Abstract
Azelastine nasal spray (Allergodil®, Lastin®, Afluon®; Meda AB, Stockholm, Sweden) is a fast-acting, efficacious and well-tolerated H1-receptor antagonist for the treatment of rhinitis. In addition it also has mast-cell stabilizing and anti-inflammatory properties, reducing the concentration of leukotrienes, kinins and platelet activating factor in vitro and in vivo, as well as inflammatory cell migration in rhinitis patients. Well-controlled studies in patients with seasonal allergic rhinitis (SAR), perennial rhinitis (PR) or vasomotor rhinitis (VMR) confirm that azelastine nasal spray has a rapid onset of action, and improves nasal symptoms associated with rhinitis such as nasal congestion and post-nasal drip. Azelastine nasal spray is effective at the lower dose of 1 spray as well at a dose of 2 sprays per nostril twice daily, but with an improved tolerability profile compared to the 2-spray per nostril twice daily regimen. Compared with intranasal corticosteroids, azelastine nasal spray has a faster onset of action and a better safety profile, showing at least comparable efficacy with fluticasone propionate (Flonase®; GSK, USA), and a superior efficacy to mometasone furoate (Nasonex®; Schering Plough, USA). In combination with fluticasone propionate, azelastine nasal spray exhibits greater efficacy than either agent used alone, and this combination may provide benefit for patients with difficult to treat seasonal allergic rhinitis. In addition, azelastine nasal spray can be used on an as-needed basis without compromising clinical efficacy. Compared with oral antihistamines, azelastine nasal spray also demonstrates superior efficacy and a more rapid onset of action, and is effective even in patients who did not respond to previous oral antihistamine therapy. Unlike most oral antihistamines, azelastine nasal spray is effective in alleviating nasal congestion, a particularly bothersome symptom for rhinitis sufferers. Azelastine nasal spray is well tolerated in both adults and children with allergic rhinitis. Bitter taste which seems to be associated with incorrect dosing technique is the most common side effect reported by patients, but this problem can be minimized by correct dosing technique.
Introduction
Rhinitis is an inflammatory disease of the upper airways, affecting approximately 58 million people only in the United States alone (CitationSettipane 2001) and its prevalence is increasing. The cost of the disease is significant with between US$2 and US$5 billion incurred annually in both direct and indirect costs (CitationRay et al 1999; CitationReed et al 2004). In the US, the number of lost workdays is estimated as approximately 3.5 million a year (CitationMahr and Sheth 2005). It can be classified as allergic, non-allergic or mixed upper respiratory disorder (Berstein 2007). It is classified as allergic if symptoms occur in association with a specific IgE-mediated response; as non-allergic if symptoms are induced by irritant triggers, but without an IgE-mediated response; and as of mixed etiology if IgE-mediated responses occur in conjunction with symptoms induced by both allergens and non-allergic irritant triggers. Allergic rhinitis (AR) is further classified as seasonal or perennial (CitationDykewicz et al 1998). Seasonal allergic rhinitis (SAR) symptoms are induced by exposure to pollens from trees, grass, weeds or seasonal mould spores, whilst perennial rhinitis (PR) is associated with environmental allergens which are generally present on a year-round basis such as house dust, animal dander and insect droppings (CitationDykewicz et al 1998). In contrast, the “Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines” recommend a classification in intermittent allergic rhinitis and persistent allergic rhinitis according to the frequency and persistence of symptoms (CitationBousquet et al 2001).
Symptoms of SAR include nasal congestion, runny nose, nasal and nasopharyngeal itching, ear symptoms, sneezing and ocular symptoms in many patients, including itchy and watery eyes (CitationBielory and Ambrosio 2002). The symptoms of sneezing, itching and rhinorrhea are less common with PR (CitationEconomides and Kaliner 2002). As many as half of all patients diagnosed with rhinitis have non-allergic disease (sometimes called vasomotor rhinitis [VMR]) where an allergic component cannot be identified (CitationDykewicz et al 1998). Symptoms are often induced by irritant triggers such as tobacco smoke, strong odors and temperature and pressure changes (CitationDevyani and Corey 2004). The symptoms of VMR are similar to those of AR (CitationDevyani and Corey 2004). To further complicate rhinitis classification, as many as half of all patients with AR are also sensitive to non-allergic triggers; a condition referred to as mixed rhinitis (CitationSettipane and Settipane 2002; CitationLiberman et al 2005). Symptoms of rhinitis can have a major impact on patients’ quality of life (QoL) by interfering with sleep which causes fatigue, and impairing daily activities and cognitive function (CitationDykewicz et al 1998). Patients often complain of an inability to concentrate, and in the case of SAR often avoid outdoor activities in order to avoid exposure to symptom-inducing allergen(s). The Joint Task Force on Allergy Practice and Parameters advises that improving the negative impact on daily life in rhinitis patients defines successful treatment as much as providing symptom relief (CitationDykewicz et al 1998). Indeed, CitationJuniper (1997) recommends that for most patients with rhinitis, improving patient well-being and QoL should be the primary goal of treatment.
Treatment guidelines from the Joint Task Force and WHO recommend that antihistamines, both topical (eg, azelastine [Allergodil®; Meda AB, Stockholm, Sweden]) and oral second-generation (eg, loratadine [Claritin®, Schering Plough, USA], desloratadine [Clarinex®; Schering Plough, USA], fexofenadine [Allegra®; SanofiAventis, USA] or cetirizine [Zyrtec®; Pfizer, USA], and levocetirizine [Xyzall®; UCB, EU]) be used as first-line therapy for AR (CitationDykewicz et al 1998; CitationBousquet et al 2001). Intranasal corticosteroids (eg, fluticasone propionate [Flonase®, GSK, USA], mometasone furoate [Nasonex®; Schering Plough, USA]) may also be considered as initial therapy for AR in patients with more severe symptoms, particularly nasal congestion [(CitationDykewicz et al 1998; CitationLaForce 1999). The Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines recommend a stepped approach to therapy based upon the frequency and severity of symptoms () (CitationBousquet et al 2001). Interestingly, a recent US nationwide survey incorporating approximately 2500 adult allergy sufferers, revealed that 66% were dissatisfied with their current allergy medication due to lack of effectiveness (CitationAnon 2006). Furthermore, more than two-thirds of primary care physicians reported patient dissatisfaction with therapy as the main reason for stopping or switching medications (CitationAnon 2001). Clearly, effective therapies with a good safety profile are needed to treat AR sufferers.
Table 1 Summary of ARIA allergic rhinitis management guidelines
Azelastine
Azelastine nasal spray is a topically administered second-generation antihistamine and selectively antagonizes the H1-receptor (CitationZechel et al 1981) being approximately tenfold more potent than chlorpheniramine in this regard (CitationCasale 1989). It has one of the fastest onsets of action (15 min with nasal spray and up to 3 min with eye drops) among the currently available rhinitis medications (CitationBaumgarten et al 1994; CitationGreiff et al 1997). The effect of azelastine lasts at least 12 hours, thus allowing for a once or twice daily dosing regimen (CitationGreiff et al 1997). It has proven efficacy in treating both allergic and non-allergic rhinitis, and is the only prescription antihistamine approved in the US, Portugal and the Netherlands for the treatment of both SAR (1996) and VMR (1999). In SAR patients azelastine therapy (two sprays per nostril twice daily), improved both total symptom and major symptom complex scores to a significantly greater extent than placebo (CitationMcTavish and Sorkin 1989; CitationStorms et al 1994; CitationLaForce et al 1996; CitationRatner and Sacks 2007). Similarly, in PR patients, azelastine nasal spray significantly improved sleeping, reduced daytime somnolence and nasal congestion compared with placebo (CitationGolden et al 2000). CitationLiberman et al (2005) were the first to show that azelastine was also effective in the management of VMR and even in mixed rhinitis. Azelastine nasal spray significantly (p < 0.01) reduced the total VMR symptom score (TVRSS) compared with placebo after 21-day double-blind treatment, and was associated with clinical improvement in each symptom of the TVRSS (ie, rhinorrhea, sneezing, nasal congestion, and post-nasal drip). In a large open-label trial 4364 patients received azelastine nasal spray (2 sprays per nostril twice daily) as monotherapy for 2 weeks. 78% of VMR patients reported some or complete control of post-nasal drip which rose to 90% of SAR patients for the symptom of sneezing. Of patients reporting sleep difficulties or impaired daytime activities because of rhinitis symptoms, 85% experienced improvements in these parameters with azelastine. Baseline sleep difficulties and impairment of daytime activities were significant (p < 0.01) predictors of a positive treatment effect with azelastine nasal spray. Female patients (p = 0.02), patients with SAR (p < 0.01) and patients with SAR plus sensitivity to non-allergic triggers (p = 0.03) were identified as being most likely to respond to azelastine nasal spray (CitationLiberman et al 2005) Due to its rapid onset of action, azelastine nasal spray continues to control rhinitis symptoms when used on an as-needed basis (CitationCiprandi et al 1997). This property of azelastine is discussed later. First marketed in the UK in 1991 for the treatment of both SAR and PR, it is currently available in more than 70 countries world-wide.
Mode of action
However, azelastine is more than just an anti-histamine. It exhibits a very fast and long-acting effect based on a triple mode of action, with anti-inflammatory and mast cell stabilizing properties in addition to its anti-allergic effects (CitationBernstein 2007; CitationLee and Corren 2007). For example, azelastine inhibits the activation of cultured mast cells and release of interleukin (IL)-6, tryptase, and histamine (CitationKempuraj et al 2002). It also reduces mediators of mast cell degranulation such as leukotrienes which are involved in the late phase allergic response (CitationHowarth 1997), in the nasal lavage fluid of patients with rhinitis (CitationShin et al 1992). It does this possibly by reducing the production of leukotriene (LT)B4 synthase and LTC4, inhibiting phospholipase A2 and LTC4 (CitationHamasaki et al 1996). Leukotrienes are associated with dilation of vessels, increased vascular permeability and edema which results in nasal congestion, mucus production and recruitment of inflammatory cells (CitationGolden et al 2006). Substance P and bradykinin concentrations which are formed in biological fluids and tissues during inflammation, are also reduced by azelastine (CitationShin et al 1992; CitationNieber et al 1993; CitationShinoda et al 1997). These agents are associated with the AR symptoms of nasal itching and sneezing, but may also contribute to the onset of non-allergic VMR symptoms. Other anti-inflammatory properties of azelastine include inhibition of tumor necrosis factor alpha (TNFα) release (CitationHide et al 1997; CitationMatsuo and Takayama 1998), reduction of granulocyte macrophage colony-stimulating factor (GM-CSF) generation, as well as a reduction in the number of a range of inflammatory cytokines including interleukin (IL)-1β, IL-6, IL-4 and IL-8 (CitationYoneda et al 1997; CitationIto et al 1998; CitationBeck et al 2000). These cytokines perpetuate the inflammatory response (CitationSettipane 2001). Finally, in SAR patients, azelastine nasal spray has been shown to lower neutrophil and eosinophil counts and decrease intercellular adhesion molecule-1 (ICAM-1) expression on nasal epithelial cell surfaces in both the early and late phases of the allergic reaction (CitationCiprandi et al 1996). It also decreases free-radical production by human eosinophils and neutrophils (CitationBusse et al 1989; CitationUmeki 1992) and calcium influx induced by platelet-activating factor in vitro (CitationNakamura et al 1988; CitationMorita et al 1993).
The use of a topical treatment has many advantages over a systemic treatment. Firstly, with a nasal spray, medication can be delivered directly to the site of allergic inflammation. Secondly, the higher concentrations of antihistamines that can be achieved in the nasal mucosa by topical as opposed to oral administration should enhance the anti-allergic and potential anti-inflammatory effects of these agents. Thirdly, a dose of 0.28 mg intranasally has a faster onset of action than a dose of 2.2 mg administrated orally (CitationHorak et al 1994). And finally, with topical administration the risk of interaction with concomitant medication is minimized (CitationDavies et al 1996) and the potential of systemic effects reduced.
Dosage
Recent results from 2 studies have shown that azelastine nasal spray at a dosage of 1 spray per nostril twice daily is effective and has a better tolerability profile compared to 2 sprays per nostril twice daily in patients (≥12 years; n = 554) with moderate to severe SAR (CitationLumry et al 2007). The total nasal symptom score (TNSS) improved by 14.1% in study 1 and by 22.1% in study 2 with azelastine nasal spray (1 spray per nostril twice daily) compared with 4.5% and 12.0% with placebo in study 1 (p = 0.01) and 2 (p < 0.01) respectively. This compares with a 24%–29% improvement in rhinitis symptoms scores with a 2-spray dosage of azelastine (CitationRatner et al 1994; CitationStorms et al 1994; CitationLaForce et al 1996). For individual symptoms, itchy nose, runny nose, sneezing, and nasal congestion were all significantly improved after the 1-spray azelastine regimen compared with placebo. One spray per nostril twice daily of azelastine was also associated with significant improvements in the Rhinitis Quality of Life Questionnaire (RQLQ) daily activity and nasal symptoms domains and patient global evaluations compared with placebo. In addition, the incidence of a bitter taste after azelastine application more than halved and the incidence of somnolence decreased almost 30 times in the 1-spray group versus the labeled incidence with the 2-spray regimen (CitationLumry et al 2007). Although an earlier study showed an improvement in rhinitis symptoms versus placebo with azelastine 1 spray per nostril twice daily, this improvement failed to reach statistical significance. However, a global evaluation noted a significant clinical improvement versus placebo (49%) in the 1-spray regimen (75%, p < 0.001) as well as a 2-spray once daily (89%, p = 0.028) and a 2-spray twice daily regimen (83%, p < 0.001) (CitationWeiler et al 1994).
From these results one can conclude that a greater degree of effectiveness would be expected with two sprays per nostril twice daily. Although one spray per nostril twice daily may provide somewhat less efficacy this is compensated for by an improved tolerability profile compared with the 2-spray regimen. Therefore, the choice of dosage of azelastine nasal spray should be based on the severity and persistence of symptoms as well as the patient’s acceptance of the nasal spray (CitationBernstein 2007). For example, the 2-spray dose could be used as the starting dose for patients with severe symptoms of SAR, and either maintained or tapered to the 1-spray dose as required. The 1-spray dose could be used as a starting dose in patients with mild-to-moderate symptoms, and if necessary the dose increased to 2 sprays per nostril twice daily if symptom control proved to be inadequate (CitationLumry et al 2007).
As-needed
Because azelastine starts working within 15 minutes of application investigators wondered how effective an as-needed regimen would be in controlling the symptoms of rhinitis (CitationCiprandi et al 1997). A randomized controlled study was carried out in 30 patients sensitized to Parietaria pollen or grass. Patients were treated with the standard European dose of azelastine (0.56 mg/day), half this dose (0.22 mg/day), or as-needed. Both groups who received the standard and half-standard doses showed an improvement in their rhinitis symptoms, with a concomitant reduction in markers of inflammation, namely neutrophil and eosinophil counts as well as ICAM-1 expression in nasal scrapings. However, patients who used azelastine nasal spray on an as-needed basis also showed an improvement in their rhinitis symptoms, but without a reduction in the markers of inflammation. The results of this small study suggest that although regular treatment with azelastine is superior at controlling symptoms, as-needed therapy may be useful in the treatment of clinical symptoms (CitationCiprandi et al 1997). The use of azelastine nasal spray on an as-needed basis would be expected to improve drug tolerability and has important implications for patient compliance. Another option is to use azelastine as-needed in addition to an oral antihistamine treatment on days with severe symptoms of SAR.
Comparisons with other agents used to treat rhinitis
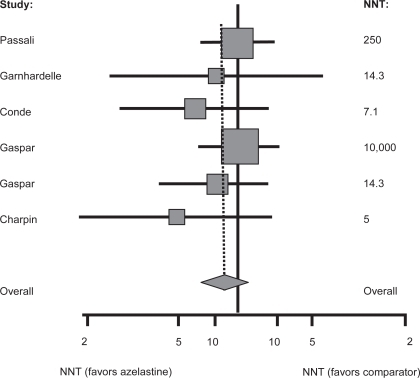
The complexity of rhinitis as a disease and the multiple pathways involved in its pathophysiology mean that there are several classes of drugs available to treat it. These include intranasal corticosteroids, oral antihistamines, intranasal antihistamines and mast-cell stabilizers (eg, cromolyn compounds). A useful metric to compare each of these treatment modalities is the number needed to treat (NNT), which estimates the number of patients who must be treated with a particular drug in order to have one positive outcome. Obviously, drugs with low a NNT are considered more effective than those with a higher NNT. One report estimated the NNT range for oral antihistamines as 9–35, 3–6 for intranasal corticosteroids, 5–6.3 for azelastine, and 4.6 for immunotherapy (CitationPortnoy et al 2004). However, in that study the NNT was calculated using only a single trial for each drug, and so not all of the evidence was considered. A more recent meta-analysis systematically reviewed the efficacy of azelastine nasal spray, in terms of global assessment of efficacy, versus active comparators using NNTs as the outcome measure (CitationLee and Pickard 2007). The active comparators included beclomethasone (Beconase®; GSK, USA) and budesonide nasal sprays (Rhinocort®; Astra Zeneca, USA), loratadine, terfenadine (Seldane®; SanofiAventis, USA), cetirizine, ebastine (Kestine®), and levocabastine. Forty-six studies were initially identified and 21 separate publications were included in the analysis. In 5 comparisons azelastine was more efficacious than placebo with a summary NNT of 5.0. No statistically significant difference was found between azelastine nasal spray and the other treatments, including intranasal corticosteroids, in terms of their efficacy in treating rhinitis. However, when the analysis was limited to studies in which an oral allergy treatment was the comparator, the point estimate of the pooled results favored azelastine nasal spray (). The results were consistent across both SAR and nonallergic VMR, and across trials of different durations. The risk difference would have been even more favourable for azelastine if only results for azelastine at a dose of 1.12 mg/day were included in the analysis, but the small number of studies available for the meta-analysis precluded that stratification (CitationLee and Pickard 2007).
Figure 1 Number needed to treat a global assessment of efficacy as an outcome for azelastine nasal spray compared with oral agents for the treatment of allergic rhinitis. Reprinted with permission from CitationLee T, Pickard S. 2007. Meta-analysis of azelastine nasal spray for the treatment of allergic rhinitis. Pharmcotherapy, 27:852–9. Copyright © 2007 Pharmcotherapy Publications.
Comparisons with intranasal corticosteroids
Azelastine nasal spray is a non-steroidal treatment and has some advantages over intranasal corticosteroids in the treatment of SAR, even though its anti-inflammatory effect is not as strong (CitationWang et al 1997). It has a faster onset of action compared with intranasal corticosteroids (CitationBerkowitz et al 1999; CitationHorak et al 2006), with at least comparable (in the case of intranasal fluticasone propionate) or superior (in the case of intranasal mometasone furoate) efficacy, and has a better safety profile (CitationBehncke et al 2006; CitationPatel et al 2007). Like intranasal corticosteroids, azelastine is effective in treating the symptom of nasal congestion. Whereas intranasal corticosteroid therapy should begin before the onset of symptoms in order to obtain optimal benefit from therapy antihistamines can also be taken on an as needed basis. But in contrast to intranasal corticosteroids azelastine may induce a bitter taste and nasal burning after application.
Azelastine versus mometasone furoate
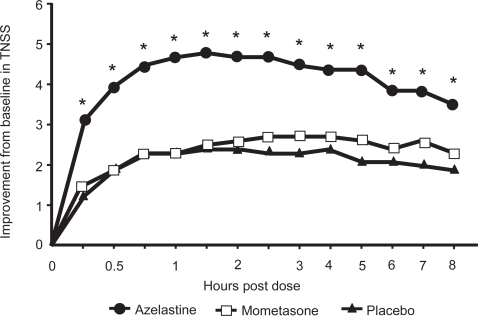
Azelastine nasal spray is superior to the topical corticosteroid mometasone nasal spray in reducing nasal symptoms (CitationPatel et al 2007). Patients with a history of SAR and symptomatic while exposed to ragweed pollen in an environmental exposure chamber (EEC) were randomized to 2 sprays per nostril of azelastine nasal spray (137 μg/spray; n = 150), mometasone nasal spray (50 μg/spray; n = 150), or placebo. At 15 minutes after administration of study drugs, azelastine nasal spray significantly reduced the TNSS from baseline by 29.5% compared with 12.3% with placebo (p < 0.001) and this significant superiority of azelastine over placebo persisted at each time point throughout the 8-hour allergen exposure (). At 8-hour post-allergen challenge, azelastine had reduced the TNSS by 33.9% from baseline versus 18.6% with placebo. Conversely, mometasone furoate nasal spray did not significantly reduce the TNSS from baseline compared with placebo at any time point (p ≥ 0.09), and azelastine nasal spray was significantly more effective than mometasone at each time point during the 8-hour study period (p ≤ 0.001; ) (CitationPatel et al 2007). A previously published study has shown a 12- to 72-hour onset of action for mometasone (CitationBerkowitz et al 1999) which would explain why mometasone did not significantly improve SAR symptoms compared to placebo within 8 hours after allergen exposure. An online survey by the American College of Allergy, Asthma and Immunology showed that 77% of allergists and 69% of primary care physicians thought rapid onset of action was an essential element of therapy (CitationPhysician Survey 2001). A rapid onset of action within 15 minutes, as shown with azelastine nasal spray, may enhance compliance with therapy.
Figure 2 Onset of action of azelastine hydrochloride nasal spray in relieving nasal symptoms of seasonal allergic rhinitis. *p < 0.001 azelastine vs placebo; *p ≤ 0.001 azelastine vs mometasone; mometasone vs placebo = not significant. Reprinted with permission from Patel P, D’Andrea C, Sacks HJ. 2007. Onset of action of azelastine nasal spray compared with mometasone nasal spray and placebo in subjects with seasonal allergic rhinitis evaluated in an environmental exposure chamber. Am J Rhinol, 21:499–503.
Copyright © 2007 Oceanside Publications.
Azelastine versus fluticasone propionate
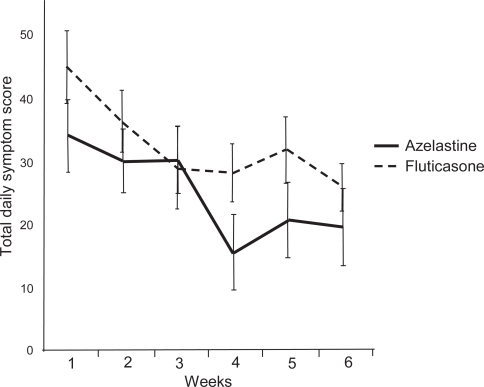
A study in geriatric patients with allergic or non-allergic rhinitis showed that azelastine nasal spray (2 sprays per nostril twice daily; 1.1 mg) was just as effective as fluticasone propionate nasal spray 2 sprays per nostril daily; 200 μg) at improving patients’ RQLQ scores () and rhinitis symptoms () (CitationBehncke et al 2006). Azelastine nasal spray and oral antihistamines are often used concurrently with an intranasal corticosteroid spray in patients with difficult to treat rhinitis symptoms. Several studies with oral antihistamines in combination with intra-nasal corticosteroids showed no increased clinical benefit with these drugs in combination (CitationWeiner et al 1998; CitationNielsen and Dahl 2003). However, a recent proof-of-concept study showed that azelastine nasal spray and fluticasone nasal spray in combination provided a substantial therapeutic benefit for patients with SAR compared with therapy with either agent alone (CitationRatner and Sacks 2007). Patients were randomized to receive either azelastine nasal spray (2 sprays per nostril twice daily), fluticasone nasal spray (2 sprays per nostril twice daily), or both agents together (same dosage). All three groups had statistically significant (p < 0.01) improvement from baseline in TNSS after 2 weeks’ treatment, but the improvement was significant (p < 0.05) with the combination regimen (38%) versus either agent alone (azelastine: 25%; fluticasone: 27%) (CitationRatner and Sacks 2007).
Figure 3 Effect of azelastine nasal spray or fluticasone propionate nasal spray on Rhinitis Quality of Life Questionnaire (RQLQ) scores in geriatric patients with either allergic or non-allergic rhinitis. Reprinted with permission from CitationBehncke VB, Alemar GO, Kaufman DA, et al 2006. Azelastine nasal spray and fluticasone nasal spray in the treatment of geriatric patients with rhinitis. J Allergy Clin Immunol, 117:263. Copyright © 2006 Elsevier.
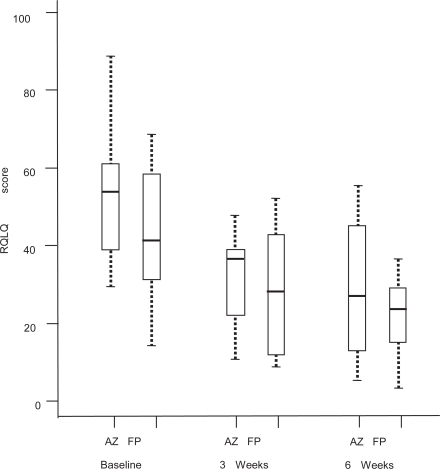
Figure 4 Effect of azelastine nasal spray or fluticasone propionate nasal spray on Total Daily Symptom Score (TDSS) in geriatric patients with either allergic or non-allergic rhinitis. Reprinted with permission from CitationBehncke VB, Alemar GO, Kaufman DA, et al 2006. Azelastine nasal spray and fluticasone nasal spray in the treatment of geriatric patients with rhinitis. J Allergy Clin Immunol, 117:263. Copyright © 2006 Elsevier.
Comparisons with oral antihistamines
Well-controlled studies in patients with rhinitis have shown that azelastine nasal spray demonstrates superior efficacy and a more rapid onset of action compared to oral antihistamines (CitationMcNeely and Wiseman 1998; CitationCorren et al 2005; CitationBerger et al 2006; CitationHorak et al 2006; CitationMeltzer and Sacks 2006; CitationSher and Sacks 2006). Azelastine is a potent drug and has been shown to be effective in patients suffering from rhinitis who have not responded to previous oral antihistamine therapy (CitationBerger and White 2003; CitationLaForce et al 2004). Additionally, it significantly reduces nasal congestion (CitationHerman et al 1997), a particularly bothersome symptom for rhinitis sufferers. Unlike some of the earlier antihistamines, topical application of azelastine produces very low plasma concentrations of the drug which reduces the sedative potential. Indeed, compared with cetirizine and loratadine, azelastine actually increased alertness in patients with seasonal or perennial rhinitis (CitationSpaeth et al 1996).
Azelastine versus desloratadine
Desloratadine is a new anti-histamine tablet. In contrast to antihistamines of earlier generations, these drugs are thought to noticeably reduce nasal congestion (CitationMcClellan and Jarvis 2001; CitationHorak et al 2002b; CitationHorak et al 2003), are non-sedating and do not cause cardiac side-effects. A recently published study was the first to assess the efficacy and onset of action of azelastine nasal spray (one spray per nostril) compared to desloratadine tablets (5 mg) in patients with SAR (CitationHorak et al 2006). Results showed that azelastine nasal spray was significantly better than desloratadine tablets in reducing the symptoms of SAR including ‘nasal congestion’ induced by allergen challenge in the Vienna Challenge Chamber (VCC). Both azelastine nasal spray and desloratadine tablets significantly (p < 0.001) reduced the Major Nasal Symptom Score (MNSS; defined as the sum of scores of sneezing, rhinorrhea and nasal itching) compared to placebo () (CitationHorak et al 2006), with azelastine significantly (p = 0.005) superior to desloratadine in this regard (). In addition, the onset of action of azelastine nasal spray was 15 min. compared with 150 min. for desloratadine tablets. Regarding desloratadine tablets, the onset of action of 150 min reported by CitationHorak et al (2006) was notably longer than that previously described (CitationHorak et al 2002a). This may have been due to the encapsulation of desloratadine tablets for the purpose of blinding.
Figure 5 Major nasal symptom scores averaged over treatment and time for the per protocol population after administration of azelastine (1 spray per nostril), desloratadine (5 mg), or placebo in patients with SAR. Reprinted with permission from CitationHorak F, Zieglmayer UP, Zidglmayer R, et al 2006. Azelastine nasal spray and desloratadine tablets in pollen-induced seasonal allergic rhinitis: a pharmacodynamic study of onset of action and efficacy. Curr Med Res Opion, 22:151–7. Copyright © 2006 LibraPharm.
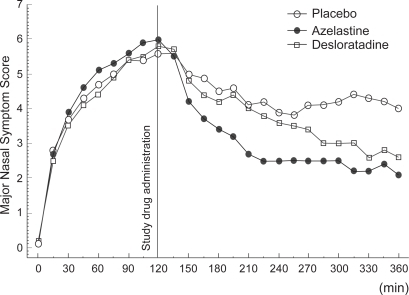
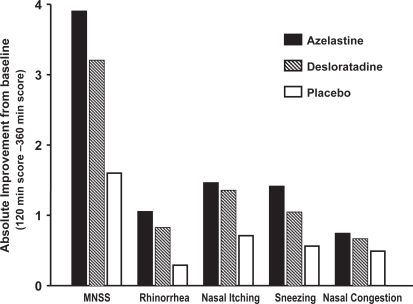
The mean 4- to 6-hour change in TNSS, which comprised a score for nasal congestion, were consistent with those for MNSS. The largest improvement with azelastine was observed for nasal itching followed by sneezing, rhinorrhea and nasal congestion () (CitationHorak et al 2006). In fact, azelastine nasal spray was superior to desloratadine tablets at alleviating nasal congestion when comparing absolute scores at the end of the challenge. This result was unexpected since to date, antihistamines have been found to have little decongestant activity, whereas reduction of nasal congestion is one of the main clinical advantages of third-generation anti-histamines (CitationHorak and Stübner 2002; CitationMurdoch et al 2003). Significant decongestant activity has previously been reported for azelastine nasal spray, but only at the higher dosage of 2 sprays per nostril (CitationThomas et al 1992). Therefore, these results suggest that azelastine at a dosage of 1 spray per nostril is just as effective as 2 sprays. However, one should be reminded that the improvement in nasal congestion following azelastine therapy is a subjective one, and further objective studies, measuring nasal flow or nasal resistance, are required to confirm these findings.
Figure 6 Major nasal symptom and mean nasal symptom scores after administration of azelastine nasal spray (1 spray per nostril), desloratadine (5 mg) or placebo in patients with SAR: absolute changes of last value (6 hours after the start of challenge) compared to predose (ie, 2 hours after the start of the challenge). Reprinted with permission from CitationHorak F, Zieglmayer UP, Zidglmayer R, et al 2006. Azelastine nasal spray and desloratadine tablets in pollen-induced seasonal allergic rhinitis: a pharmacodynamic study of onset of action and efficacy. Curr Med Res Opion, 22:151–7. Copyright © 2006 LibraPharm.
Azelastine versus cetirizine
Cetirizine hydrochloride is an oral second-generation antihistamine indicated for the treatment of SAR and PR. It also has demonstrated inhibitory effects on other mediators of inflammation including leukotrienes (CitationCheria-Sammari et al 1995), prostaglandins (CitationCharlesworth et al 1989), ICAM-1 expression, and eosinophil chemotaxis (CitationCiprandi et al 1995). The first Azelastine Cetirizine Trial (ACT 1) carried out in the autumn of 2004, examined the effectiveness and tolerability of azelastine (2 sprays per nostril) and cetirizine tablets (10 mg once daily) in 307 patients with moderate to severe SAR (CitationCorren et al 2005). During the 2-week double-blind treatment period, azelastine nasal spray significantly (p = 0.02) improved the overall TNSS compared with cetirizine. All four symptom components of the TNSS were improved after azelastine therapy, with a significantly greater improvement versus cetirizine for rhinorrhea (p = 0.003). Differences in the TNSS between azelastine nasal spray and cetirizine were sustained throughout the study period and became more evident as the study progressed, with statistically significant differences favoring azelastine nasal spray on study days 8 through 14. In addition, compared with cetirizine, azelastine nasal spray significantly (p = 0.049) improved patients’ HRQoL as assessed by the RQLQ (CitationCorren et al 2005).
More recently, two 2-week, double-blind, multi-center studies were conducted which compared the efficacy and safety of azelastine nasal spray (2 sprays/nostril twice daily) with oral cetirizine (10 mg daily) in the treatment of patients with moderate to severe SAR (CitationSher and Sacks 2006). A combined analysis of results showed that azelastine nasal spray significantly improved the TNSS (p < 0.001) and each of the four individual symptoms of the TNSS (p < 0.01) compared with cetirizine. Patients in the azelastine spray group experienced an improvement in TNSS of 32.5% compared with 24.6% for those patients taking oral cetirizine. The most common side effect reported by patients in the azelastine group was bitter taste (5.7%). Somnolence was reported by 1.5% of patients taking cetirizine (CitationSher and Sacks 2006).
In addition to nasal symptoms, patients with SAR can experience impairment in HRQoL. Two 2-week, double-blind, multicenter studies were conducted during autumn 2004 and spring 2005 comparing the improvement with azelastine nasal spray (2 sprays per nostril twice daily) versus cetirizine (10 mg daily) on symptoms and HRQoL in SAR patients (CitationMeltzer and Sacks 2006). Results from these studies revealed that azelastine nasal spray improved the overall RQLQ score to a significantly (p < 0.05) greater degree than cetirizine tablets. When results from both studies were pooled, the combined analysis confirmed the significant superiority of azelastine spray both in terms of the overall RQLQ score (p < 0.001) as well as each RQLQ domain (p < 0.03) including the nasal symptoms domain (p < 0.001). More patients in the azelastine nasal spray group experienced a clinically important improvement from baseline in HRQoL (ie, ≥2 units on the 0–6 rating scale) compared with patients in the cetirizine group (35% vs 20% respectively) (CitationMeltzer and Sacks 2006).
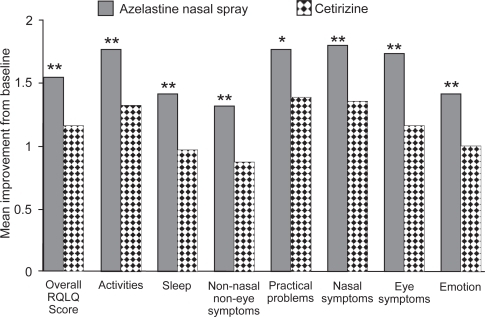
CitationBerger et al (2006) also showed that azelastine nasal spray (2 sprays per nostril) and oral cetirizine (10 mg once daily) effectively treated nasal symptoms in patients with SAR (n = 360). Rapid relief of rhinitis symptoms was evident in both groups at the first evaluation after initial administration and continued during the 14 study days, with the azelastine patients showing the greatest degree of improvement during the second week of treatment. Improvements in the TNSS and individual symptoms favored azelastine over cetirizine (), with significant differences for nasal congestion (p = 0.049) and sneezing (p = 0.01). Azelastine nasal spray improved TNSS by a mean of 4.6 (23.9%) compared with 3.9 (19.6%) with cetirizine. The positive effect of azelastine nasal spray on congestion was observed despite the fact that the cetirizine group had the added benefit of daily use of a placebo saline spray. Azelastine nasal spray also significantly improved the RQLQ overall (p = 0.002) and individual domain (p ≤ 0.05) scores compared with cetirizine (CitationBerger et al 2006). Although oral cetirizine significantly improved RQLQ scores, patients treated with azelastine nasal spray reported additional statistically significant improvement beyond that reported with cetirizine for each individual RQLQ domain including activities, sleep, non-nose/non-eye symptoms, practical problems, nasal symptoms, eye symptoms, and emotions (). Although it is often assumed that patients prefer oral medications to sprays in both the ACT I and ACTII trials, patients reported superior improvements in QoL variables with azelastine nasal spray compared with oral cetirizine (CitationCorren et al 2005).
Figure 7 Mean daily improvements from baseline to day 14 in combined morning and evening 12-hour reflective total nasal symptom scores (TNSSs). *p < 0.05 versus cetirizine. Reprinted with permission from CitationBerger W, Hampel F, Bernstein J, et al 2006. Impact of azelastine nasal spray on symptoms and quality of life compared with cetirizine oral tablets in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol, 97:375–81. Copyright © 2006 American College of Allergy, Asthma and Immunology.
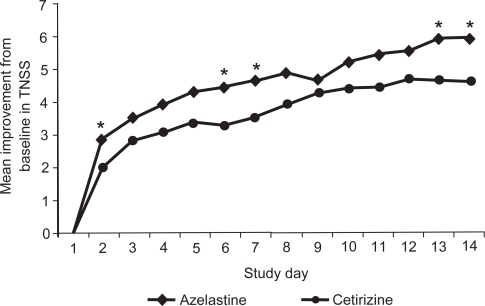
Figure 8 Mean improvement from baseline to day 14 in overall Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ) score and individual RQLQ domain scores (intention-to-treat population). *p ≤ 0.05 vs cetirizine; **p < 0.01 vs cetirizine. Reprinted with permission from CitationBerger W, Hampel F, Bernstein J, et al 2006. Impact of azelastine nasal spray on symptoms and quality of life compared with cetirizine oral tablets in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol, 97:375–81. Copyright © 2006 American College of Allergy, Asthma and Immunology.
Azelastine nasal spray and cetirizine were well tolerated in this study (CitationBerger et al 2006). Relatively high incidences of somnolence and bitter taste have been previously reported in early trials with azelastine nasal spray (CitationMeltzer et al 1994; CitationStorms et al 1994; CitationWeiler et al 1994; CitationLaForce et al 2004; CitationRatner and Sacks 2007). However, subsequent trials in patients with VMR (CitationBanov and Liberman 2001) and post-marketing studies in patients who remained symptomatic after treatment with loratadine (CitationBerger and White 2003) or fexofenadine (CitationLaForce 1999) reported somnolence rates with azelastine nasal spray that were similar to those with placebo.
Non-responders
As many as 20% of all AR patients do not respond to blockers at all (CitationBerger and White 2003). Two oral H1 studies assessed the efficacy of azelastine in patients with moderate-to-severe SAR who had an unsatisfactory response to oral second generation antihistamines (CitationBerger and White 2003; CitationLaForce et al 2004). The first study comprised 435 patients who had a sub-optimal response to loratadine and showed that both azelastine monotherapy and azelastine plus loratadine significantly (p < 0.001) improved total symptoms compared with placebo (CitationBerger and White 2003). The second study comprised 334 patients who had failed to response to 1 week treatment with fexofenadine. Similar results were obtained, in that patients in both the azelastine and combination groups showed significant (p < 0.01) improvement in their symptoms compared with placebo.
Azelastine versus other intranasal antihistamines
Azelastine versus levocabastine
Levocabastine is a potent and selective histamine H1-receptor antagonist. Previous limited data indicated equivalent efficacy of levocabastine to that of oral loratadine, oral cetirizine or azelastine nasal spray (CitationNoble and McTavish 1995). More recently, the efficacy and tolerability of azelastine nasal spray (1.12 mg, 2 sprays twice daily) was shown to be statistically superior to that of topical intranasal levocabastine (0.4 mg, 2 sprays twice daily) in a 4-week, double-blind, parallel-group study in 180 patients (CitationFalser et al 2001). Results showed that azelastine was significantly (p < 0.001) superior at reducing both morning and evening nasal symptoms compared to levocabastine, and was judged to be significantly (p < 0.007) more efficacious in a global evaluation by the investigator. Global efficacy was judged by physicians as either ‘very good’ or ‘good’ for 90% of azelastine patients compared to 74% of the levocabastine group; moreover, 92% of azelastine patients judged the treatment as either ‘good’ or ‘very good’ compared with just 76% of levocabastine patients (CitationFalser et al 2001).
Safety and tolerability
The advantages of intranasal delivery include lower risk of systemic side effects and drug interactions (CitationSalib and Howarth 2003). In controlled studies, azelastine nasal spray was well-tolerated for treatment durations up to 4 weeks in both adults and children (≥12 years) (CitationStorms et al 1994; CitationMeltzer et al 1994; CitationRatner et al 1994; CitationWeiler et al 1994; CitationLaForce et al 1996). Bitter taste, headache, somnolence and nasal burning were the most frequently reported adverse events, but most of these were mild or moderate in nature. These studies reported a greater incidence of somnolence compared with placebo (11.5% vs 5.4%, p < 0.05). However, the incidence of somnolence between azelastine- and placebo-treated patients (3.2% vs 1.0%) did not differ in VMR studies (CitationBanov and Liberman 2001). Post-marketing surveillance studies also reported a similar degree of somnolence (approx 2%) in both azelastine and placebo groups (CitationBerger and White 2003; CitationLaForce et al 2004; CitationCorren et al 2005; CitationBerger et al 2006). The lower incidence of azelastine-related adverse events in later trials is most likely due to correct dosing technique, when the drug is administered without tipping back the head or deeply inhaling the spray, both of which would increase systemic absorption and could result in bitter taste and somnolence. As the incidence of somnolence whilst using azelastine nasal spray has been reported to be greater than placebo in certain studies, US prescribing recommendations warn against concurrent use of alcohol and/or other CNS suppressants. However, to date no studies have been designed to assess specifically the effects of azelastine nasal spray on the CNS in humans.
Abbreviations
| ACT 1 | = | first Azelastine Cetirizine Trial |
| AR | = | allergic rhinitis |
| ARIA | = | allergic rhinitis and its impact on asthma |
| EEC | = | environmental exposure chamber |
| GM-CSF | = | granulocyte macrophage-colony stimulating factor |
| HRQoL | = | health-related quality of life |
| ICAM-1 | = | intercellular adhesion molecule-1 |
| IL | = | interleukin |
| LT | = | leukotriene |
| MNSS | = | major nasal symptom score |
| NNT | = | number needed to treat |
| PR | = | perennial rhinitis |
| QoL | = | quality of life |
| RQLQ | = | Rhinoconjunctivitis Quality of Life Questionnaire |
| SAR | = | seasonal allergic rhinitis |
| TNFα | = | tumor necrosis factor alpha |
| TNSS | = | total nasal symptom score |
| TVRSS | = | Total VMR Symptom Scale |
| VCC | = | Vienna Challenge Chamber |
| VMR | = | vasomotor rhinitis. |
Disclosures
The author has no conflicts of interest to report.
References
- Al SuleimaniYMWalkerMJA2007Allergic rhinitis and its pharmacologyPharmacol Ther1142336017433446
- Anon.2001Physician survey sponsored by the American College of Allergy, Asthma and ImmunologyRochester (NY)Harris Interactive Inc1019–21
- Anon.2006Allergies in America: a landmark survey of nasal allergy sufferersHealthSTAR Communications, Inc Sponsored by Altana Pharma US, Inc. March 1.
- BanovCHLibermanP2001Efficacy of azelastine nasal spray in the treatment of vasomotor (perennial nonallergic) rhinitisAnn Allergy Asthma Immunol86283511206234
- BaumgartenCRPetzoldUDokicD1994Modification of allergen-induced symptoms and mediator release by intranasal azelastineJ Pharmacol Ther34351
- BeckGMansurAAfzalM2000Effect of azelastine nasal spray on mediators of inflammation in patients with seasonal allergic rhinitis (SAR)American Academy of Allergy, Asthma and Immunology 56th Annual MeetingSan Diego (CA)33–8
- BehnckeVBAlemarGOKaufmanDA2006Azelastine nasal spray and fluticasone nasal spray in the treatment of geriatric patients with rhinitisJ Allergy Clin Immunol11726316461125
- BergerWHampelFBernsteinJ2006Impact of azelastine nasal spray on symptoms and quality of life compared with cetirizine oral tablets in patients with seasonal allergic rhinitisAnn Allergy Asthma Immunol973758117042145
- BergerWEWhiteMV2003Efficacy of azelastine nasal spray in patients with an unsatisfactory response to loratadineAnn Allergy Asthma Immunol912051112952117
- BerkowitzTBBernsteinDILaForceC1999Onset of action of mometasone furoate nasal spray (NASONEX) in seasonal allergic rhinitisAllergy5464910195359
- BernsteinJA2007Azelastine hydrochloride: a review of pharmacology, pharmacokinetics, clinical efficacy and tolerabilityCurr Med Res Opin2324415217723160
- BieloryLAmbrosioP2002Conjunctivitis and allergic eye diseasesKalinerMACurrent Reviews of RhinitisPhiladelphiaCurrent Medicine, Inc11522
- BousquetJvan CauwenbergePBKhaltaevN2001Allergic rhinitis and its impact on asthma: ARIA workshop reportJ Allergy Clin Immunol108S147S33411707753
- BusseWRandleyBSedgwickJ1989The effect of azelastine on neutrophil and eosinophil generation of superoxideJ Allergy Clin Immunol8340052563742
- CasaleTB1989The interaction of azelastine with human lung histamine H1, beta, and muscarinic receptor binding sitesJ Allergy Clin Immunol8377162540229
- CharlesworthENKagey-SobotkaANormanPS1989Effect of cetirizine on mast cell-mediator release and cellular traffic during the cutaneous late-phase reactionJ Allergy Clin Immunol83905122469708
- Cheria-SammariSAlouiRGormandB1995Leukotriene B4 production by blood neutrophils in allergic rhinitis: effects of cetirizineClin Exp Allergy25729367584684
- CiprandiGBuscagliaSPesceG1995Cetirizine reduces inflammatory cell recruitment and ICAM-1 (or CD54) expression on conjunctival epithelium in both early-and late-phase reactions after allergen-specific challengeJ Allergy Clin Immunol95612217531732
- CiprandiGPronzatoCPassalacquaG1996Topical azelastine reduces eosinophil activation and intercellular adhesion molecule-I expression on nasal epithelial cells: an anti-allergic activityJ Allergy Clin Immunol981088968977510
- CiprandiGRiccaVPassalaquaG1997Seasonal rhinitis and azelastine: long- or short-term treatment?J Allergy Clin Immunol9930179058684
- CorrenJStormsWBernsteinJ2005Effectiveness of azelastine nasal spray compared with oral cetirizine in patients with seasonal allergic rhinitisClin Ther275435315978303
- DaviesRJBagnallACMcCabeRN1996Antihistamines: topical vs oral administrationClin Exp Allergy26S11S17
- DevyaniLCoreyJP2004Vasomotor rhinitis updateCurr Opin Otolaryngol Head Neck Surg1224324715167037
- DykewiczMSFinemanSSkonerDP1998Diagnosis and management of rhinitis: complete guidelines of the Joint ask Force on Practice Parameters in Allergy, Asthma and ImmunologyAnn Allergy Asthma Immunol814785189860027
- EconomidesAKalinerMA2002Allergic rhinitisKalinerMACurrent Reviews of RhinitisPhiladelphiaCurrent Medicine, Inc3551
- FalserNWoberWRahlfsVW2001Comparative efficacy and safety of azelastine and levocabastine nasal sprays in patients with seasonal allergic rhinitisArzeimittelforschung5138793
- GoldenMPGleasonMMTogiasA2006Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitisClin Exp Allergy3668970316776669
- GoldenSTeetsSJLehmanEB2000Effect of topical nasal azelastine on the symptoms of rhinitis, sleep and daytime somnolence in perennial allergic rhinitisAnn Allergy Asthma Immunol8553710923605
- GreiffLAnderssonMSvenssonC1997Topical azelastine has a 12-hour duration of action as assessed by histamine challenge-induced exudation of alpha 2-macroglobulin into human nasal airwaysClin Exp Allergy27438449146938
- HamasakiYShafigehMYamamotoS1996Inhibition of leukotriene synthesis by azelastineAnn Allergy Asthma Immunol76469758630722
- HermanDGarayRLegalM1997A randomized double-blind placebo controlled study of azelastine nasal spray in children with perennial rhinitisInt J Pediatr Otorhinolaryngol39189051434
- HideIToriuNNuibeT1997Suppression of TNF-α secretion by azelastine in a rat mast (RBL-2H3) cell lineJ Immunol1592932409300717
- HorakFJagerSTothJ1994Azelastine in pollen-induced allergic rhinitis – a pharmacodynamic study of onset of action and efficacyDrug Invest73440
- HorakFStübnerPZieglmayerR2003Comparison of the effect of Desloratadine 5mg daily and placebo on nasal airflow and seasonal allergic rhinitis symptoms induced by grass pollen exposureAllergy58481512757447
- HorakFStübnerP2002Decongestant activity of desloratadine in controlled-allergen-exposure trialsClin Drug Invest22Suppl 21320
- HorakFStübnerUPZieglmayerR2002aEffect of desloratadine versus placebo on nasal airflow and subjective measures of nasal obstruction in patients with grass pollen-induced allergic rhinitis in an allergen-exposure unitJ Allergy Clin Immunol1099566112063524
- HorakFStübnerUPZieglmayerR2002bEffect of desloratadine versus placebo on nasal airflow and subjective measures of nasal obstruction in subjects with grass pollen-induced allergic rhinitis in an allergen-exposure unitJ Allergy Clin. Immunol1099566112063524
- HorakFZieglmayerUPZieglmayerR2006Azelastine nasal spray and Desloratadine tablets in pollen-induced seasonal allergic rhinitis: a pharmacodynamic study of onset of action and efficacyCurr Med Res Opion221517
- HowarthPH1997Mediators of nasal blockage in allergic rhinitisAllergy52Suppl 401289353555
- ItoHNakamuraYTakagiS1998Effects of azelastine on the level of serum interluekin-4 and soluble CD23 antigen in the treatment of nasal allergyArzneim-Forsch48114379893928
- JuniperEF1997Measuring health-related quality of life in rhinitisJ Allergy Clin Immunol99S742S99042066
- KempurajDHuangMKandereK2002Azelastine is more potent than olopatadine in inhibiting interleukin-6 and tryptase release from human umbilical cord blood derived cultured mast cellsAnn Allergy Asthma Immunol88501612027072
- LaForceCDockhornRJPrennerBM1996Safety and efficacy of azelastine nasal spray (Astelin NS) for seasonal allergic rhinitisAnn Allergy Asthma Immunol7618188595539
- LaForceC1999Use of nasal steroids in managing allergic rhinitisJ Allergy Clin Immunol103S388S9410069899
- LaForceCFCorrenJWheelerWJ2004Efficacy of azelastine nasal spray in seasonal allergic rhinitis patients who remain symptomatic after treatment with fexofenadineAnn Allergy Asthma Immunol93154915328675
- LeeCCorrenJ2007Review of Azelastine nasal spray in the treatment of allergic and non-allergic rhinitisExpert Opin Pharmacother8701917376024
- LeeTPickardS2007Meta-analysis of azelastine nasal spray for the treatment of allergic rhinitisPharmcotherapy278529
- LibermanPKalinerMAWheelerWJ2005Open-label evaluation of azelastine nasal spray in patients with seasonal allergic rhinitis and nonallergic vasomotor rhinitisCurr Med Res Opin21611815899111
- LumryWPrennerBCorrenJ2007Efficacy and safety of azelastine nasal spray at a dose of 1 spray per nostril twice dailyAnn Allergy Asthma Immunol992677217910331
- MahrTAShethK2005Update on allergic rhinitisPediatr Rev26284916061526
- MatsuoSTakayamaS1998Influence of the anti-allergic agent, azelastine, on tumor necrosis factor-alpha (TNF-α) secretion from cultured mouse mast cellsIn Vivo1248149827354
- McClellanKJarvisB2001DesloratadineDrugs617899611398910
- McNeelyWWisemanLR1998Intranasal azelastine – a review of its efficacy in the management of allergic rhinitisDrugs56911149664202
- McTavishDSorkinEM1989Azelastine – A review of its pharmaco-dynamic and pharmacokinetic properties, and therapeutic PotentialDrugs387788002574665
- MeltzerEOSacksH2006Azelastine nasal spray significantly improves health related quality of life compared to cetirizine tablets in patients with seasonal allergic rhinitis (SAR)J Allergy Clin Immunol117S324
- MeltzerEOWeilerJMDockhornRJ1994Azelastine nasal spray in the management of seasonal allergic rhinitisAnn Allergy7235497908778
- MoritaMOhshimaYAkutagawaH1993Inhibitory effects of azelastine hydrochloride on CA2+ influx, actin polymerization and release of eosinophils cationic protein of an eosinophilic leukaemia cell line EoL-1Curr Med Res Opin13163748222744
- MurdochDGoaKLKeamSJ2003Desloratadine – an update of its efficacy in the management of allergic disordersDrugs6320517712962522
- NakamuraTNishizawaYSatoT1988Effect of azelastine on the intracellular Ca2+ mobilization in guinea pig peritoneal macrophagesEur J Pharmacol14835412898372
- NieberKBaumgartenCRathsackR1993Effect of azelastine on Substance P content in bronchoalveolar and nasal lavage fluids of patients with allergic asthmaClin Exp Allergy2369717679943
- NielsenLPDahlR2003Comparison of intranasal corticosteroids and antihistamines in allergic rhinitisAm J Respir Med2556514720022
- NobleSMcTavishD1995Levocabastine. An update of its pharmacology, clinical efficacy and tolerability in the topical treatment of allergic rhinitis and conjunctivitisDrugs501032498612470
- PatelPD’AndreaCSacksHJ2007Onset of action of azelastine nasal spray compared with mometasone nasal spray and placebo in subjects with seasonal allergic rhinitis evaluated in an environmental exposure chamberAm J Rhinol2149950317882923
- Physician Survey.2001Physician Survey sponsored by the American College of Allergy, Asthma and ImmunologyHarris Interactive, Inc.Rochester, NY1019–29
- PortnoyJMVan OsdolTWilliamsPB2004Evidence-based strategies for treatment of allergic rhinitisCurr Allergy Asthma Rep44394615462709
- RatnerPSacksH2007Randomized, double-blind trial of azelastine nasal spray plus fluticasone nasal spray compared to either agent alone in patients with allergy to Texas Mountain CedarAnn Allergy Asthma Immunol98Suppl 1A20
- RatnerPHFindlaySRHampelF1994A double-blind, controlled trial to assess the safety and efficacy of azelastine nasal spray in seasonal allergic rhinitisJ Allergy Clin Immunol94818257963150
- RayNFBaraniukJNThamerM1999Direct expenditures for the treatment of allergic rhinoconjunctivitis in 1996, including the contributions of related airway illnessesJ Allergy Clin Immunol103401710069872
- ReedSDLeeTAMcCroryDC2004The economic burden of allergic rhinitis: a critical evaluation of the literaturePharmacoeconomics223456115099121
- SalibJRHowarthPH2003Safety and tolerability profiles of intranasal antihistamines and intranasal corticosteroids in the treatment of allergic rhinitisDrug Saf268639312959630
- SettipaneRASettipaneGA2002Nonallergic rhinitisKalinerMACurrent Reviews of RhinitisPhiladelphiaCurrent Medicine, Inc5365
- SettipaneRA2001Demographics and epidemiology of allergic and nonal-lergic rhinitisAllergy Asthma Proc22185911552666
- SherESacksH2006Azelastine nasal spray compared to cetirizine in the treatment of patients with seasonal allergic rhinitis: a pooled analysis of two double-blind, multicenter studiesJ Allergy Clin Immunol117S319
- ShinMHBaroodyFProudD1992The effect of azelastine on the early allergic responseClin Exp Allergy22289951349259
- ShinodaMWatanabeNSukoT1997Effects of anti-allergic drugs on substance P (SP) and vasoactive intestinal peptide (VIP) in nasal secretionsAm J Rhinol11237419209598
- SpaethJKlimekLMoesgesR1996Sedation in allergic rhinitis is caused by the condition and not by antihistamine treatmentAllergy518939069020417
- StormsWWPearlmanDSChervinskyP1994Effectiveness of azelastine nasal solution in seasonal allergic rhinitisENT J733829
- ThomasKEOllierSFergusonH1992The effect of intranasal azelastine, Rhinolast®, on nasal airway obstruction and sneezing following provocation testing with histamine and allergenClin Exp Allergy2264271393763
- UmekiS1992Effects of antiallergic drops on human neutrophil superoxide generating NADPH oxidaseBiochem Pharmacol431109171372806
- WangDSmitzJDe WaeleM1997Effect of topical applications of budesonide and azelastine on nasal symptoms, eosinophil count and mediator release in atopic patients after nasal allergen challenge during the pollen seasonInt Arch Allergy Immunol114185929338613
- WeilerJMMeltzerEOBensonPM1994A dose-ranging study of the efficacy and safety of azelastine nasal spray in the treatment of seasonal allergic rhinitis with an acute modelJ Allergy Clin Immunol94972807798545
- WeinerJMAbramsonMJPuyRM1998Intranasal corticosteroids versus oral H1-receptor antagonists in allergic rhinitis: systematic review of randomized controlled trialsBMJ317162499848901
- YonedaKYamamotoTUetaE1997Suppression by azelastine hydrochloride of NF-κB activation involved in generation of cytokines and nitric oxideJpn J Pharmacol73145539074948
- ZechelHJBrockNLenkeD1981Pharmacological and toxicological properties of azelastine, a novel anti-allergic agentArzneim-Forsch311184936170296
