Abstract
This 8-week, multicenter study evaluated the efficacy and safety of candesartan cilexetil (CC, 8–16 mg) in elderly (>65 years) hypertensive patients. Patients (n=3013) received CC 8 mg during 8 weeks which eventually doubled to CC 16 mg at week 4 if blood pressure remained uncontrolled (≥140/90 mmHg). At week 8, 65.5% of patients were normalized (BP <140/90 mmHg). Mean changes at week 8 were −25.8, −13.2, and −12.7 mmHg for systolic, diastolic, and pulse pressure, respectively. Age, sex, and diabetic status did not influence the antihypertensive effect of CC. 68% of the patients treated with, but uncontrolled or intolerant of, prior antihypertensive treatment were normalized by CC 8–16 mg. In summary, CC 8–16 mg once daily was effective and well tolerated in the management of arterial hypertension in elderly subjects.
Introduction
Hypertension is the most prevalent epidemic disease with a major impact on morbidity and mortality in the current world. Its prevalence is increasing in the adult population, and is estimated to be 30% in developed countries (CitationAsmar et al 2001; CitationESH 2003).
With increasing longevity, there is a shift from diastolic to systolic high blood pressure (BP). Diastolic BP (DPB) increases until about the age of 60, whereas systolic BP (SBP) continues to rise with age (CitationVasan et al 2002). Isolated systolic hypertension affects 10%–20% of the elderly and becomes the predominant type of hypertension (nearly 60%) in both treated and untreated elderly subjects (CitationChobanian et al 2003; CitationThijs et al 2004). In older patients with isolated systolic hypertension there is an increased risk of developing cardiovascular disease. Clinical trials have demonstrated that control of isolated systolic hypertension reduces global mortality, cardiovascular mortality, stroke, and heart failure events (CitationChobanian et al 2003). Randomized studies have demonstrated that treating hypertensive older persons is useful in decreasing mortality and morbidity (CitationMulrow et al 1994). There is strong evidence from clinical trials to support the treatment of systolic hypertension in older person with SBP of at least 160 mmHg (CitationChaudhry et al 2004).
Despite this knowledge, there is an important gap between the number of hypertensive patients and the percentage of normalized patients (CitationChaudhry et al 2004). Two principal reasons could explain this gap: on the one hand, there is a poor patient adherence to treatment, and on the other hand, physicians are not aggressive enough in the management of hypertension (CitationBerlowitz et al 1998). Therapeutic approaches include increased doses of antihypertensive agents, the use of combination therapy, or introduction of an alternative class of therapeutic agent.
Initial therapeutic approaches include beta-blockers, diuretics, angiotensin converting enzyme (ACE) inhibitors, calcium channel blockers, angiotensin II receptor antagonists, and low dose combinations (CitationReif et al 1998; CitationHAS 2005).
The development of angiotensin II receptor antagonists represented an important advance in the treatment of hypertension. Candesartan cilexetil (CC) is an angiotensin II type 1 (AT1) receptor antagonist. In controlled clinical trials, candesartan has proven to be effective in lowering BP; its efficacy increases up to a dose of 32 mg po once daily (CitationReif et al 1998; CitationMeredith 2000; CitationNeldam and Forsen 2001). The antihypertensive effect of CC in doses up to 16 mg/day has been confirmed (CitationElmfeldt et al 1997) with acceptable tolerability in numerous patient groups, including women, diabetics, and patients with severe hypertension (CitationOparil et al 1999; CitationTrenkwalder 2000).
Materials and methods
Patients
This study included outpatients, over 65 years old, with a diagnosis of essential hypertension (SBP ≥140 mmHg and/or DBP ≥90 mmHg). Hypertension was untreated, treated with poor tolerability, or treated but not normalized. Patients were enrolled by general practitioners in France.
The exclusion criteria were as follows: age <65 years; orthostatic hypotension; poor tolerance to angiotensin II inhibitors; secondary arterial hypertension; cardiac arrhythmia; congestive cardiac failure; valvular stenosis; ischemic cardiomyopathy or stenosis of a clinically important cerebral artery; surgery or gastrointestinal pathology potentially affecting the absorption or elimination of the treatment study; severe renal or hepatic insufficiency.
Methodology
This 8-week, multicenter study evaluated the efficacy and tolerability of CC in treating elderly hypertensive patients. During the study, the investigator examined the patient at three visits: at inclusion, and after 4 and 8 weeks of treatment. At inclusion, all patients were given CC at a dose of 8 mg once daily. If BP remained uncontrolled (SBP ≥140 and/or DBP ≥90 mmHg) at week 4, CC was increased to 16 mg once a day. If BP was controlled at week 4, patients remained on CC 8 mg for an additional 4 weeks.
The study was conducted according to the Declaration of Helsinki for biomedical research. The protocol was approved by the French Independent Ethics Committee (IE). Written informed consent was obtained from each patient.
Efficacy and safety criteria
The primary efficacy endpoint was the proportion of patients normalized (BP <140/90 mmHg) by CC at the end of week 8. The secondary efficacy criteria were the proportions of patients normalized at week 4, and the mean BP changes from baseline to week 4 and week 8.
Sitting BP was measured according to guidelines (CitationO'Brien et al 2003) from the dominant arm (arm with the higher SBP) 3 times at 2-minute intervals after the patient had been sitting for at least 5 minutes.
Cardiovascular risk was calculated following ESH guidelines (CitationESH 2003). Safety was assessed by monitoring the incidence of adverse events during the treatment period, whether reported as related or unrelated to the use of CC. In addition, orthostatic hypotension was surveyed.
Statistical analysis
Efficacy was determined for an intent-to-treat (ITT) population which included all patients who took at least one dose of treatment and for whom the baseline BP value was available. Per-protocol (PP) population consisted of all patients from the ITT population not presenting major protocol deviations. Qualitative variables were compared using the Chi-square test. Groups were compared by analysis of variance (ANOVA). A p value ≤0.05 was considered statistically significant.
Results
Patient
A total of 3077 patients were included in the study. Among them, 64 patients were excluded from the analysis (no treatment intake and/or missing BP values at baseline). During follow-up, 2884 patients (95.7%) completed the study, and 129 patients (4.3%) withdrew prematurely (adverse events, n=28; protocol deviation, n=27; consent withdrawal, n=13; lost to follow-up, n=13; not determined, n=17; inefficacy, n=4; other reasons, n=27).
ITT population consisted of 3013 patients. At the end of the study (week 8), 58% of patients had received CC 8/8 mg (CC 8 mg the first 4 weeks followed by 8 mg the last 4 weeks), and 42% of patients received CC 8/16 mg (CC 8 mg the first 4 weeks followed by 16 mg the last 4 weeks).
Demographics and baseline characteristics
Baseline clinical characteristics of the patients are presented in . The arterial hypertension was principally systolic (99.8%). The mean ± SD hypertension duration was 4.7±6.5 years, with 43.5% of patients diagnosed during the last year and 16.2% of patients diagnosed more than 10 years ago. Patients with grade I (SBP 140–159 mmHg or DBP 90–99 mmHg), grade II (SBP 160–179 mmHg or DBP 100–109 mmHg), and grade III (SBP ≥180 mmHg or DBP ≥110 mmHg) hypertension at entry was 27.7%, 61.2%, and 8.9%, respectively. Hypertension was previously treated in 59% of patients; previous antihypertensive therapy was diuretics (28.6%), calcium inhibitors (23.6%), ACE inhibitors (18.6%), and beta-blockers (8.5%). 85% of patients switched to CC 8 mg because of poor therapeutic response to previous therapy. 99.4% of patients had at least one cardiovascular risk factor.
Table 1 Baseline characteristics of patients
Antihypertensive effect of candesartan cilexetil
BP normalization
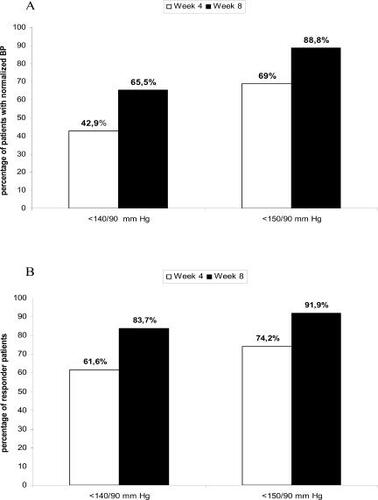
The target of SBP <140 mmHg and DBP <90 mmHg was achieved at week 4 by 1267 patients (42.9%) and at week 8 by 1865 patients (65.5%) (). The dose adjustment at week 4 from CC 8 mg to CC 16 mg increased the proportion of responders for both SBP/DBP <140/90 mmHg by 22.6%. Among the patients not responding to CC 8 mg at week 4, 47.9% of patients responded when treated by CC 16 mg. With reference to a target of SBP/DBP <150/90 mmHg, 69% and 88.8% of patients were normalized at week 4 and week 8 respectively (). Similar results with higher proportions of normalized patients were observed in the PP population analysis: the target of SBP/DBP <140/90 mmHg was achieved by 53.4% and 70.5% at week 4 and week 8, respectively.
Figure 1 Patients with normalized blood pressure (BP) (A) and responders (B) to candesartan cilexetil (CC) (8–16 mg) at week 4 and week 8. Patients were considered as normalized according to two systolic (S) and diastolic (D) BP targets: SBP/DBP <140/90 mmHg, and SBP/DBP <150/90 mmHg. Patients were considered as responders if they achieved SBP <140 mmHg or a reduction of 20 mmHg on the SBP compared with the baseline value and DBP <90 mmHg or a reduction of 10 mmHg on the DBP compared with the baseline value. Two BP targets were evaluated: SBP/DBP <140/90 mmHg, and SBP/DBP <150/90 mmHg. Week 4, n=2951 and week 8, n=2847. At week 8, 1659 patients received CC 8/8 mg and 1187 patients received CC 8/16 mg.
BP responders
At the end of the study, 83.7% patients showed a clinically significant response (SBP <140 mmHg or reduction of 20 mmHg compared with the baseline value and DBP <90 mmHg or reduction of 10 mmHg compared with the baseline value) (). Comparable results were observed in the PP population: the target was achieved by 63.8% and 85.6% at week 4 and week 8, respectively.
BP reduction
BP values showed a significant decrease at week 4 following CC 8 mg treatment. The changes over time in SBP, DBP, and PP values are shown in . The most important change occurred between baseline and week 4 (SBP/DBP: −21/−10 mmHg); BP values continued to decrease up to week 8 (SBP/DBP: −26/−13 mmHg). Patients not normalized at week 4 by CC 8 mg, and in whom the dose of CC was increased to 16 mg, showed a decrease in their SBP/DBP values of −11/−6 mmHg at week 8.
Table 2 Mean blood pressure (BP) values (mean ± SD) at baseline, and after 4 weeks and 8 weeks of candesartan cilexetil (CC)
BP control was inversely related to the degree of hypertension at baseline. Hypertensive patients of grade I, grade II, and grade III were normalized at week 4 in 57.5%, 37.1%, and 27.9% of cases, respectively, and at week 8 in 75.5%, 62.6%, and 47.4% of the cases, respectively.
Previous antihypertensive therapy at baseline did not influence the percentage of normalized patients (68% and 64.1% for treated and untreated, respectively) at the end of the study. Despite different SBP baseline values between previously treated vs untreated patients (161±11 and 164±11 mmHg, p<0.001) no differences were found after treatment (136±9 and 137±9 mmHg, treated vs untreated patients).
Antihypertensive effect of CC according to previous treatment, age, sex, and diabetes
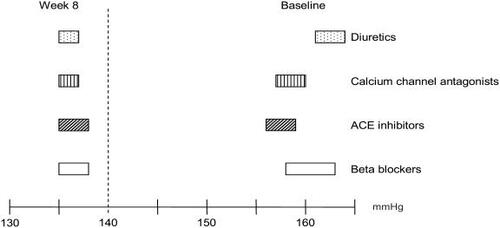
After 8 weeks of treatment with CC, significant decreases of SBP were observed for all patients, a mean of 136 mmHg with 95% confidence interval ranging from 135 to 138 mmHg, irrespective of previous treatment ().
Figure 2 Effect of candesartan cilexetil on systolic blood pressure according to previous antihypertensive treatment. Values are presented as confidence interval 95%.
Variation of SBP and DBP were similar for men and women. Neither age (≥80 or >80 years of age) nor diabetes were related to the antihypertensive effect of CC 8–16 mg ().
Table 3 Influence of age, sex, and diabetes on the systolic and diastolic blood pressure (SBP, DBP) changes (mean ± SD) of patients treated with candesartan cilexetil (CC)
Safety results
A total of 219 adverse events (AEs) were reported by 174 patients (5.8%). 51 AEs experienced by 49 patients (1.6%) were considered. 36 AEs resulted in discontinuation in 28 patients (0.9%); 2 of these were severe AEs (breast cancer and severe arterial hypotension). The most common AEs resulting in study discontinuation were vertigo (n=5), rash (n=4), headache (n=3), and nausea (n=2).
Orthostatic hypotension was reported in 4 patients (0.1%) at week 4 and 3 patients (0.1%) at week 8.
Discussion
Treatment of hypertensive patients may be considered to be the achievement of SBP/DBP <140/90 mmHg (CitationReif et al 1998; CitationHAS 2005). This study demonstrated that CC is an effective treatment for BP control in elderly patients (aged >65 years). Administration of CC 8 mg for 4 weeks and doubling CC dose if the patients did not achieve BP normalization resulted in 65.5% of patients being normalized after 8 weeks of treatment.
At baseline, 59% of patients were already treated by one antihypertensive treatment (28.6% diuretics, 23.6% calcium channel blockers, 18.6% ACE inhibitors, and 8.5% betablockers). The results observed in these elderly hypertensive patients corroborate those observed with similar studies performed in middle-aged populations. A European multicenter study showed that switching to angiotensin II receptor blocker (ARB) treatment (candesartan 8–16 mg once daily) was associated with a significant decrease of BP with a higher percentage of responders (CitationAsmar et al 2004). An analysis of the patient subgroup aged >65 years of age in the Switch study (n=236) showed that BP benefit was observed irrespective of age or to previous treatment (CitationAsmar et al 2004).
In patients aged over 60 years, systolic hypertension is a more important cardiovascular disease risk factor than diastolic hypertension. Consequently the control of SBP should be the focus of treatment in this population (CitationChobanian et al 2003; CitationHAS 2005).
In the present study population, hypertension was principally systolic (99.8% of the patients).
SBP values decreased by an average of 25.8 mmHg. Studies have shown that a small decrease in mean SBP has benefit in terms of cardiovascular morbidity and mortality (CitationTurnbull 2003). Antihypertensive treatment has demonstrated efficacy in primary prevention of cardiac events and stroke in high-risk patients >60 years of age, particularly by lowering SBP (CitationDahlof et al 1991; CitationSHEP 1991; CitationStaessen et al 1997; CitationAndrawes et al 2005). A recent study conducted by the Study on Cognition and Prognosis in the Elderly Group (CitationTrenkwalder et al 2005) indicated a reduction in major cardiovascular events and stroke in elderly people (70–89 years) treated with candesartan.
One objective of treating elderly subjects (more than 80 years old) is to achieve SBP <150 mmHg in the absence of orthostatic hypotension (CitationHAS 2005). CitationThomas et al (2006) highlighted the benefit of ARBs in elderly patients with hypertension. In this study, 88.2% of patients were normalized <150 mmHg after being treated for 8 weeks with candesartan 8–16 mg. Orthostatic hypotension was rarely observed (0.1%).
During the last decade, the role of high pulse pressure as an independent marker of cardiovascular morbidity and mortality has been largely described in both treated and untreated hypertensive patients aged over 50 years. Antihypertensive agents have varying effects. ARBs decrease high pulse pressure in hypertensive patients. These results demonstrated a significant reduction of 13 mmHg for PP, which confirm previous studies in hypertensive patients (CitationVaccarino et al 2001).
Hypertension is often associated other risk factors (sex, age, diabetes). In the present study, the SBP values at baseline demonstrated a significant difference according to age (<80 vs ≥80 years, p=0.004) and with the existence of a previous antihypertensive treatment (treated vs untreated, p<0.001). After 8 weeks of treatment with CC, no subgroup differences were found for the final BP values (sex, age, diabetes). The results are interesting to compare with the ALLHAT study, where predictive factors for antihypertensive treatment inefficacy included female sex and diabetes (CitationCushman et al 2002). In the LIFE study, diabetic patients needed more medication than non diabetics for hypertension (CitationKjeldsen et al 2000). In this study the key factor to predict normalization of both SBP and DBP was the severity of hypertension.
Elderly subjects have an increased susceptibility to adverse reactions from pharmacological treatment. All subjects participating in the present study were more than 65 years old, 470 subjects (16%) were over 80 years old. In this study 1.6% of patients developed an AE related to the treatment and 0.9% discontinued the study. The most common AEs resulting in study discontinuation were vertigo, rash, headache, and nausea. These results suggest that CC is generally tolerated in elderly patients with an acceptable safety profile (CitationTrenkwalder 2000; CitationNeldam and Forsen 2001; CitationSkoog et al 2005). A limitation of the present study is that it was not a controlled study, and it was conducted in general practice.
In conclusion, this large-scale study in elderly hypertensive patients in France, demonstrated that candesartan (8–16 mg once daily) is suitable therapy for effective control of blood pressure and enhanced patient compliance.
Disclosure
Dr Asmar has no conflict of interest. Dr Nisse-Durgeat is an employee of Laboratoire Takeda.
References
- AndrawesWFBussyCBelminJPrevention of cardiovascular events in elderly peopleDrugs Aging2005228597616245959
- AsmarRPorcellatiCDusingRSwitch from ABCD pre-treatment to A-II-A treatment: a multinational open, centrally randomized, prospective parallel group comparisonDrugs Exp Clin Res2004301536115553661
- AsmarRVolSPannierBHigh blood pressure and associated cardiovascular risk factors in FranceJ Hypertens20011917273211593091
- BerlowitzDRAshASHickeyECInadequate management of blood pressure in a hypertensive populationN Engl J Med19983391957639869666
- ChaudhrySIKrumholzHMFoodyJMSystolic hypertension in older personJAMA200429210748015339901
- ChobanianAVBakrisGLBlackHRThe Seventh Report of the Joint National Committee on Preventions, Detection, Evaluation, and Treatment of High Blood PressureJAMA200328925607212748199
- CushmanWCFordCECutlerJASuccess and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT)J Clin Hypertens20024393404
- DahlofBLindholmLHHanssonLMorbidity and mortality in the Swedish Trial and Old Patients with Hypertension (STOP Hypertension)Lancet1991265128151682683
- ElmfeldtDGeorgeMHubnerRCandesartan cilexetil, a new generation angiotensin II antagonist, provides dose dependent antihypertensive effectJ Hum Hypertens199711S49539331007
- [ESH] Guidelines CommitteeEuropean Society of Hypertension – European Society of Cardiology guidelines for the management of arterial hypertensionJ Hypertens20032110115312777938
- [HAS] Haute Authorité de SantéPrise en charge des patients adultes atteints d'hypertension artérielle essentielle. Actualisation des recommendations2005 Private communication
- KjeldsenSEDahlofBDevereuxRBLowering of blood pressure and predictors of response in patients with left ventricular hypertrophy: the LIFE study. Losartan intervention for endpointAm J Hypertens20001389990610950398
- MeredithPAchieving quality 24-h blood pressure control with candesartan cilexetilBlood Press Suppl2000123611059632
- MulrowCDCornellJAHerreraCRHypertension in the elderly. Implications and generalizability of randomized trialsJAMA1994272193287990246
- NeldamSForsenBMulticentre Study Group. Antihypertensive treatment in elderly patients aged 75 years or over: a 24-week study of the tolerability of candesartan cilexetil in relation to hydrochlorothiazideDrugs Aging2001182253211302289
- O'BrienEAsmarRBeilinLEuropean Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurementJ Hypertens2003218214812714851
- OparilSLevineJHZuschkeCAEffects of candesartan cilexetil in patients with severe systemic hypertension. Candesartan Cilexetil Study InvestigatorsAm J Cardiol1999842899310496437
- ReifMWhiteWBFaganTCEffects of candesartan cilexetil in patients with systemic hypertension. Candesartan Cilexetil Study InvestigatorsAm J Cardiol19988296159794352
- SHEP Cooperative Research GroupPrevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP)JAMA19912653255642046107
- SkoogILithellHHanssonLEffect of baseline cognitive function and antihypertensive treatment on cognitive and cardiovascular outcomes: Study on Cognition and Prognosis in the Elderly (SCOPE)Am J Hypertens2005181052916109319
- StaessenJAFagardRThijsLRandomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial InvestigatorsLancet1997350757649297994
- ThijsLHondEDNawrotTPrevalence, pathophysiology and treatment of isolated systolic hypertension in the elderlyExpert Rev Cardiovasc Ther20042761915350177
- ThomasGNChanPTomlinsonBThe role of angiotensin II type 1 receptor antagonists in elderly patients with hypertensionDrugs Aging2006231315516536636
- TrenkwalderPEfficacy and tolerability of candesartan cilexetil in special patient groupsBlood Press Suppl20001273011059633
- TrenkwalderPElmfeldtDHofmanAThe Study on COgnition and Prognosis in the Elderly (SCOPE) - major CV events and stroke in subgroups of patientsBlood Press20051431715823945
- TurnbullFBlood Pressure Lowering Treatment Trialists' Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trialsLancet200336215273514615107
- VaccarinoVBergerAKAbramsonJPulse pressure and risk of cardiovascular events in the systolic hypertension in the elderly programAm J Cardiol200188980611703993
- VasanRSBeiserASeshadriSResidual life time risk for developing hypertension in middle-aged women and men: the Framingham Heart StudyJAMA200228710031011866648
