Abstract
Blockade of the renin–angiotensin system is an important approach in managing high blood pressure, and has increasingly been shown to affect cardiovascular disease processes mediated by angiotensin II throughout the cardiovascular and renal continua. Telmisartan is an angiotensin II receptor blocker (ARB) displaying unique pharmacologic properties, including a longer half life than any other ARB, that result in large and sustained reductions of blood pressure. In patients with mild-to-moderate hypertension, telmisartan has proved superior to other antihypertensive agents (valsartan, losartan, ramipril, perindopril, and atenolol) in controlling blood pressure particularly towards the end of the dosing interval. There is also clinical evidence that telmisartan reduces left ventricular hypertrophy, reduces arterial stiffness and the recurrence of atrial fibrillation, and confers renoprotection. The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET®) study has demonstrated that telmisartan has similar cardiovascular protective effects to ramipril in a large, high-risk patient population but was better tolerated. The powerful and sustained blood pressure control apparent in clinical trials, together with cardiovascular protection and tolerability demonstrated in ONTARGET® means that telmisartan may be a preferred option for patients with hypertension.
Introduction
Angiotensin II, which is generated by the renin–angiotensin system (RAS), plays a pivotal role in hypertension and cardiovascular disease. Thus, pharmacologic regulation of angiotensin II is central to the control of blood pressure and prevention of its pathophysiologic effects on the cardiovascular system, including the kidney and the brain.
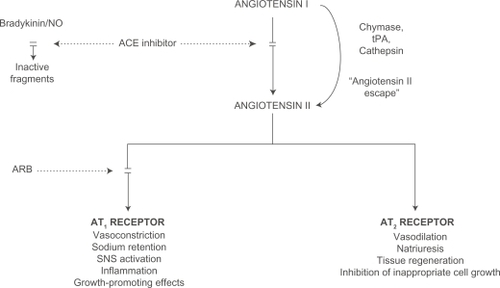
The angiotensin-converting enzyme (ACE) inhibitors target one of the enzymes that generate angiotensin II from angiotensin I (). However, angiotensin II is not produced exclusively by this mechanism; other enzymes, such as chymase, are also able to generate angiotensin II.Citation1 The angiotensin II receptor blockers (ARBs) overcome the detrimental effects of angiotensin II by preventing it binding to the type 1 receptors (AT1). This review examines evidence for the efficacy of telmisartan in the treatment of high blood pressure, and explores the body of the evidence that telmisartan prevents disease mediated by angiotensin II throughout the cardiovascular and renal continua.
Pharmacology of telmisartan
There are currently seven commercially available ARBs, with telmisartan offering unique pharmacologic features compared with the other agents of its class. Telmisartan displays insurmountable, but reversible binding to the AT1 receptor, and it has the highest binding affinity for this receptor among commercially available ARBs.Citation2 As well as providing long-term blockade of the AT1 receptor, telmisartan has minimal affinity for the AT2 receptor (K > 10,000 nM) or for acetylcholine, catecholamine, dopamine, histamine, serotonin, or imipramine receptors.Citation3 Telmisartan is also highly lipophilic, which facilitates oral absorption and benefits tissue and cell penetration, as demonstrated by its large volume of distribution of approximately 500 L,Citation4,Citation5 thereby blocking both systemic and local RAS. Unlike other ARBs, which are excreted to varying extents via the kidneys,Citation6,Citation7 more than 90% of telmisartan is eliminated in the feces.Citation8 An important distinguishing feature of telmisartan is its long terminal elimination half-life of about 24 hours, suggesting a long duration of action.Citation5 It has been shown in healthy volunteers that, at peak plasma concentrations, telmisartan 80 mg reduces the response to exogenous angiotensin II by about 90%, and approximately 40% inhibition persists for 24 hours.Citation9
Telmisartan modulates peroxisome proliferator-activated receptor γ (PPARγ), an established therapeutic target in the treatment of insulin resistance, diabetes, and metabolic syndrome.Citation10 It has effects that are characteristic of PPARγ ligands on metabolism.Citation11–Citation14 In addition, there is a growing body of evidence that PPARγ activation raises adiponectin production and exerts anti-inflammatory, anti-oxidative and anti-proliferative effects on vascular walls, thus decreasing the risks for atherosclerosis.Citation15,Citation16 Although PPARγ activation has been reported for other commercially available ARBs,Citation17–Citation19 the effects on PPARγ activity have been shown to be considerably weaker than achieved with telmisartan and occur at much higher concentrations.Citation19,Citation20 Thus, the unique PPARγ-inducing properties of telmisartan, which are achievable at therapeutic doses, may have the capacity for targeting both diabetes and cardiovascular disease.
The importance of sustained blood pressure control
Hypertension is well recognized as a major risk factor for cardiovascular and renal morbidity and mortality. The importance of blood pressure lowering has been established through epidemiologic and clinical studies, and has led to a broad consensus from guideline bodies on the targets for blood pressure control. Improved control of blood pressure is vital to obtain maximum benefit.
Patients typically prefer to take their medication in the morning. To optimize patient compliance, once-daily dosing is important. However, for a once-daily drug taken in the morning, early morning is the time of trough efficacy and may pose a problem in the management of hypertension. In one study, approximately 60% of patients with apparently controlled hypertension when measured in the office during the day had, in reality, uncontrolled blood pressure (systolic blood pressure [SBP]/diastolic blood pressure [DBP] > 130/85 mmHg) determined by ambulatory blood pressure monitoring (ABPM) in the early morning.Citation21 An antihypertensive agent’s duration of action must be sufficient to control blood throughout the dosing interval and, ideally, if the next dose is delayed or missed.Citation22
A further consideration is that, during the morning, incidences of cardiovascular events increase dramatically and are more frequent than at any other time of the day.Citation23–Citation25 Blood pressure follows a circadian rhythm, being lowest at night and increasing suddenly in the morning upon awakening.Citation26 This early morning blood pressure surge (EMBPS) is caused primarily by orthostatic changes but is also linked to circadian changes in the RAS.Citation27–Citation29
Antihypertensive efficacy of telmisartan
The efficacy of telmisartan in the primary care setting has recently been demonstrated in the MICARDIS® Community Access Trial (MICCAT-2) involving 1619 patients.Citation30 The patients had uncontrolled hypertension, 675 having blood pressure that was not controlled despite prior receipt of conventional therapy. The patients in the trial were treated with telmisartan 40 mg, titrated to 80 mg or a combination of telmisartan 80 mg plus hydrochlorothiazide (HCTZ) 12.5 mg. Office SBP/DBP fell by 22.7/12.6 mmHg in the previously untreated patients and by 16.8/10.3 mmHg in the previously treated patients. After telmisartan treatment, blood pressure was controlled in 79% of the patients.
An accurate reflection of the extent of blood pressure control at different stages of the dosing interval is provided by self-measurement of blood pressure in the home or by 24-hour ABPM using an automated device.Citation31 In MICCAT-2, ABPM showed that telmisartan alone or in combination with HCTZ produced significant reductions in blood pressure as shown in both day-time and night-time mean SBP/DBP. Furthermore, telmisartan reduced SBP/DBP by 17.2/10.1 mmHg in the 4 hours post-awakening in the 95 patients who had an EMBPS of SBP > 30 mmHg.Citation32
A large number of clinical studies have demonstrated the antihypertensive efficacy of telmisartan versus other antihypertensive agents. Key studies, as described below, are summarized in . It should be noted that relative efficacy in fixed-dose studies depends upon the doses employed, which typically related to the doses approved or intended for clinical practice when the study was conducted. Results should be interpreted with caution in cases where the doses employed are less than the current, clinically-available maximal dose.
Table 1 Summary of studies comparing the antihypertensive efficacy of telmisartan
Telmisartan versus other ARBs
In Japanese hypertensive patients, home blood pressure measurement confirmed that telmisartan reduces blood pressure more than other ARBs.Citation33 At the lower doses typically used in Japan, once-daily telmisartan 10 to 40 mg taken in the morning achieved greater blood pressure reductions in the early morning than once-daily valsartan 40 to 80 mg, candesartan 2 to 12 mg, or losartan 25 to 100 mg. Comparison of the morning effect on blood pressure versus the evening effect on blood pressure showed that, in particular, the effect of losartan did not persist for 24 hours.
Ambulatory blood pressure monitoring has shown that telmisartan 80 mg confers significantly greater blood pressure lowering than several other ARBs. When compared with valsartan 160 mg, telmisartan provided sustained anti-hypertensive efficacy and superior control of blood pressure during the early morning period.Citation34,Citation35 Differences between the treatments were also apparent for seated SBP. This measure was significantly reduced by telmisartan compared with valsartan (12.1 vs 8.2 mmHg, respectively; P = 0.0281), while the reduction in DBP was also numerically greater with telmisartan.Citation35 Pooled data from two studies showed that, after active therapy, last 6-hour mean DBP was reduced by 7.6 mmHg with telmisartan compared with 5.8 mmHg with valsartan (P = 0.0044) and last 6-hour mean SBP was reduced by 11.1 mmHg with telmisartan as opposed to 9.1 mmHg with valsartan (P = 0.0066).Citation35 After a dose was deliberately missed, 24-hour mean DBP was reduced by 7.2 mmHg with telmisartan compared with 5.5 mmHg with valsartan (P = 0.0004), and the reduction in 24-hour mean SBP after a missed dose was 10.7 mmHg with telmisartan and 8.7 mmHg with valsartan (P = 0.0024).
Similarly, 3 ABPM studies comparing telmisartan 40 or 80 mg with losartan 50 or 100 mg demonstrated that telmisartan provided greater reductions than losartan in both the 24-hour mean SBP and DBP and in the in last 6 hours of the dosing interval.Citation36–Citation38
There are fewer data comparing the antihypertensive efficacy of telmisartan with ARBs other than valsartan and losartan. A 1-year comparative study in patients with mild hypertension and type 2 diabetes showed that telmisartan produced a superior reduction in blood pressure compared with eprosartan.Citation39 Two small-scale clinical studies have compared the blood pressure lowering effects of telmisartan 40 mg versus olmesartan 20 mg in Japanese patients. In one open-label study of 20 patients with early-stage type 2 diabetes and hypertension, olmesartan was shown to provide greater blood pressure reductions than telmisartan.Citation40 Conversely, in a separate study, telmisartan was shown to be more effective than olmesartan for controlling early morning blood pressure, in addition to improving glucose and cholesterol levels in patients with hypertension, chronic heart failure and metabolic syndrome.Citation41 A PubMed search identified no clinical trials directly comparing the antihypertensive effects of telmisartan versus irbesartan.
Telmisartan versus ACE inhibitors
Other evidence for telmisartan providing effective blood pressure control comes from two 14-week studies of identical design – Prospective, Randomized Investigation of the Safety and efficacy of MICARDIS® versus ramipril using ABPM (PRISMA™) – conducted in 1613 hypertensive patients in Europe and South Africa (PRISMA™ I) and in the USA and Canada (PRISMA™ II). In PRISMA™ I, telmisartan titrated from 40 to 80 mg and given in the morning provided superior blood pressure control than ramipril titrated from 2.5 to 5 to 10 mg.Citation42 Notably, this difference was observed throughout all periods of the 24-hour dosing interval and resulted in significantly greater reduction in SBP/DBP than ramipril during the last 6 hours (P < 0.001).Citation42 Similar results were recorded in PRISMA™ II.Citation43 The pooled analysis of the PRISMA™ I and II trials documented that 24-h mean SBP/DBP reductions were significantly greater with telmisartan than ramipril (−14.1/−9.6 vs −11.1/−7.2, respectively) and superiority of telmisartan over ramipril was also apparent during the last 6 hours (difference: 4.8/3.3 mmHg (P < 0.0001)).Citation44 Furthermore, the findings of a meta-analysis of individual data from 1 million patients in 61 prospective studies suggest that the statistically significant greater reduction in last 6-hour mean SBP in patients treated with telmisartan in the PRISMA™ studies is of clinical relevance in improving long-term prognosis.Citation45
The antihypertensive effect of telmisartan was examined in a double-blind comparison of telmisartan 80 mg and perindopril 4 mg. Both agents produced similar reductions in 24-hour mean SBP/DBP at the end of the 8-week study.Citation46 However, telmisartan provided significantly greater reductions in hourly mean DBP in each of the last 8 hours of the dosing period. Telmisartan 40 mg was also compared with perindopril 4 mg in a 12-week, open-label study, with the dose being doubled in patients who failed to respond (DBP ≥ 90 mmHg) at week 6.Citation47 Reductions in trough SBP/DBP from baseline were significantly greater with telmisartan at both 6 and 12 weeks.
Using both 24-hour ABPM and clinic blood pressure measurements, telmisartan 80 mg was found to be as effective as lisinopril 20 mg in reducing SBP and DBP, with telmisartan provide sustained blood pressure control throughout the 24-hour dosing interval.Citation48 Higher doses of telmisartan (40, 80, and 160 mg) and lisinopril (10, 20, and 40 mg) were compared in another, larger titration-to-response study measuring trough clinic blood pressure and comprising 578 patients who could also receive HCTZ up to a dose of 25 mg.Citation49 Control of DBP was similar in patients receiving either telmisartan or lisinopril.
As well as telmisartan generally producing greater reductions in SBP and DBP that were particularly evident towards the end of the dosing period, telmisartan is better tolerated than ACE inhibitors. Comparative studies have consistently shown that incidences of cough were lower with telmisartan than with perindopril,Citation46,Citation47 lisinopril,Citation49 or ramipril.Citation42,Citation43 The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET®) study, which was an outcome study in a broad cross-section of patients who were at high risk for cardiovascular diseases and who could tolerate ACE inhibitors, the rates of cough and angioedema were significantly lower with telmisartan than with ramipril.Citation50 Moreover, telmisartan was associated with better tolerability and greater treatment adherence. The differences in tolerability and adherence between telmisartan and ramipril may well have implications for patients who need long-term treatment to reduce their cardiovascular risk.
Telmisartan versus beta (β)-blockers
Beta-blockers have been compared with telmisartan in several studies of short or longer duration. In a titration-to-response study of 533 patients (with HCTZ added as needed to achieve blood pressure control; mean baseline seated BP 165.8/101.8 mmHg), full SBP response (≤89 mmHg and/or ≥10% reduction from baseline) was achieved by 84% of telmisartan-treated patients and 78% of atenolol-treated patients (nonsignificant).Citation51 In addition, 80% achieved a ≥10 mmHg reduction in trough SBP with telmisartan 40 to 80 to 120 mg compared with only 68% of patients receiving atenolol 50 to 100 mg (P = 0.003).Citation51 In addition, telmisartan had the advantage of being better tolerated: over the 26-week study, side effects were experienced by 53% of patients receiving telmisartan but by 61% of those treated with the β-blocker. Most notably, there were fewer incidences of fatigue and male impotence. The superiority of telmisartan was also demonstrated in an 8-week open-label comparison of telmisartan 80 mg and atenolol 50 mg in 58 patients.Citation52
Telmisartan was compared with carvedilol in a multicenter study on their effects on left ventricular mass (LVM) in patients with mild-to-moderate hypertension.Citation53 As part of the study, ABPM was performed at baseline and after 44 weeks’ treatment with telmisartan 80 mg or carvedilol 25 mg in 82 patients. The 24-hour mean SBP/DBP reductions were similar in both treatment groups. However, night-time and last 6-hour mean reductions were numerically greater with telmisartan, although statistical significance was not achieved.
Telmisartan versus calcium channel blockers
When telmisartan 40 mg (titrated to 80 mg at 4 weeks and to 120 mg at 8 weeks for patients whose DBP remained >90 mmHg) and amlodipine 5 mg (5 mg at 4 weeks to 10 mg at 8 weeks for patients whose DBP remained >90 mmHg)Citation54 were compared, ABPM demonstrated that both agents produced similar, significant decreases in 24-hour mean SBP/DBP (P < 0.0001). Telmisartan, however, was superior to amlodipine with respect to the reductions in DBP at night and during the early morning hours: reduction in DBP in the last 4 hours of the dosing interval was 3.4 mmHg greater with telmisartan than with amlodipine (P < 0.05). In addition, a 24-hour mean DBP < 85 mmHg were observed in 71% of telmisartan-treated patients but only in 55% of those receiving amlodipine. Telmisartan was also better tolerated: the incidence of adverse events, particularly edema, was lower with telmisartan (5%) than with amlodipine (22%; P = 0.05).
Another 12-month study, primarily designed to evaluate left ventricular hypertrophy (LVH), compared the antihypertensive efficacy of telmisartan 40 mg with that of nifedipine gastrointestinal therapeutic system (GITS) 20 mg.Citation55 Similar and significant reductions from baseline in SBP/DBP were observed in the two treatment arms.
Telmisartan versus HCTZ
Telmisartan has been shown to provide more effective control of high blood pressure than HCTZ. In an 8-week factorial study comparing telmisartan (20, 40, 80, or 160 mg), 3 doses of HCTZ (6.25, 12.5, or 25 mg) and combinations of these doses, telmisartan 40 and 80 mg resulted in greater reductions in SBP and DBP than HCTZ 12.5 mg.Citation56
In a 12-month study to determine the effect of telmisartan and HCTZ on LVH in hypertensive patients, 24-hour ABPM was performed at baseline and after 12 months’ double-blind treatment with telmisartan 80 mg or HCTZ 25 mg.Citation57 At the end of the study, significant reductions from baseline in 24-hour mean SBP/DBP were detected in both treatment groups, but the blood pressure-lowering effect of 24/13 mmHg with telmisartan versus 10/8 mmHg with HCTZ was significantly superior (P < 0.01).
Another study was performed in 1039 patients with isolated systolic hypertension.Citation58 Trough office SBP was reduced by 15.6 mmHg and 17.9 mmHg in the telmisartan 40 and 80 mg arms after 6 weeks, respectively. This lowering was similar to that of 15.7 mmHg recorded with HCTZ 12.5 mg. However, significantly more patients achieved the target reduction in SBP (<140 mmHg or >20 mmHg reduction) with telmisartan 80 mg than with HCTZ 12.5 mg (P = 0.03).
Combination treatment in difficult-to-treat patients and high-risk populations
Blood pressure in some patients is ineffectively controlled with monotherapy, and they require a combination of anti-hypertensive agents to achieve target blood pressure. The combination of telmisartan and HCTZ has been shown to provide greater reductions in blood pressure than either component alone. After a 4-week, placebo run-in period, patients were randomized to receive placebo, telmisartan 20, 40, 80 or 160 mg/day, HCTZ 6.25, 12.5 or 25 mg/day, or one of 12 combinations of the two agents in a trial involving 818 patients with mild-to-moderate hypertension.Citation56 The analysis focused on two combinations: telmisartan 40 mg/HCTZ 12.5 mg and telmisartan 80 mg/HCTZ 12.5 mg. After 8 weeks, telmisartan 80 mg/HCTZ 12.5 mg significantly reduced mean supine trough blood pressures by 23.9/14.9 mmHg compared with placebo, which represented a 8.5/3.4 mm Hg greater decrease than that achieved with telmisartan 80 mg alone and a 17.0/7.7 mmHg greater decrease than HCTZ 12.5 mg alone (both P < 0.01). There was a significant reduction in SBP of 18.8 mmHg with telmisartan 40 mg/HCTZ 12.5 mg compared with placebo, and this decrease was significantly greater than that achieved with either monotherapy.
Data from two studies evaluating the combination of telmisartan and HCTZ showed that it produced significantly greater SBP and DBP reductions in the last 6 hours of the dosing interval compared with losartan/HCTZ.Citation59 Two studies of identical design have also shown that the fixed-dose combination of telmisartan 80 mg/HCTZ 25 mg lowered trough blood pressure to a greater extent than valsartan 160 mg/HCTZ 25 mg in patients with stages 1 and 2 hypertension.Citation60,Citation61 In a comparison of telmisartan 80 mg/HCTZ 12.5 mg with olmesartan 20 mg/HCTZ 12.5 mg, the telmisartan/HCTZ combination gave a greater reduction in 24-h blood pressure, and this difference was also seen in daytime and night-time blood pressure values.Citation62
There have been several studies that have investigated the combination of telmisartan and HCTZ in patients whose blood pressure is not adequately controlled by telmisartan alone. In one such study, patients whose DBP remained above 90 mmHg after 8 weeks of treatment with telmisartan 80 mg were randomized to telmisartan 80 mg or telmisartan 80 mg/HCTZ 12.5 mg for a further 8 weeks.Citation63 Greater reductions in blood pressure were achieved with the combination, such that blood pressure had been normalized (defined as SBP < 140 mmHg and DBP < 90 mmHg) in 41.5% of patients receiving the combination versus 26.1% of patients receiving monotherapy.
Patients who are at a particular risk of cardiovascular disease include those who are obese or have type 2 diabetes. It often proves especially difficult to achieve the rigorous control of blood pressure required in these patients. Superior blood pressure lowering of telmisartan 80 mg plus HCTZ 12.5 mg, compared with valsartan 160 mg/HCTZ 12.5 mg over 24 hours and during the early morning hours was demonstrated in the Study of MICARDIS® on Obese/Overweight Type 2 diabetics with Hypertension (SMOOTH®).Citation64
The elderly, another group in which it can be difficult to achieve satisfactory blood pressure control, were recruited into ATHOS® (A comparison of Telmisartan plus HCTZ with amlodipine plus HCTZ in Older patients with predominantly Systolic hypertension). In 1000 patients (≥60 years) with isolated systolic hypertension, telmisartan 40 to 80 mg plus HCTZ 12.5 mg was compared with amlodipine 5 to 10 mg plus HCTZ 12.5 mg.Citation65 Although there was no significant difference between the two groups in the change from baseline in SBP during the last 6 hours of the dosing interval (which was the primary end point), telmisartan/HCTZ resulted in significantly greater reductions in 24-hours, morning and daytime SBP than amlodipine/HCTZ. The ATHOS study indicates that the combination of telmisartan plus HCTZ provides effective blood pressure control in elderly patients.
A common finding of these studies was that the placebo-like tolerability profile of telmisartan was maintained when it was given in combination with HCTZ. In an analysis of 50 trials involving 16,416 patients, the overall incidence of adverse events was low and similar between telmisartan monotherapy and the telmisartan/HCTZ combination.Citation66
The combination of telmisartan and amlodipine has also been demonstrated to provide more powerful reductions in blood pressure than monotherapy with either telmisartan or amlodipine.Citation67 In a factorial design study, patients with stage 1 or 2 hypertension received placebo, telmisartan (20 to 80 mg), amlodipine (2.5 to 10 mg) or a combination of the two agents. The reductions in the in-clinic DBP and SBP observed with the combinations of most clinical interest (40 or 80 mg plus amlodipine 5 or 10 mg) were all significant. The greatest overall reductions (−26.4/−20.1 mmHg) were achieved with the telmisartan 80 mg/amlodipine 10 mg combination. This was also associated with the greatest response rates and blood pressure control. In the study, the treatments were well tolerated and, notably, the high incidence of edema with amlodipine 10 mg monotherapy (17.8%) was reduced by 37% to 65% when telmisartan was used in combination.Citation68 Therefore, the combination of telmisartan and amlodipine represents a treatment option that delivers large reductions in blood pressure and thereby likely reducing the risk of cardiovascular events.
Cardiovascular protective effects of telmisartan
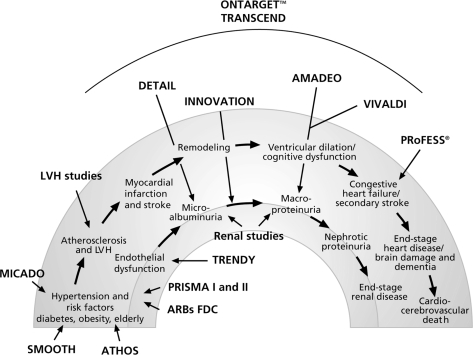
The concept of the cardiovascular and renal continua was introduced to explain the pathologic processes connecting risk factors to clinical events of increasing severity and ultimately resulting in end-organ damage and death (). Hypertension is one such risk factor. There is a large body of evidence, from ex vivo and in vivo studies to demonstrate that modulation of the RAS with ARBs and ACE inhibitors interferes with several of the pathophysiological mechanisms that lead to target organ damage (TOD), which, if uncontrolled, can be life-threatening.
Figure 2 The cardiovascular and renal continua of disease and studies evaluating the efficacy of telmisartan.
The cardioprotective properties of ARBs have yet to be determined for all agents in this class and direct comparisons on the effects of ARBs on target organ protection are sparse. Furthermore, within-class comparisons are made difficult given that cardiovascular outcome studies of ARBs have been conducted in very different patient populations, ranging from low risk patients with hypertension (eg, the Losartan Intervention For Endpoint reduction in hypertension [LIFE]Citation69 and Valsartan Antihypertensive Long-term Use Evaluation [VALUE]Citation70 trials) through to patients with severe underlying cardiovascular disease (eg, the Valsartan Heart Failure Trial [ValHeFT],Citation71 Candesartan in Heart Failure Assessment of Reduction in Mortality and morbidity [CHARM] trial,Citation72 and Valsartan in Acute Myocardial Infarction Trial [VALIANT]).Citation73
Current evidence focusing on telmisartan suggest that pleiotropic effects manifest as improvements in endothelial dysfunction, reductions in LVH, renoprotection in normotensive and hypertensive subjects, improvements in metabolic parameters, and potential benefits in cerebrovascular disease, as discussed below.
Telmisartan and endothelial function
One mechanism by which telmisartan may prevent TOD is by reducing or reversing endothelial dysfunction, which is one of the first signs of vascular damage and is partly driven by oxidative stress.Citation74 Telmisartan reduced superoxide production, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity, and markers of oxidative stress in apolipoprotein E-deficient mice.Citation75 In addition, telmisartan decreased the size of atherosclerotic lesions.Citation75 In spontaneously hypertensive rats, telmisartan expression of NADPH oxidase is reduced and there was increased expression of endothelial nitric oxide synthase, which is likely to contribute to reduced oxidative stress.Citation76
Oxidative stress also promotes the accumulation of advanced glycation end (AGE) products.Citation77 Together with their cell-surface receptor (RAGE), AGEs are a major cause of the microvascular damage that accompanies the hyperglycemia of diabetes. In cultured endothelial cells, telmisartan prevents angiotensin II-induced upregulation of RAGE expression.Citation78 Corroboration for this effect is provided by studies in telmisartan-treated spontaneously hypertensive rats in which the RAGE expression that would normally accompany intra-ocular age expression did not occur.Citation79
Platelet-derived growth factor (PDGF) is a mitogen that is upregulated by oxidative stress and inflammatory stimuli.Citation80 It is known to be produced by smooth muscle cells and is one of the most potent growth factors that is involved in the progression of macroangiopathy as seen in diabetes. Telmisartan has been shown to reduce angiotensin II-induced oxidative stress and thereby suppressed the expression of PDGF-B in cultured bovine retinal pericytes.Citation81,Citation82
Clinical evidence for improvements in endothelial function with telmisartan is provided by the Telmisartan versus Ramipril in renal ENdothelial DYsfunction (TRENDY®) study.Citation83 Both telmisartan 40 mg and ramipril 5 mg improved endothelial function, assessed by measuring renal plasma flow in response to the infusion of NG-monomethyl-l-arginine acetate (l-NMMA), in patients with mild-to-moderate hypertension and normo- or microalbuminuria. Another measure of endothelial function, brachial artery flow-mediated dilation, was improved by 36% by ramipril 2.5 mg, 96% by telmisartan 40 mg, and by 111% with the combination in nonhypertensive patients with well-controlled type 2 diabetes mellitus, but without coronary artery disease, left ventricular dysfunction, or microalbuminuria.Citation84
Telmisartan and arterial stiffness
Arterial stiffness is an important risk factor for cardiovascular mortalityCitation85 and is increased on acute infusion of angiotensin II.Citation86 Prior administration of telmisartan significantly attenuated this acute response, as indicated by changes in systemic vascular resistance and the pulse wave stiffness index.Citation87 Furthermore, in patients with type 2 diabetes and mild-to-moderate hypertension, telmisartan 40 mg for 3 weeks reduced arterial stiffness measured by pulse wave velocity along the carotid–femoral route.Citation88 Another study in patients with hypertension suggests that the improvement in pulse wave velocity is greater than predicted on the basis of blood pressure changes.Citation89
Metabolic effects of telmisartan
Vascular risk factors of hypertension, hyperglycemia, and atherogenic dyslipidemia are prevalent abnormalities in subjects with type 2 diabetes. Diabetes increases cardiovascular risk to the same extent as a prior myocardial infarction (MI) in a nondiabetic subject.Citation90
Studies in hypertensive patients have shown consistently that telmisartan improves insulin sensitivity and lipid profiles. For example, in patients with type 2 diabetes (managed with diet and exercise) and mild hypertension, telmisartan 40 mg was significantly more effective than eprosartan 600 mg in reducing low-density lipoprotein (LDL)-cholesterol, total cholesterol, and triglycerides.Citation39 In another study conducted in patients with type 2 diabetes treated with oral hypoglycemics, telmisartan 40 mg produced significantly greater reductions than nifedipine GITS 20 mg in LDL-cholesterol and total cholesterol.Citation55 The effects of telmisartan on lipid parameters have been also been observed in smaller studyCitation91 and in a post-marketing surveillance study in which people with and without diabetes were treated with telmisartan 40 to 80 mg for at least 1 year.Citation92 In the latter study, triglycerides were reduced by 17.4 mg/dL and cholesterol by 16.4 mg/dL in the population as a whole and were 22.7 mg/dL and 23.8 mg/dL, respectively, in patients with hypercholesterolemia. Among patients with diabetes, the reductions were 22.7 mg/dL and 17.4 mg/dL, respectively.
Telmisartan has been demonstrated to improve markers of glycemic control, such as glycosylated hemoglobinCitation93,Citation94 and insulinCitation91 in patients with type 2 diabetes. Reductions in insulin resistance with telmisartan have also been demonstrated in nondiabetic subjects.Citation95,Citation96 Moreover, telmisartan 80 mg lowered insulin resistance, as measured by the homeostasis model assessment method, to a significantly greater extent than losartan 50 mg in hypertensive patients with metabolic syndrome.Citation97 Free plasma glucose, glycosylated hemoglobin and response to the oral glucose tolerance test were also significantly improved by telmisartan.
Telmisartan in renal impairment
The progression of renal disease can be halted by RAS blockade mediated through reductions in glomerular pressure and through decreased inflammation and oxidative stress. Evidence for the renoprotective effect of telmisartan comes from studies that together have demonstrated positive benefits on renal function in the renal continuum from endothelial dysfunction through to reductions in macroalbuminuria.
In the TRENDY® study, telmisartan not only improved renal endothelial function in patients with type 2 diabetes but also preserved renal function.Citation83 In comparison with ramipril, telmisartan significantly improved resting renal plasma flow, renal vascular resistance, and lowered albuminuria.
The Diabetics Exposed to Telmisartan And enalaprIL (DETAIL®) study showed the long-term benefit of telmisartan in patients with type 2 diabetes and either micro- or macro-albuminuria.Citation98 Glomerular filtration rate (GFR) declined in the first year with both treatments, but this effect has also been observed with ACE inhibitors and other ARBs, and has been attributed to a hemodynamic effect.Citation99,Citation100 Thereafter, the rate of decline was markedly reduced, such that by year 3, the annual decline in GFR had stabilized to approximately 2 mL/min/1.73 m2, which is substantially lower than the 10 to 12 mL/min/1.73 m2 that is typical in untreated diabetics with macroalbuminuria.Citation101
Telmisartan has also been shown to reduce albuminuria compared with HCTZ in nondiabetic patients with isolated systolic hypertensionCitation102 and to reduce microalbuminuria by 69% over the course of a 12-month, noncomparative study in hypertensive patients.Citation103 Other studies confirmed that telmisartan reduced macroalbuminuria in patients with mild and moderate renal failure.Citation104,Citation105 The effects of telmisartan on proteinuria may well be additive to those of ACE inhibitors.Citation106
Several large-scale clinical studies have been completed that demonstrate the beneficial effects of telmisartan on renal function. The Incipient to Overt: Angiotensin II Blocker, Telmisartan, Investigation on Type 2 Diabetic Nephropathy (INNOVATION®) study was performed in normotensive, as well as in hypertensive, Japanese patients.Citation107 Over a mean of 1.3 years’ treatment, both telmisartan 40 and 80 mg significantly reduced transition rates to overt nephropathy in comparison with placebo. Reduced transition rates to overt nephropathy remained after adjustment for changes in SBP and in normotensive patients, suggesting telmisartan had a blood pressure-independent effect.
The sister studies, A trial to compare telMisartan 40 mg titrated to 80 mg versus losArtan 50 mg titrated to 100 mg in hypertensive type 2 DiabEtic patients with Overt nephropathy (AMADEO™), and inVestigate the efficacy of MICARDIS® versus VALsartan in hypertensive type 2 DIabetic patients with overt nephropathy (VIVALDI®) evaluated the effect of telmisartan on macroalbuminuria. In AMADEO™, telmisartan reduced urinary protein:creatinine significantly more than losartan after 52 weeks (29% versus 20% from baseline, respectively; P = 0.03), despite similar blood pressure control.Citation108 This suggests that there are intra-class difference in the renal effects of ARBs, which is consistent with additional properties beyond the blood pressure-lowering effect. In VIVALDI®, telmisartan 80 mg provided identical reductions in urinary protein excretion (33% from baseline) to valsartan 160 mg and there were no significant differences between the two agents in serum creatinine, creatinine clearance, or estimated GFR changes.Citation109 These studies suggest that telmisartan may slow the progression of diabetic nephropathy in this group of patients.
Cardiac disease
The presence of LVH in patients with established hypertension nearly triples the incidence of coronary heart disease and stroke, and increases the incidence of heart failure about seven-fold.Citation110 Reducing LVM significantly reduces cardiovascular risk.Citation111 Angiotensin II plays a central role in cardiac hypertrophy, causing a trophic response to increased blood pressure and having direct proliferative effects.Citation112 The clinical evidence that telmisartan reduces LVM comes from several studies. For example, telmisartan reduced LVM from 151.6 to 135.1 g/m2, largely due to decreased thickness of the left ventricular wall, in hypertensive patients.Citation113 Telmisartan has been compared with other antihypertensives, including diuretics, β-blockers, ACE inhibitors, and other ARBs. Telmisartan 80 mg proved superior to HCTZ 25 mg, with the reduction in LVM being significantly greater with telmisartan for a given percentage change in blood pressure.Citation57 Telmisartan 80 mg was more effective in reducing LVM than carvedilol 25 mg, despite there being no significant difference in 24-hour mean SBP/DBP reductions between the two treatments.Citation53 Addition of telmisartan 80 mg to ramipril 5 mg provided further beneficial effects on LVM, although there were similar reductions in blood pressure with either monotherapy or combination.Citation114
A 12-week study showed that replacing twice-daily enalapril 10 mg with once-daily telmisartan 10 to 80 mg does not produce any acute deterioration of exercise capacity or clinical status in patients with mild-to-moderate congestive heart failure (CHF) (New York Heart Association [NYHA] Class II or III and left ventricular ejection fraction ≤40%).Citation115 The study also found no differences in changes of other parameters, such as ejection fraction, NYHA classification, and mean SBP between the treatment groups.
Atrial fibrillation
ARBs and ACE inhibitors have been shown to be effective in preventing atrial fibrillation in patients with heart failure or left ventricular dysfunction, as seen in the meta-analysis by Healey and colleagues.Citation116 The RAS plays an important role facilitating new onset or recurrence of atrial fibrillation. It mediates atrial remodeling by increasing blood pressure, intracavitary atrial pressure, and arrhythmogenic atrial remodeling, by facilitating coronary atherosclerosis and by increasing reactive oxygen substances and favoring atrial fibrosis. Blocking the RAS may prevent left atrial dilatation, atrial fibrosis, dysfunction, and conduction velocity slowing.
There are different clinical scenarios involving prevention of atrial fibrillation in the hypertensive patient (ie, those who have not had any previous episodes of atrial fibrillation, and those with parossistic or persistent atrial fibrillation who either do not need any anti-arrhythmic therapy, or those with persistent atrial fibrillation who do require anti-arrhythmic therapy to maintain sinus rhythm following cardioversion). Previous studies suggest that inhibition of RAS with ARBs or ACE inhibitors may prevent new onset atrial fibrillation in patients without any previous episodes of atrial fibrillation,Citation117 and recurrence after cardioversion in hypertensive patients requiring antiarrhythmic therapy.Citation118 Previously, we investigated whether telmisartan prevented the recurrence of atrial fibrillation in hypertensive patients who did not require anti-arrhythmic therapy. We compared the efficacy of telmisartan and carvedilol in preventing the recurrence of atrial fibrillation in 154 hypertensive patients with a recent history of atrial fibrillation.Citation119 There was an atrial fibrillation episode in 14.2% (10/70) of patients who received telmisartan compared with 37% (23/62) of those receiving carvedilol (P < 0.005). In addition to preventing recurrence of atrial fibrillation, the time to a recurrence of atrial fibrillation was longer with telmisartan than with carvedilol. This difference in the rates of new episodes of atrial fibrillation between the agents was not related to changes in blood pressure, left atrial size, although a greater left ventricular mass reduction in the telmisartan group was observed. This suggests preventive properties of telmisartan were a pharmacologic effect. It is possible that telmisartan favorably interferes with electrical and structural atrial remodeling in hypertensive patients.
Cerebrovascular disease
For each 2 mmHg increase in SBP, the risk of stroke is increased by 10%.Citation45 Angiotensin II pathways appear not only to be implicated in blood pressure control and body fluid homeostasis, but may also contribute to the pathogenesis of stroke via the stimulation of AT1 receptors.Citation120 The use of ARBs may not only prevent the ischemic effect of angiotensin II mediated via AT1 receptors, but also stimulate the unoccupied AT2 receptors with a consequent improvement of brain ischemia. Intra-cerebroventricular infusion of an ARB for 5 days has been shown to induce neuronal regrowth after cerebral ischemia and to reduce expression of transcription factors c-Fos and c-Jun that are associated with programmed cell death and neurodegeneration.Citation121 To date, evidence of possible beneficial effects of telmisartan on cerebrovascular disease are provided by studies in animals.
In rats, telmisartan is able to cross the blood–brain barrier and block the effects of centrally administered angiotensin II.Citation122 Furthermore, at doses that had no effect on blood pressure, telmisartan delayed the onset of stroke in spontaneously hypertensive, stroke-prone animals.Citation123 In cerebral arterioles, telmisartan reversed the vasoconstrictor effect of angiotensin II, changing the response to a vasodilatory oneCitation124 and overcame the cerebral arterial remodeling occurring in spontaneously hypertensive rats.Citation125
Although the effect of telmisartan on stroke has yet to be demonstrated in clinical studies, the effects of telmisartan on cognitive function have been examined in elderly subjects with hypertension.Citation126 In addition to providing superior blood pressure control compared with lisinopril 20 mg plus HCTZ, telmisartan 80 mg given with HCTZ 12.5 mg improved performance on cognitive tests significantly more than lisinopril/HCTZ.
Telmisartan outcome trials
The ONTARGET® program consists of two long-term, large-scale, double-blind, multinational outcome studies – the ONTARGET® studyCitation50 and the parallel Telmisartan Randomized AssessmeNt Study in aCE iNtolerant subjects with cardiovascular Disease (TRANSCEND®) study.Citation127
The ONTARGET® study compared telmisartan 80 mg monotherapy to ramipril 10 mg monotherapy and the combination to ramipril alone. The primary endpoint was a composite of cardiovascular mortality, nonfatal MI, nonfatal stroke, and hospitalization for CHF. Secondary endpoints included newly diagnosed CHF, cardiovascular revascularization, newly diagnosed diabetes, cognitive decline/dementia, new onset of atrial fibrillation, and nephropathy. ONTARGET® was conducted in patients who could tolerate ACE inhibitor therapy, whereas TRANSCEND® compared telmisartan with placebo in addition to best standard of care in patients intolerant of this class using the same endpoints as ONTARGET®.
In the ONTARGET® study, the primary outcome occurred in 1423 patients (16.7%) in the telmisartan group, 1412 patients (16.5%) in the ramipril group, and in 1386 (16.3%) in the combination-therapy group.Citation50 Telmisartan was non-inferior to ramipril, and the combination offered no additional protective effect. The results for the secondary outcome of death from cardiovascular causes, myocardial infarction or stroke were consistent with those of the primary outcome.
Even though individuals who were intolerant to ACE inhibitors had been excluded from the trial, 360 patients in the ramipril group stopped their medication because of cough compared with only 93 patients in the telmisartan group. Angioedema resulted in 25 patients discontinuing ramipril compared with 10 patients in the telmisartan group. Rates of cough and angioedema were also higher in the combination group than in the telmisartan group. In the combination group, significantly more patients stopped because of hypotensive symptoms, diarrhea, or renal impairment than in the ramipril group. The incidence of these events was also numerically higher than in the telmisartan group, although no statistics were reported for this comparison.
On the basis of the ONTARGET® results, telmisartan is the only ARB proven to have cardiovascular protective effects in a broad cross section of high-risk patients. It is as effective as ramipril but is associated with less angioedema and cough. The combination offers no additional efficacy advantage compared with the monotherapies. As the authors of the ONTARGET® publication state, the choice between telmisartan and ramipril ‘will depend on the preferences of the patients and physicians and the individual’s susceptibility to specific adverse events’.
In the TRANSCEND® study, telmisartan was well tolerated among patients who were unable to tolerate ACE inhibitors. Although the reduction in the primary outcome (which included hospitalizations for heart failure) with telmisartan did not achieve statistical significance, it did significantly reduce the risk of the composite outcome of cardiovascular death, myocardial infarction, or stroke by 13%.Citation127 Moreover, adherence to telmisartan was high and better in the comparison arm, in which patients received the best standard of care. It is reasonable to assume that the greater tolerability and treatment adherence observed with telmisartan in both ONTARGET® and TRANSCEND® will be of benefit for many patients who are likely to require life-long treatment.
The potential cerebroprotective efficacy of telmisartan was evaluated in the Prevention Regimen For Effectively avoiding Second Strokes (PRoFESS®) study.Citation128 The 4-year study compared telmisartan and placebo on top of usual care, including antihypertensives to control blood pressure, in 20,000 patients with known prior ischemic stroke. The study had a 2 × 2 factorial design, with patients also receiving either aspirin plus dipyridamole extended release or clopidogrel alone. The primary outcome was time to recurrent stroke, while secondary outcomes included the onset of vascular events including bleeding events or CHF. There was non significant trend favoring telmisartan over usual care for the primary endpoint.Citation129 Exploratory analyses indicate that after excluding the first 6 months of treatment, the incidence of recurrent stroke or major vascular events was significantly lower with telmisartan. The mean treatment period was 2.5 years and a longer treatment period may have allowed the trends that were observed to become significant.
Conclusions
The pharmacologic features of telmisartan enable it to provide greater and more sustained antihypertensive efficacy than many other antihypertensive agents, and compared with other antihypertensive in other classes, telmisartan is well tolerated. Telmisartan in combination with HCTZ or amlodipine provides greater reductions in blood pressure than the respective monotherapies, and these combinations are well tolerated. The antihypertensive efficacy of telmisartan monotherapy and combinations should translate into increased protection against cardiovascular events. There is also growing evidence that telmisartan and ARBs have beneficial effects on various stages of the cardiovascular and renal continua that may not be solely explained by the lowering of blood pressure. ONTARGET® has shown that telmisartan provides similar cardiovascular protection to ramipril in high-risk patients, while being better tolerated and associated with greater treatment adherence; the latter property is likely to be important in the long-term management of cardiovascular risk.
The attributes of telmisartan and the clinical evidence of its efficacy suggest that it should be one of the preferred options for the treatment of hypertension in mild to moderate hypertensive patients and make it an attractive foundation for use in combination therapy. The findings of both ONTARGET® and TRANSCEND® demonstrate that telmisartan provides a protective benefit when added to other therapies. Its effect on cardiovascular endpoints combined with its proven tolerability suggest that telmisartan could be considered as a potential treatment for patients with vascular disease or high-risk diabetes, irrespective of whether or not they can tolerate ACE inhibitors.
Acknowledgements
Editorial assistance and journal fees were provided by PAREXEL MMS. This work was supported by Boehringer Ingelheim GmbH.
Disclosures
The authors disclose no conflicts of interest.
References
- WeberMAInterrupting the renin-angiotensin system: the role of angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists in the treatment of hypertensionAm J Hypertens199912189S194S10619571
- KakutaHSudohKSasamataMTelmisartan has the strongest binding affinity to angiotensin II type 1 receptor: comparison with other angiotensin II type 1 receptor blockersInt J Clin Pharmacol Res200525414615864875
- WienenWHauelNvan MeelJCPharmacological characterization of the novel nonpeptide angiotensin II receptor antagonist, BIBR 277Br J Pharmacol19931102452528220885
- WienenWEntzerothMvan MeelJCAA review on telmisartan: a novel, long-acting angiotensin II-receptor antagonistCardiovasc Drug Rev200018127156
- StangierJSuCAPFRothWPharmacokinetics of orally and intravenously administered telmisartan in healthy young and elderly volunteers and in hypertensive patientsJ Int Med Res20002814916711014323
- BrunnerHRThe new oral angiotensin II antagonist olmesartan medoxomil: a concise overviewJ Hum Hypertens200216Suppl 2S13S1611967728
- BurnierMBrunnerHRAngiotensin II receptor antagonistsLancet200035563764510696996
- EbnerTHeinzelGProxADisposition and chemical stability of telmisartan 1-O-acylglucuronideDrug Metab Dispos1999271143114910497140
- StangierJSuCAPFvan HeiningenPNMInhibitory effect of telmisartan on the blood pressure response to angiotensin II challengeJ Cardiovasc Pharmacol20013867268511602814
- PershadsinghHAKurtzTWInsulin-sensitizing effects of telmisartan: implications for treating insulin-resistant hypertension and cardiovascular diseaseDiabetes Care200427101515047668
- BensonSCPershadsinghHAHoCIIdentification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARγ-modulating activityHypertension200443993100215007034
- FujimotoMMasuzakiHTanakaTAn angiotensin II AT1 receptor antagonist, telmisartan augments glucose uptake and GLUT4 protein expression in 3T3-L1 adipocytesFEBS Lett200457649249715498586
- ClasenRSchuppMForyst-LudwigAPPARγ-activating angiotensin type-1 receptor blockers induce adiponectinHypertension20054613714315939809
- SchuppMClemenzMGinesteRMolecular characterization of new selective peroxisome proliferator-activated receptor-γ modulators with angiotensin receptor blocking activityDiabetes2005543442345216306360
- BrodyRPelegEGrossmanEProduction and secretion of adiponectin from 3T3-L1 adipocytes: comparison of antihypertensive drugsAm J Hypertens2009221126112919730415
- NakamuraTKawachiKSaitoYEffects of ARB or ACE-inhibitor administration on plasma levels of adlosterone and adiponectin in hypertensionInt Heart J20095050151219609054
- KappertKTsuprykovOKaufmannJChronic treatment with losartan results in sufficient serum levels of the metabolite EXP3179 for PPAR {gamma} activationHypertension20095473874319687349
- JankeJSchuppMEngeliSAngiotensin type 1 receptor antagonists induce human in-vitro adipogenesis through peroxisome proliferator-activated receptor-gamma activationJ Hypertens2006241809181616915030
- MarshallTGLeeREMarshallFECommon angiotensin receptor blockers may directly modulate the immune system via VDR, PPAR and CCR2bTheor Biol Med Model2006118617421746
- CostaFVTelmisartan: standing out in a crowded contest?High Blood Press Cardiovasc Prev2006138594
- RedónJRoca-CusachsAMora-MaciaJUncontrolled early morning blood pressure in medicated patients: the ACAMPA study. Analysis of the control of blood pressure using ambulatory blood pressure monitoringBlood Press Monit2002711111612048428
- IskedjianMEinarsonTRMacKeiganLDRelationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: evidence from a meta-analysisClin Ther20022430231611911560
- MullerJEStonePHTuriZGCircadian variation in the frequency of onset of acute myocardial infarctionN Engl J Med1985313131513222865677
- MarlerJRPriceTRClarkGLMorning increase in onset of ischemic strokeStroke1989204734762648651
- ElliottWJCircadian variation in the timing of stroke onset – a meta-analysisStroke1998299929969596248
- Millar-CraigMWBishopCNRafteryEBCircadian variation of blood-pressureLancet1978i79579785815
- SternNSowersJRMcGintyDCircadian rhythm of plasma renin activity in older normal and essential hypertensive men: relation with inactive renin, aldosterone, cortisol and REM sleepJ Hypertens198645435503540117
- CasigliaEPalatiniPColangeliG24 h rhythm of blood pressure and forearm peripheral resistance in normotensive and hypertensive subjects confined to bedJ Hypertens199614475212013494
- LearyACStruthersADDonnanPTThe morning surge in blood pressure and heart rate is dependent on levels of physical activity after wakingJ Hypertens20022086587012011646
- WhiteWBGilesTBakrisGLMeasuring the efficacy of anti-hypertensive therapy by ambulatory blood pressure monitoring in the primary care settingAm Heart J2006a15117618416368314
- PhillipsRAWeinbergJMHypertension 2005: an evidence-based approach to diagnosis and treatment – an American perspectiveExpert Rev Cardiovasc Ther2005369170416076279
- WhiteWBWeberMADavidaiGAmbulatory blood pressure monitoring in the primary care setting: assessment of therapy on the circadian variation of blood pressure from the MICCAT-2 TrialBlood Press Monit20051015716315923818
- NishimuraTHashimotoJOhkuboTEfficacy and duration of action of the four selective angiotensin II subtype 1 receptor blockers, losartan, candesartan, valsartan and telmisartan, in patients with essential hypertension determined by home blood pressure measurementsClin Exp Hypertens20052747748916081340
- WhiteWBLacourcièreYDavidaiGEffects of the angiotensin II receptor blockers telmisartan versus valsartan on the circadian variation of blood pressure: impact on the early morning periodAm J Hypertens20041734735315062889
- LacourcièreYKrzesinskiJMWhiteWBSustained antihypertensive activity of telmisartan compared with valsartanBlood Press Monit2004920321015311147
- MallionJMSichéJPLacourcièreYThe Telmisartan Blood Pressure Monitoring GroupABPM comparison of the antihypertensive profiles of the selective angiotensin II receptor antagonists telmisartan and losartan in patients with mild-to-moderate hypertensionJ Hum Hypertens19991365766410516734
- SmithDHCramerMJNeutelJMComparison of telmisartan versus losartan: meta-analysis of titration-to-response studiesBlood Press Monit2003811111712900588
- DingPYChuKMChiangHTA double-blind ambulatory blood pressure monitoring study of the efficacy and tolerability of once-daily telmisartan 40 mg in comparison with losartan 50 mg in the treatment of mild-to-moderate hypertension in Taiwanese patientsInt J Clin Pract Suppl200458162215617454
- DerosaGRagonesiPDMugelliniAEffects of telmisartan compared with eprosartan on blood pressure control, glucose metabolism and lipid profile in hypertensive, type 2 diabetic patients: a randomized, double-blind, placebo-controlled 12-month studyHypertens Res20042745746415302981
- NakayamaSWatadaHMitaTComparison of effects of olmesartan and telmisartan on blood pressure and metabolic parameters in Japanese early-stage type-2 diabetics with hypertensionHypertens Res20083171318360012
- SasakiTNodaYYasuokaYComparison of the effects of telmisartan and olmesartan on home blood pressure, glucose, and lipid profiles in patients with hypertension, chronic heart failure, and metabolic syndromeHypertens Res20083192192918712048
- WilliamsBGossePLoweLThe prospective, randomized investigation of the safety and efficacy of telmisartan versus ramipril using ambulatory blood pressure monitoring (PRISMA I)J Hypertens20062419320016331118
- LacourcièreYNeutelJMDavidaiGA multicenter, 14-week study of telmisartan and ramipril in patients with mild-to-moderate hypertension using ambulatory blood pressure monitoringAm J Hypertens20061910411216461201
- GossePNeutelJMSchumacherHThe effect of telmisartan and ramipril on early morning blood pressure surge: a pooled analysis of two randomized clinical trialsBlood Press Monit20071214114717496463
- LewingtonSClarkeRQizilbashNAge-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studiesLancet20023601903191312493255
- NalbantgilINalbantgilSÖzerkanFThe efficacy of telmisartan compared with perindopril in patients with mild-to-moderate hypertensionInt J Clin Pract2004585054
- RagotSEzzaherAMeunierAComparison of trough effect of telmisartan vs perindopril using self blood pressure measurement: EVERESTE studyJ Hum Hypertens20021686587312522468
- StergiouGSEfstathiouSPRoussiasLGBlood pressure- and pulse pressure-lowering effects, trough:peak ratio and smoothness index of telmisartan compared with lisinoprilJ Cardiovasc Pharmacol20034249149614508234
- NeutelJMFrishmanWHOparilSComparison of telmisartan with lisinopril in patients with mild-to-moderate hypertensionAm J Ther1999616116610423659
- ONTARGET InvestigatorsYusufSTeoKKTelmisartan, ramipril, or both in patients at high risk for vascular eventsN Engl J Med20083581547155918378520
- FreytagFSchellingAMeinickeTComparison of 26-week efficacy and tolerability of telmisartan and atenolol, in combination with hydrochlorothiazide as required, in the treatment of mild-to-moderate hypertension: a randomized, multicenter studyClin Ther20012310812311219471
- AlcocerLFernandez-BonettiPCamposEClinical efficacy and safety of telmisartan 80 mg once daily vs. atenolol 50 mg once daily in patients with mild-to-moderate hypertensionInt J Clin Pract Suppl200458353915617457
- GalzeranoDTammaroPdel ViscovoLThree-dimensional echocardiographic and magnetic resonance assessment of the effect of telmisartan compared with carvedilol on left ventricular mass a multicenter, randomized, longitudinal studyAm J Hypertens2005181563156916364826
- LacourcièreYLenisJOrchardRA comparison of the efficacy and duration of action of the angiotensin II receptor blocker telmisartan to amlodipineBlood Press Monit1998329530210212369
- DerosaGCiceroAFGBertoneGComparison of the effects of telmisartan and nifedipine gastrointestinal therapeutic system on blood pressure control, glucose metabolism, and the lipid profile in patients with type 2 diabetes mellitus and mild hypertension: A 12-month, randomized, double-blind studyClin Ther2004261228123615476904
- McGillJBReillyPATelmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group trialClin Ther20012383385011440284
- GalzeranoDTammaroPCercielloAFreehand three-dimensional echocardiographic evaluation of the effect of telmisartan compared with hydrochlorothiazide on left ventricular mass in hypertensive patients with mild-to-moderate hypertension: a multicentre studyJ Hum Hypertens200418535914688811
- ManolisAJReidJLde ZeeuwDAngiotensin II receptor antagonist telmisartan in isolated systolic hypertension (ARAMIS) study: efficacy and safety of telmisartan 20, 40 or 80 mg versus hydrochlorothiazide 12.5 mg or placeboJ Hypertens2004221033103715097245
- LacourcièreYNeutelJMSchumacherHComparison of fixed-dose combinations of telmisartan/hydrochlorothiazide 40/12.5 mg and 80/12.5 mg and a fixed-dose combination of losartan/hydrochlorothiazide 50/12.5 mg in mild to moderate essential hypertension: Pooled analysis of two multicenter, prospective, randomized, open-label, blinded-end point (PROBE) trialsClin Ther2005271795180516368450
- WhiteWBPunziHAMurwinDEffects of the angiotensin II receptor blockers telmisartan vs valsartan in combination with hydrochlorothiazide 25 mg once daily for the treatment of hypertensionJ Clin Hypertens (Greenwich)2006862663316957424
- WhiteWBMurwinDChrysantSGEffects of the angiotensin II receptor blockers telmisartan versus valsartan in combination with hydrochlorothiazide: a large, confirmatory trialBlood Press Monit200813212718199920
- FogariRZoppiAMugelliniAEffectiveness of hydrochlorothiazide in combination with telmisartan and olmesartan in adults with moderate hypertension not controlled with monotherapy: a prospective, randomized, open-label, blinded end point (PROBE), parallel-arm studyCurr Ther Res200869115
- LacourcièreYTytusRO’KeefeDEfficacy and tolerability of a fixed-dose combination of telmisartan plus hydrochlorothiazide in patients uncontrolled with telmisartan monotherapyJ Hum Hypertens20011576377011687919
- SharmaADavidsonJKovalSTelmisartan/hydrochlorothiazide versus valsartan/hydrochlorothiazide in obese hypertensive patients with type 2 diabetes: the SMOOTH studyCardiovasc Diabetol200762817910747
- NeldamSEdwardsCTelmisartan plus HCTZ vs amlodipine plus HCTZ in older patients with systolic hypertension: results from a large ambulatory blood pressure monitoring studyAm J Geriatr Cardiol20061615116016687967
- SchumacherHManciaGThe safety profile of telmisartan as monotherapy or combined with hydrochlorothiazide: a retrospective analysis of 50 studiesBlood Press Suppl20081324018705533
- LittlejohnTMajulCOlveraRResults of treatment with telmisartan-amlodipine in hypertensive patientsJ Clin Hypertens20091117
- LittlejohnTMajulCOlveraREffect of telmisartan addition to amlodipine on reduction of incidence of peripheral oedema: safety analysis from a factorial study in hypertensive patientsJ Hypertens200826Suppl 1S460
- DahlofBDevereuxRBKjeldsenSECardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenololLancet2002359995100311937178
- JuliusSKjeldsenSEWeberMOutcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trialLancet20043632022203115207952
- CohnJNTognoniGfor the Valsartan Heart Failure Trial InvestigatorsA randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failureN Engl J Med3451667167511759645
- PfefferMASwedbergKGrangerCBEffects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programmeLancet200336275976613678868
- PfefferMAMcMurrayJJVelazquezEJValsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or bothN Engl J Med20033491893190614610160
- PortaluppiFBoariBManfrediniROxidative stress in essential hypertensionCurr Pharm Des2004101695169815134566
- TakayaTKawashimaSShinoharaMAngiotensin II type 1 receptor blocker telmisartan suppresses superoxide production and reduces atherosclerotic lesion formation in apolipoprotein E-deficient miceAtherosclerosis200618640241016157344
- TakaiSKirimuraKJinDSignificance of angiotensin II receptor blocker lipophilicities and their protective effect against vascular remodelingHypertens Res20052859360016335888
- ThornalleyPJCell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEsCell Mol Biol199844101310239846883
- NakamuraKYamagishiSNakamuraYTelmisartan inhibits expression of a receptor for advanced glycation end products (RAGE) in angiotensin-II-exposed endothelial cells and decreases serum levels of soluble RAGE in patients with essential hypertensionMicrovasc Res20057013714116271939
- YamagishiSTakeuchiMMatsuiTAngiotensin II augments advanced glycation end product-induced pericyte apoptosis through RAGE overexpressionFEBS Lett20055794265427016051229
- EyriesMCollinsTKhachigianLMModulation of growth factor gene expression in vascular cells by oxidative stressEndothelium20041113313915370072
- AmanoSYamagishiSInagakiYAngiotensin II stimulates platelet-derived growth factor-B gene expression in cultured retinal pericytes through intracellular reactive oxygen species generationInt J Tissue React200325515514518593
- YamagishiSAmanoSInagakiYAngiotensin II-type 1 receptor interaction upregulates vascular endothelial growth factor messenger RNA levels in retinal pericytes through intracellular reactive oxygen species generationDrugs Exp Clin Res200329758012951837
- SchmiederREDellesCMimranAImpact of telmisartan versus ramipril on renal endothelial function in patients with hypertension and type 2 diabetesDiabetes Care2007301351135617337492
- SymeonidesPKoulourisSTriantafyllouKFavourable pleiotropic effects of ramipril and telmisartan on vascular endothelium of diabetics [abstract]J Am Coll Cardiol200545Suppl A428A
- LaurentSBoutouyriePAsmarRAortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patientsHypertension2001371236124111358934
- WilkinsonIBMacCallumHHupperetzPCChanges in the derived central pressure waveform and pulse pressure in response to angiotensin II and noradrenaline in manJ Physiol200153054155011158283
- VingerhoedtNMGillesRHowesJBHemodynamic and pulse wave responses to intravenous infusions of angiotensin II during chronic telmisartan therapy in normal volunteersJ Renin Angiotensin Aldosterone Syst2003424424814689372
- AsmarRGossePTopouchianJEffects of telmisartan on arterial stiffness in Type 2 diabetes patients with essential hypertensionJ Renin Angiotensin Aldosterone Syst2003317618012563568
- UchidaHNakamuraYKaiharaMPractical efficacy of telmisartan for decreasing morning home blood pressure and pulse wave velocity in patients with mild-to-moderate hypertensionHypertens Res20042754555015492473
- HaffnerSMRisk constellations in patients with metabolic syndrome: epidemiology, diagnosis and treatment patternsAm J Med2006119Suppl 5AS3S916563945
- MiuraYYamamotoNTsunekawaSReplacement of valsartan and candesartan by telmisartan in hypertensive patients with type 2 diabetes: metabolic and antiatherogenic consequencesDiabetes Care20052875775815735228
- MichelMCBohnerHKösterJSafety of telmisartan in patients with arterial hypertension. An open-label observational studyDrug Saf20042733534415061687
- HonjoSNichiYWadaYPossible beneficial effect of telmisartan on glycemic control in diabetic subjectsDiabetes Care20052849815677828
- NegroRHassanHThe effects of telmisartan and amlodipine on metabolic parameters and blood pressure in type 2 diabetic, hypertensive patientsJ Renin Angiotensin Aldosterone Syst2006a724324617318795
- NagelJMTietzABGokeBThe effect of telmisartan on glucose and lipid metabolism in nondiabetic, insulin-resistant subjectsMetabolism2006551149115416919531
- BenndorfRARudolphTAppelDTelmisartan improves insulin sensitivity in nondiabetic patients with essential hypertensionMetabolism2006551159116416919533
- VitaleCMercuroGCastiglioniCMetabolic effect of telmisartan and losartan in hypertensive patients with metabolic syndromeCardiovasc Diabetol20054615892894
- BarnettAHBainSCBouterPAngiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathyN Engl J Med20043511952196115516696
- LeveyASAdlerSCaggiulaAWEffects of dietary protein restriction on the progression of advanced renal disease in the Modification of Diet in Renal Disease StudyAm J Kidney Dis1996276526638629624
- LacourcièreYBelangerAGodinCLong-term comparison of losartan and enalapril on kidney function in hypertensive type 2 diabetics with early nephropathyKidney Int20005876276910916100
- ParvingHHAndersenSJacobsenPAngiotensin receptor blockers in diabetic nephropathy: renal and cardiovascular end pointsSemin Nephrol20042414715715017527
- VogtLNavisGKösterJThe angiotensin II receptor antagonist telmisartan reduces urinary albumin excretion in patients with isolated systolic hypertension: results of a randomized, double-blind, placebo-controlled trialJ Hypertens2005232055206116208149
- RedónJLuque-OteroMMartellNRenin-angiotensin system gene polymorphisms: relationship with blood pressure and microalbuminuria in telmisartan-treated hypertensive patientsPhamacogenomics J200551420
- HannedoucheTChanardJBaumelouBEvaluation of the safety and efficacy of telmisartan and enalapril, with the potential addition of frusemide, in moderate-renal failure patients with mild-to-moderate hypertensionJ Renin Angiotensin Aldosterone Syst2001224625411881131
- CupistiARizzaGMD’AlessandroCEffect of telmisartan on the proteinuria and circadian blood pressure profile in chronic renal patientsBiomed Pharmacother20035716917212818479
- SengulAMAltuntasYKurkluABeneficial effect of lisinopril plus telmisartan patients with type 2 diabetes, microalbuminuria and hypertensionDiabetes Res Clin Pract20067121021916112244
- MakinoHHanedaMBabazonoTPrevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetesDiabetes Care2007301577157817389334
- BakrisGBurgessEWeirMTelmisartan is more effective than losartan in reducing proteinuria in patients with diabetic nephropathyKidney Int20087436436918496508
- GalleJSchwedhelmEPinnettiSBögerRHWannerCAntiproteinuric effects of angiotensin receptor blockers: telmisartan versus valsartan in hypertensive patients with type 2 diabetes mellitus and overt nephropathyNephrol Dial Transplant2008233174318318450829
- KannelWBLeft ventricular hypertrophy as a risk factor in arterial hypertensionEur Heart J199213Suppl D82881396865
- DevereuxRBWachtellKGerdtsEPrognostic significance of left ventricular mass change during treatment of hypertensionJAMA20042922350235615547162
- BouzegrhaneFThibaultGIs angiotensin II a proliferative factor of cardiac fibroblasts?Cardiovasc Res20025330431211827680
- IvanovaOVFomichevaOASergakovaLMAngiotensin II receptor blocker telmisartan: Effect on blood pressure profile and left ventricular hypertrophy in patients with arterial hypertensionJ Int Med Res200533Suppl 121A29A15651713
- PetrovicJPetrovicDVukovicNVentricular and vascular remodelling – effects of the angiotensin II receptor blocker telmisartan and/or the angiotensin-converting enzyme inhibitor ramipril in hypertensive patientsJ Int Med Res200533Suppl 139A49A
- DunselmanPHJMthe replacement of angiotensin converting enzyme inhibition (REPLACE) investigatorsEffects of the replacement of the angiotensin converting enzyme inhibitor enalapril by the angiotensin II receptor blocker telmisartan in patients with congestive heart failureInt J Cardiol20017713113811182175
- HealeyJSBaranchukACrystalEPrevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysisJ Am Coll Cardiol2005451832183915936615
- WachtellKLehtoMGerdtsEAngiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) studyJ Am Coll Cardiol20054571271915734615
- MadridAHBuenoMGRebolloJMUse of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized studyCirculation200210633133612119249
- GalzeranoDCaselliSBreglioRA multicentre, randomized study comparing efficacy of telmisartan versus carvedilol in preventing atrial fibrillation recurrence in hypertensive patientsCirculation2007116Suppl II556557
- ChrysantSGPossible pathophysiologic mechanisms supporting the superior stroke protection of angiotensin receptor blockers compared to angiotensin-converting enzyme inhibitors: clinical and experimental evidenceJ Hum Hypertens20051992393116049519
- DaiWJFunkAHerdegenTBlockade of central angiotensin AT(1) receptors improves neurological outcome and reduces expression of AP-1 transcription factors after focal brain ischemia in ratsStroke1999302391239810548676
- GohlkePWeissSJansenAAT1 receptor antagonist telmisartan administered peripherally inhibits central responses to angiotensin II in conscious ratsJ Pharmacol Exp Ther2001298627011408526
- XuJCulmanJBlumeATreatment with telmisartan and lithium of stroke-prone spontaneously hypertensive rats: survival studyNaunyn Schmiedebergs Arch Pharmacol2002365Suppl 1R67
- VincentJMKwanYWLungCSConstrictor and dilator effects of angiotensin II on cerebral arteriolesStroke2005362691269516269635
- DupuisFAtkinsonJLiminanaPComparative effects of the angiotensin II receptor blocker, telmisartan, and the angiotensin-converting enzyme inhibitor, ramipril, on cerebrovascular structure in spontaneously hypertensive ratsJ Hypertens2005231061106615834293
- FogariRMugelliniAZoppiAEffect of telmisartan/hydrochlorothiazide vs lisinopril/hydrochlorothiazide combination on ambulatory blood pressure and cognitive function in elderly hypertensive patientsJ Hum Hypertens20062017718516306998
- TRANSCEND® InvestigatorsEffects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trialLancet20083721174118318757085
- DienerHCSaccoRYusufSRationale, design and baseline data of a randomized, double-blind, controlled trial comparing two antithrombotic regimens (a fixed-dose combination of extended-release dipyridamole plus ASA with clopidogrel) and telmisartan versus placebo in patients with strokes: The Prevention Regimen for Effectively Avoiding Second Strokes Trial (PRoFESS®)Cerebrovasc Dis20072336838017337887
- YusufSDienerHCSaccoRLTelmisartan to prevent recurrent stroke and cardiovascular eventsN Engl J Med20083591225123718753639