330531317260179
- InskipPDTaroneREHatchEECellular-telephone use and brain tumorsN Engl J Med20013442798611150357
- VilchezRAButelJSEmergent human pathogen simian virus 40 and its role in cancerClin Microbiol Rev200417349550815258090
- ReussDvon DeimlingAHereditary tumor syndromes and gliomasRecent Results Cancer Res20091718310219322539
- KrexDKlinkBHartmannCLong-term survival with glioblastoma multiformeBrain2007130Pt 102596260617785346
- SmithJSJenkinsRBGenetic alterations in adult diffuse glioma: occurrence, significance, and prognostic implicationsFront Biosci20005D213D23110702383
- LacroixMAbi-SaidDFourneyDRA multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survivalJ Neurosurg200195219019811780887
- HessKRExtent of resection as a prognostic variable in the treatment of gliomasJ Neurooncol199942322723110433106
- MetcalfeSEGrantRBiopsy versus resection for malignant gliomaCochrane Database Syst Rev20013CD00203411687008
- BloomHJCombined modality therapy for intracranial tumorsCancer1975351111120162849
- SalazarOMRubinPFeldsteinMLPizzutielloRHigh dose radiation therapy in the treatment of malignant gliomas: final reportInt J Radiat Oncol Biol Phys197951017331740231023
- WalkerMDAlexanderEJrHuntWEEvaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trialJ Neurosurg1978493333343355604
- WalkerMDGreenSBByarDPRandomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgeryN Engl J Med198030323132313297001230
- WalkerMDStrikeTAShelineGEAn analysis of dose-effect relationship in the radiotherapy of malignant gliomasInt J Radiat Oncol Biol Phys197951017251731231022
- FineHADearKBLoefflerJSBlackPMCanellosGPMeta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adultsCancer1993718258525978453582
- CairncrossJGMacdonaldDRSuccessful chemotherapy for recurrent malignant oligodendrogliomaAnn Neurol19882343603643382171
- StuppRMasonWPvan den BentMJRadiotherapy plus concomitant and adjuvant temozolomide for glioblastomaN Engl J Med20053521098799615758009
- WongETHessKRGleasonMJOutcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trialsJ Clin Oncol19991782572257810561324
- WHLThe vascular pattern of tumorsJohns Hopkins Hosp Bull192741156162
- FolkmanJTumor angiogenesis: therapeutic implicationsN Engl J Med197128521118211864938153
- FolkmanJAnti-angiogenesis: new concept for therapy of solid tumorsAnn Surg197217534094165077799
- FerraraNHillanKJGerberHPNovotnyWDiscovery and development of bevacizumab, an anti-VEGF antibody for treating cancerNat Rev Drug Discov20043539140015136787
- SholleyMMFergusonGPSeibelHRMontourJLWilsonJDMechanisms of neovascularization. Vascular sprouting can occur without proliferation of endothelial cellsLab Invest19845166246346209468
- FolkmanJAngiogenesis in psoriasis: therapeutic implicationsJ Invest Dermatol197259140435039962
- MillerJWAdamisAPShimaDTVascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate modelAm J Pathol199414535745847521577
- MoultonKSAngiogenesis in atherosclerosis: gathering evidence beyond speculationCurr Opin Lipidol200617554855516960504
- FerraraNVEGF and the quest for tumour angiogenesis factorsNat Rev Cancer200221079580312360282
- BalukPHashizumeHMcDonaldDMCellular abnormalities of blood vessels as targets in cancerCurr Opin Genet Dev200515110211115661540
- HanahanDFolkmanJPatterns and emerging mechanisms of the angiogenic switch during tumorigenesisCell19968633533648756718
- FolkmanJAngiogenesis: an organizing principle for drug discovery?Nat Rev Drug Discov20076427328617396134
- FolkmanJIs angiogenesis an organizing principle in biology and medicine?J Pediatr Surg200742111117208533
- KerbelRSTumor angiogenesisN Engl J Med2008358192039204918463380
- FangJShingYWiederschainDMatrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor modelProc Natl Acad Sci U S A20009783884388910760260
- MaxwellPHDachsGUGleadleJMHypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growthProc Natl Acad Sci U S A19979415810481099223322
- ShweikiDItinASofferDKeshetEVascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesisNature199235963988438451279431
- PlateKHBreierGWeichHARisauWVascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivoNature199235963988458481279432
- AugustePGurselDBLemiereSInhibition of fibroblast growth factor/fibroblast growth factor receptor activity in glioma cells impedes tumor growth by both angiogenesis-dependent and -independent mechanismsCancer Res20016141717172611245488
- SalmaggiAEoliMFrigerioSIntracavitary VEGF, bFGF, IL-8, IL-12 levels in primary and recurrent malignant gliomaJ Neurooncol200362329730312777082
- SchmidtNOWestphalMHagelCLevels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesisInt J Cancer199984110189988225
- LiMRansohoffRMThe roles of chemokine CXCL12 in embryonic and brain tumor angiogenesisSemin Cancer Biol200919211111519038344
- HallbookFWilsonKThorndykeMOlinskiRPFormation and evolution of the chordate neurotrophin and Trk receptor genesBrain Behav Evol200668313314416912467
- HuangEJReichardtLFTrk receptors: roles in neuronal signal transductionAnnu Rev Biochem20037260964212676795
- NicoBMangieriDBenagianoVCrivellatoERibattiDNerve growth factor as an angiogenic factorMicrovasc Res200875213514117764704
- KraemerRHempsteadBLNeurotrophins: novel mediators of angiogenesisFront Biosci20038s1181118612957860
- HoodJDChereshDARole of integrins in cell invasion and migrationNat Rev Cancer2002229110012635172
- SunLHuiAMSuQNeuronal and glioma-derived stem cell factor induces angiogenesis within the brainCancer Cell20069428730016616334
- SengerDRGalliSJDvorakAMPerruzziCAHarveyVSDvorakHFTumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluidScience198321945879839856823562
- FerraraNHenzelWJPituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cellsBiochem Biophys Res Commun198916128518582735925
- ConnollyDTOlanderJVHeuvelmanDHuman vascular permeability factor. Isolation from U937 cellsJ Biol Chem19892643320017200242584205
- HouckKAFerraraNWinerJCachianesGLiBLeungDWThe vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNAMol Endocrinol1991512180618141791831
- LeungDWCachianesGKuangWJGoeddelDVFerraraNVascular endothelial growth factor is a secreted angiogenic mitogenScience19892464935130613092479986
- de VriesCEscobedoJAUenoHHouckKFerraraNWilliamsLTThe fms-like tyrosine kinase, a receptor for vascular endothelial growth factorScience199225550479899911312256
- ShibuyaMYamaguchiSYamaneANucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms familyOncogene1990545195242158038
- OlssonAKDimbergAKreugerJClaesson-WelshLVEGF receptor signalling – in control of vascular functionNat Rev Mol Cell Biol20067535937116633338
- TermanBIDougher-VermazenMCarrionMEIdentification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factorBiochem Biophys Res Commun19921873157915861417831
- TakahashiMMatsuiAInaoMMochidaSFujiwaraKERK/MAPK-dependent PI3K/Akt phosphorylation through VEGFR-1 after VEGF stimulation in activated hepatic stellate cellsHepatol Res200326323223612850696
- TakahashiTShibuyaMThe 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma pathway and partially induces mitotic signals in NIH3T3 fibroblastsOncogene19971417207920899160888
- JonesMKItaniRMWangHActivation of VEGF and Ras genes in gastric mucosa during angiogenic response to ethanol injuryAm J Physiol19992766 Pt 1G1345G135510362637
- GerberHPMcMurtreyAKowalskiJVascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activationJ Biol Chem19982734630336303439804796
- GilleHKowalskiJLiBAnalysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2). A reassessment using novel receptor-specific vascular endothelial growth factor mutantsJ Biol Chem200127653222323011058584
- HellstromMPhngLKHofmannJJDll4 signalling through Notch1 regulates formation of tip cells during angiogenesisNature2007445712977678017259973
- RidgwayJZhangGWuYInhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesisNature200644471221083108717183323
- Noguera-TroiseIDalyCPapadopoulosNJBlockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesisNature200644471221032103717183313
- BoultonMECaiJGrantMBgamma-Secretase: a multifaceted regulator of angiogenesisJ Cell Mol Med200812378179518266961
- SelkoeDKopanRNotch and Presenilin: regulated intramembrane proteolysis links development and degenerationAnnu Rev Neurosci20032656559712730322
- BoultonMECaiJGrantMBZhangYGamma-secretase regulates VEGFR-1 signalling in vascular endothelium and RPEAdv Exp Med Biol200861331331918188959
- HiranoHLopesMBLawsERJrInsulin-like growth factor-1 content and pattern of expression correlates with histopathologic grade in diffusely infiltrating astrocytomasNeuro Oncol19991210911911550306
- FerraraNGerberHPLeCouterJThe biology of VEGF and its receptorsNat Med20039666967612778165
- MatsumotoTClaesson-WelshLVEGF receptor signal transductionSci STKE20012001112RE2111741095
- BenjaminLEGolijaninDItinAPodeDKeshetESelective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawalJ Clin Invest199910321591659916127
- KuDDZaleskiJKLiuSBrockTAVascular endothelial growth factor induces EDRF-dependent relaxation in coronary arteriesAm J Physiol19932652 Pt 2H586H5928368362
- ClaussMGerlachMGerlachHVascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migrationJ Exp Med19901726153515452258694
- GabrilovichDIChenHLGirgisKRProduction of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cellsNat Med1996210109611038837607
- CarmelietPBlood vessels and nerves: common signals, pathways and diseasesNat Rev Genet20034971072012951572
- CalabreseCPoppletonHKocakMA perivascular niche for brain tumor stem cellsCancer Cell2007111698217222791
- RubensteinJLKimJOzawaTAnti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooptionNeoplasia20002430631411005565
- KunkelPUlbrichtUBohlenPInhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2Cancer Res200161186624662811559524
- PrestaLGChenHO’ConnorSJHumanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disordersCancer Res19975720459345999377574
- LinYSNguyenCMendozaJLPreclinical pharmacokinetics, interspecies scaling, and tissue distribution of a humanized monoclonal antibody against vascular endothelial growth factorJ Pharmacol Exp Ther199928813713789862791
- IgnoffoRJOverview of bevacizumab: a new cancer therapeutic strategy targeting vascular endothelial growth factorAm J Health Syst Pharm20046121 Suppl 5S21S2615552623
- CabebeEWakeleeHRole of anti-angiogenesis agents in treating NSCLC: focus on bevacizumab and VEGFR tyrosine kinase inhibitorsCurr Treat Options Oncol200781152717634832
- EscudierBCosaertJPisaPBevacizumab: direct anti-VEGF therapy in renal cell carcinomaExpert Rev Anticancer Ther20088101545155718925847
- GiantonioBJCatalanoPJMeropolNJBevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200J Clin Oncol200725121539154417442997
- HurwitzHFehrenbacherLNovotnyWBevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancerN Engl J Med2004350232335234215175435
- KabbinavarFFHambletonJMassRDHurwitzHIBergslandESarkarSCombined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancerJ Clin Oncol200523163706371215867200
- MillerKDE2100: a phase III trial of paclitaxel versus paclitaxel/bevacizumab for metastatic breast cancerClin Breast Cancer20033642142212636887
- AliSAMcHaylehWMAhmadABevacizumab and irinotecan therapy in glioblastoma multiforme: a series of 13 casesJ Neurosurg2008109226827218671639
- NarayanaAKellyPGolfinosJAntiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survivalJ Neurosurg2009110117318018834263
- PoulsenHSGrunnetKSorensenMBevacizumab plus irinotecan in the treatment patients with progressive recurrent malignant brain tumoursActa Oncol2009481525819031176
- ZunigaRMTorcuatorRJainREfficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecanJ Neurooncol200991332933618953493
- KangTYJinTElinzanoHPeereboomDIrinotecan and bevacizumab in progressive primary brain tumors, an evaluation of efficacy and safetyJ Neurooncol200889111311818438609
- NghiemphuPLLiuWLeeYBevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experienceNeurology200972141217122219349600
- NordenADYoungGSSetayeshKBevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrenceNeurology200834701077978718316689
- VredenburghJJDesjardinsAHerndonJE2ndBevacizumab plus irinotecan in recurrent glioblastoma multiformeJ Clin Oncol200725304722472917947719
- BallmanKVBucknerJCBrownPDThe relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiformeNeuro Oncol200791293817108063
- LambornKRYungWKChangSMProgression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomasNeuro Oncol2008410216217018356283
- FriedmanHSPradosMDWenPYBevacizumab alone and in combination with irinotecan in recurrent glioblastomaJ Clin Oncol200927284733474019720927
- KreislTNKimLMooreKPhase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastomaJ Clin Oncol200927574074519114704
- BatchelorTTGilbertMRSupkoJGPhase 2 study of weekly irinotecan in adults with recurrent malignant glioma: final report of NABTT 97-11Neuro Oncol200461212714769136
- PradosMDLambornKYungWKA phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium studyNeuro Oncol20068218919316533878
- MohileNAALLymberisSCKarimiSHouBLGutinPHA pilot study of bevacizumab and stereotactic intensity modulated re-irradiation for recurrent high grade gliomasASCO Meeting Abstracts20072518 Suppl2028
- NicholasMKLRArzbaecherJPaleologosNKrouwerHMalkinMBevacizumab in combination with temozolomide in the adjuvant treatment of newly diagnosed glioblastoma multiforme: Preliminary results of a phase II studyASCO Meeting Abstracts20092715S2016
- LaiAFilkaEMcGibbonBPhase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerabilityInt J Radiat Oncol Biol Phys20087151372138018355978
- NarayanaAGolfinosJGFischerIFeasibility of using bevacizumab with radiation therapy and temozolomide in newly diagnosed high-grade gliomaInt J Radiat Oncol Biol Phys200872238338918793954
- GruberMLRazaSBevacizumab in combination with radiotherapy plus concomitant and adjuvant temozolomide for newly diagnosed glioblastoma: Update progression-free survival, overall survival, and toxicityASCO Meeting Abstracts20092715S2017
- GilbertMarc R RTOG. Temozolomide and Radiation Therapy with or without Bevacizumab in treating patients with newly diagnosed glioblstoma or Gliosarcoma. http://clinicaltrials.gov/ct2/show/NCT00884741
- Hoffmann-LaRocheCTA study of Avastin in combination with Temozolomide and Radiotherapy in patients with newly diagnsoed glioblastoma. http://clinicaltrials.gov/ct2/show/NCT00943826
- AnanthnarayanSBahngJRoringJTime course of imaging changes of GBM during extended bevacizumab treatmentJ Neurooncol2008788333934718389177
- WefelJSCTZazzaliJFriedmanHSfor the BINeurocognitive function in patients with glioblastoma multiforme in first or second relapse treated with bevacizumab in the BRAIN studyASCO Meeting Abstracts20092715S20256
- PopeWBKimHJHuoJRecurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatmentRadiology2009252118218919561256
- DesjardinsABDHerndonJEIIReardonDAQuinnJARichJNDynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) evaluation in glioblastoma (GBM) patients treated with bevacizumab (BEV) and irinotecan (CPT-11)ASCO Meeting Abstracts20072518 Suppl2029
- PaldinoMDAFriedmanHSVredenburghJJBarboriakDPPrognostic significance of early changes in the apparent diffusion coefficient that occurs after treatment of patients with glioblastoma multiforme with bevacizumabASCO Meeting Abstracts20092715S2058
- Lucio-EterovicAKPiaoYde GrootJFMediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapyClin Cancer Res200915144589459919567589
- IwamotoFMAbreyLEBealKPatterns of relapse and prognosis after bevacizumab failure in recurrent glioblastomaNeurology200973151200120619822869
- QuantECNordenADDrappatzJRole of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumabNeuro Oncol200911555055519332770
- PotthastLCSPanEYuDZhuWBremSThe infiltrative, diffuse pattern of recurrence in patients with malignant gliomas treated with bevacizumabASCO Meeting Abstracts20092715S2057
