Abstract
Aim: To evaluate the association between the diameter of the superior rectal vein (dSRV) and prognosis in patients with locally advanced rectal cancer (LARC). Methods: This study included 420 LARC patients. Kaplan-Meier survival analysis and Cox regression models were used for determining the relationship between superior rectal vein diameter and survival. Results: Patients whose dSRV >3.60 mm had better 3 years disease-free survival (85.50 vs 64.2%, p < 0.001) and overall survival (91.90 vs 82.20%, p = 0.005). The multivariate Cox regression analysis showed that the dSRV was an independent prognostic factor for survival. Conclusion: The dSRV measurement is valuable in predicting the prognosis of patients with LARC, and the prognosis of patients with a smaller dSRV seems to be poor.
Plain language summary
What is this summary about?
The superior rectal vein is the main drainage vein of the rectum, and when the rectum undergoes changes due to disease, the diameter of the superior rectal vein may be altered. Can this change reflect the outcome of patients with rectal cancer? Our article reviews data from our institution over a period of more than 3 years to answer this question.
What were the results?
We found that patients whose superior rectal vein had a diameter of larger than 3.60 mm had better survival.
What do the results mean?
We should be concerned about the diameter of the superior rectal vein before treatment, and patients with smaller diameters of the superior rectal vein should be more aware of the need for regular follow-up.
Tweetable abstract
The diameter of the superior rectal vein (dSRV) measurement is valuable in predicting the prognosis of patients with locally advanced rectal cancer, and the prognosis of patients with a smaller dSRV seems to be poor.
With lifestyle changes, the prevalence of colorectal cancer increases, which is a serious threat to human life around the world. According to the Global Cancer Epidemiology Statistics (GLOBOCAN 2021) released by the International Cancer Organization Research Institute of World Health Organization in 2021, the incidence of colorectal cancer is 10% and ranked third among different types of cancers in 2020, and the death rate ranked second at 9.4% [Citation1]. However, due to the widespread use of neoadjuvant chemoradiotherapy followed by Total mesorectal excision (TME) surgery, the local recurrence of LARC has decreased significantly, and the 10-year local recurrence rate has been controlled at 5–7.1% [Citation2,Citation3]. The main pattern of treatment failure is distant metastasis, and the cumulative 10-year incidence rate is approximately 25–29.8% [Citation2,Citation3], which is an important factor affecting the survival of patients with LARC. Therefore, early identification of patients at high risk of metastasis and the use of intensive systemic treatment may improve patient survival.
When the tissue or organ undergoes pathological changes, its blood reflux and the diameter of its draining vein will change [Citation4]. The superior rectal vein collects the left and right branch veins from the rectal venous plexus, and drains the rectum and the anal canal above the dentate line [Citation5]. It has been reported that the inferior mesenteric vein of patients with rectal cancer is wider than that of patients without (5.9 mm vs 4.7 mm, p < 0.001) [Citation4], and the diameter of the superior rectal vein of patients with rectal cancer with distant metastasis is wider than that of patients with nonmetastatic cancer (4.0 mm vs 3.3 mm, p = 0.044) [Citation6].
Some studies have found that the diameter of the superior rectal vein (dSRV) can not only be used as a predictor of lymphatic vascular invasion and lymph node metastasis of rectal cancer [Citation6,Citation7] but is also valuable in determining whether liver lesions are metastatic lesions and predicting KRAS mutations in rectal cancer [Citation8,Citation9]. Although it has been reported that the dSRV in patients with metastasis is wider than that in patients without metastasis [Citation6], some studies have reported different results, and there is no significant difference in the dSRV between patients with metastasis and those without metastasis [Citation7]. At present, research on the role of the superior rectal vein in rectal cancer is still controversial, and the related studies are few. The existing studies are small sample analyses, and there may be bias. Therefore, this study intends to expand the sample size to explore the correlation between the dSRV and the prognosis of patients with LARC after neoadjuvant therapy to provide more evidence for the evaluation and prognosis of patients with LARC.
Methods
Patients
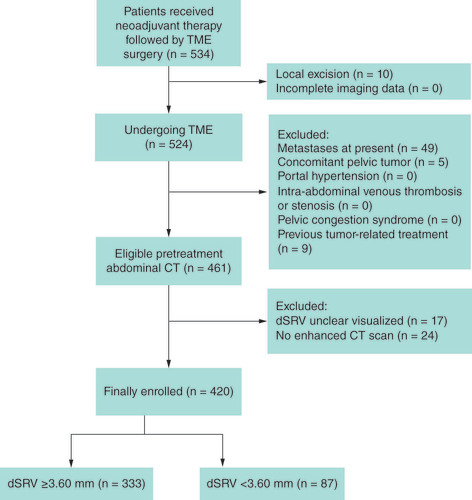
We reviewed patients with LARC who received neoadjuvant therapy followed by TME surgery in our unit from December 2014 to July 2017. The research design was conducted in accordance with the ethical guidelines for human research and was approved by the institutional review committee or the ethics committee. Patients with rectal cancer confirmed by histopathology were included in this study. Patients with complete imaging data, such as chest CT, abdominal and pelvic MRI, transrectal ultrasound and postoperative pathological data, were included in this study.
Patients with the following conditions at the time of diagnosis were excluded: distant metastasis, multiple tumors of the digestive system, intra-abdominal vein thrombosis or stenosis, pelvic congestion syndrome, portal hypertension or ascites, unoperated and local tumor resection, previous tumor-related treatment (chemotherapy or pelvic radiotherapy), without enhanced CT scan and unclear superior rectal veins ().
Measurement of the dSRV
The dSRV was measured on CT images which was took prior neoadjuvant therapy. All patients were advised to fast for 8 hours before the CT scan. The bladder was emptied one hour before the scan, 500 ml drinking water was consumed half an hour before scanning (10 ml 76% compound meglumine diatrizoate was added to the water for the development of the small intestine), and urine was held back. Approximately 90 ml contrast medium (iopromide injection, Bayer, Guangzhou, China) was injected. The GE Lightspeed large aperture CT simulator was used. The scanning distance and thickness were 0.5 cm. The CT images were transmitted to the treatment planning system (Eclipse13.5 version, Varian, USA) through the local area network for image postprocessing.
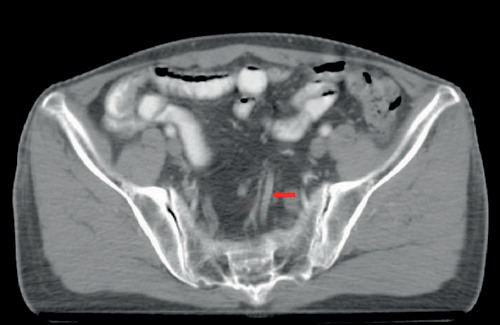
The dSRV was measured by two physicians under the guidance of a senior professional title imaging physician. We chose the second sacral vertebrae as the measured level. At this level, the vessels were horizontal in most cases, and the filling of surrounding adipose tissue made them easy to identify (). The superior rectal vein has pairs of branch veins and continues as the inferior mesenteric vein. We identified the superior rectal vein by identifying its reflux path on a series of adjacent axial CT images. The superior rectal veins can be identified by recognizing their paired tributaries, as well as their own reflux paths. After magnified the image to 400%, a clear and uniform section of the SRV was measured to determine the diameter [Citation9]. The average value of the readings of two physicians was taken as the final diameter of the superior rectal vein.
Therapy
All patients received neoadjuvant chemoradiotherapy and subsequently underwent TME surgery. The preoperative radiotherapy consisted of 50.4 Gy radiation in 25 fractions over 5 weeks, with intensity-modulated radiotherapy using 6-MV x-rays, delivered to the primary tumor and the mesentery of rectum, presacral region, pelvic sidewall, the two end portions of rectum and the pelvis internal iliac lymph drainage areas. The synchronous chemotherapy regimens included the Xelox, capecitabine and Folfox4 regimens. Surgery was performed 6–8 weeks after chemoradiotherapy, and the operation was performed in strict accordance with the principle of TME operation.
Follow-up
The last follow-up date was 1 February 2020. Number of patient deaths, tumor recurrence and metastasis were collected. Overall survival (OS) was defined as the interval from the first day of treatment to death from any cause or was censored at last follow-up. Disease-free survival (DFS) was defined as the interval from the first day after operation to the date of relapse (distant or local recurrence). Local recurrence was confined as a recurrence in the pelvic cavity, and local recurrence-free survival (LRFS) was defined as the interval from the first day after operation to the date of local regional recurrence. Distant metastasis was any tumor dissemination outside the pelvis, and metastasis-free survival (MFS) was defined as the date from the first day of treatment to the date of clinical proven distant metastasis.
Patients were followed up by outpatient services and telephone. They were required to review at regular intervals of 3 months for two years after surgery and then every 6 months for the next 3 years. Physical examination, chest CT, enhanced MRI scan of the abdomen and pelvis, serum carcinoembryonic antigen and carbohydrate antigen 19–9 were proposed at each visit. Colonoscopy was recommended 3 months after the operation and once a year thereafter. Our hospital has a professional follow-up department, and our team also has specialized follow-up staff who follow-up with patients by phone every 3 months. For patients who cannot be followed up by phone, we will contact the acquaintance or hospital where the patient is located to check the patient’s status, so there are no patients who have lost their follow-up.
Statistical analysis
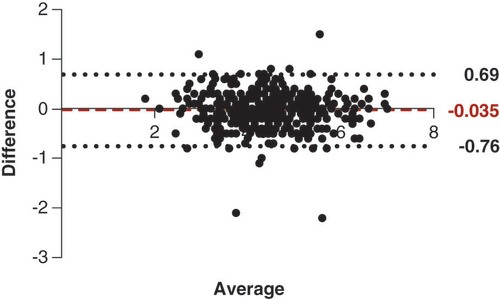
Inter-observer agreement of dSRV measurement was evaluated using intraclass correlation efficient (ICC) and Bland-Altman plot. X-tile (Yale University, X-tile Bioinformatics Software for Windows, Version 3.6.1) was used to calculate the optimal threshold value of the dSRV according to the difference in disease free survival curves. Patients were divided into two groups according to the value. The difference in baseline characteristics between the two groups was compared by chi-square or Fisher’s exact tests. Selection bias was inevitable because this study was a retrospective observational study. The propensity score matching (PSM) method was used to minimize the risk of bias. The patients were matched at a 1:1 ratio using a combination of nearest neighbor methods, and the caliper value was set to 0.05. The Kaplan–Meier method was adopted to estimate OS, DFS, MFS and LRFS. Factors affecting survival were analyzed by multivariate Cox proportional hazard regression. A p < 0.05 was considered statistically significant, and all statistical analyses were performed using the Social Sciences (SPSS) version 25.0 for Windows.
Results
Deciding the cut-off value & grouping
There was good correlation between the observers in measuring dSRV (ICC = 0.93, the Bland-Altman plot was showed in ). We used X-tile softwareto determine the threshold value of pretreatment dSRV associated with 3-year DFS in patients with LARC. The optimum cut-off value we obtained was 3.60 mm (). The patients were divided into two groups according to dSRV >3.60 mm or ≤3.60 mm.
Patients’ characteristics & PSM
The detailed parameters of the clinicopathological characteristics are summarized in . The parameters of distance to anus, tumor length, EMVI and clinical TMN stage were evaluated on baseline MRI. A total of 420 patients with LARC were enrolled in this study. The proportion of male patients was higher in the dSRV >3.60 mm group (70.9 vs 42.5%, p < 0.001). Elderly patients who had a tumor length less than 5 cm and a distance to the anus greater than 7 cm were concentrated in the dSRV ≤3.60 mm group, with a significant difference between the two groups. Considering that previous studies have found that the dSRV in EMVI positive patients is larger than that in negative patients [Citation10], EMVI and factors such as age, gender, distance from the anus and tumor length were taken as covariates of propensity score matching. Finally, 82 pairs were successfully matched. After matching, the baselines of the two groups were balanced. The results are detailed in .
Table 1. Comparison of patient characteristics according to diameter of the superior rectal vein.
Survival analysis
The median follow-up period for all patients was 45 months (7–61 months). During the follow-up, 7 patients had disease recurrence (1.60%), and 71 patients had distant metastasis (16.90%).
As shown in , there is a significant difference between two groups. The 3-year MFS, 3-year DFS, and 3-year OS rates were 87.70, 85.50 and 91.90% in the dSRV >3.60 mm group and 68.20% (p < 0.001) and 64.20% (p < 0.001) and 82.20% (p = 0.005) in the other group, respectively. In the Cox hazard model, univariate and multivariate analyses were employed to evaluate the significance of the dSRV as an independent prognostic factor. presents the results obtained from the survival analysis, the dSRV, EMVI, ypT stage, ypN stage and TRG were associated with OS, DFS and MFS. In the multivariate analysis, dSRV ≤3.60 mm was significantly correlated with poor OS (HR = 2.16, 95% CI: 1.22–3.84, p = 0.009), DFS (HR = 2.80, 95% CI: 1.80–4.36, p < 0.001) and MFS (HR = 2.95, 95% CI: 1.83–4.76, p < 0.001). After propensity score matching, the patients whose dSRV more than 3.60 mm maintained the advantage in OS, DFS and MFS. The results are detailed in .
The first row shows the survival curve prior to propensity score matching.
SRV: Superior rectal vein.
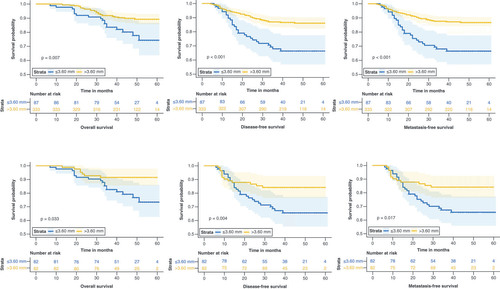
Table 2. Significant factors in survival analysis.
Discussion
We found that the dSRV was an independent prognostic factor for OS, DFS and MFS. Patients whose dSRV was greater than 3.60 mm showed better survival outcomes than those with a smaller diameter. Bakke et al. discovered that the low tumor blood flow (BF) shown by dynamic enhanced MRI was an adverse prognostic indicator of progress-free survival (PFS) and OS, suggesting a poor response to neoadjuvant therapy [Citation11]. Satoh et al. evaluated the correlation between tumor blood flow and the malignancy of gastric cancer using perfusion CT and found that patients with advanced tumor depth, peritoneal dissemination and undifferentiated subtypes had significantly reduced blood flow. This decrease in blood flow value may reflect a progressive state of gastric cancer [Citation12]. Similar results were also found in the study by Hayano et al., where tumors with low blood flow showed a significant tendency for lymph node metastasis, vascular invasion, lymphatic invasion and distant metastasis. Patients with high tumor blood flow had significantly longer survival compared with those with low tumor blood flow. Compared with arteries, venous vessels have thinner walls and greater expandability, allowing for larger volume changes with smaller pressure variations [Citation13]. Proximal venous obstruction and distal blood flow increase are the two main mechanisms of venous dilatation. Therefore, it is reasonable to speculate that the dSRV in rectal cancer is influenced by tumor blood flow, with rectal cancer patients with lower tumor blood flow having a smaller dSRV and poorer prognosis.
Metastasis is the most important cause affecting the survival of cancer patients. Hypoxia and nutrient deficiency in the tumor microenvironment affect the occurrence of tumor metastasis [Citation14]. The rapid proliferation of tumor cells requires oxygen support, but the newly formed tumor blood vessels have poor quality, incomplete vascular wall structure, disorganized vascular network and oxygen diffusion distance smaller than the capillary spacing, resulting in hypoxia within the tumor [Citation15]. The risk of metastasis and death increases with the degree of tumor hypoxia [Citation16–18]. Hypoxia induced the expression of hypoxia-inducible factors 1 (HIF-1) and 2 (HIF-2), which directly or indirectly participate in epithelial-mesenchymal transition (EMT) [Citation19,Citation20]. During the process of EMT, circulating tumor cells were generated from the primary tumor and subsequently invaded distant organs through the bloodstream and establish colonization [Citation21]. Tumors with poor blood perfusion has less oxygen and nutrients, leading to more severe tumor hypoxia, prompting tumor cells to leave the primary site and seek more suitable survival niches [Citation11]. Patients who developed metastasis after treatment had a smaller dSRV compared with non-metastatic, which may be associated with lower tumor blood flow in patients who are likely to experience metastasis.
In previous studies, larger dSRV was often associated with poor prognostic factors. Wu et al. retrospectively analyzed the differences in dSRV among 76 rectal cancer patients in various pathological states. The results revealed that patients with lymphovascular emboli had a significantly larger dSRV compared with those without (4.2 mm vs 3.0 mm, p < 0.0005). Patients with distant metastasis and lymph node positivity also exhibited a larger dSRV [Citation6]. Kang et al. also reported that patients with liver metastasis had a significantly larger dSRV compared with patients without (5.35 ± 1.49 vs 4.71 ± 1.12 mm, p = 0.0007) [Citation8]. Li et al. found that patients with lymph node metastasis had a larger dSRV compared with lymph node-negative patients (4.34 ± 0.57 vs 3.94 ± 0.66 mm, p < 0.001) [Citation7]. Song et al. discovered that the dSRV measured on CT images could predict the KRAS status, with KRAS-positive patients having a significantly larger dSRV than KRAS-negative patients (4.62 ± 0.94 vs 4.19 ± 0.82 mm, p = 0.02) [Citation9]. Interestingly, our study showed conflicting results, where patients with a smaller dSRV diameter (<3.6 mm) had a higher risk of distant metastasis and poorer prognosis during follow-up. The reason for this discrepancy may be related to the study population, as previous studies included patients who already had existing metastasis at the time of analysis, while the population selected in this study consisted of stage II–III rectal cancer patients, with distant metastasis occurring during postoperative follow-up.
EMVI has been identified as a poor prognostic factor independent of the staging system for rectal cancer [Citation22–24]. Several studies have shown that changes in the diameter of the superior rectal vein and inferior mesenteric vein (IMV) may be CT manifestations of EMVI. The superior rectal vein and inferior mesenteric vein are the main drainage veins of the rectum. Venous thrombosis and increased venous drainage may affect the diameter of the SRV and IMV in patients with rectal cancer with positive EMVI [Citation6]. It has been found that when dSRV is restricted to 3.95 mm, EMVI can be properly diagnosed with high accuracy, sensitivity (93.30%) and specificity (67.90%), and the area under the curve was 0.85 ± 0.05 (p < 0.001) [Citation10].
Extramural venous invasion (EMVI) defined by MRI was associated with risk of tumor recurrence and survival [Citation25], but due to the limited resolution of MRI images, if there are tumors in perirectal lymphatic vessels, the evaluation of EMVI on MRI may lead to false-positives. And recent studies had shown that the specificity of diffusion-weighted imaging in diagnosing EMVI can reach up to 96%, but the sensitivity was only 55% [Citation26]. In addition, on T2-weighted imaging, tumor subserosal invasion without perirectal vein invasion, connective tissue hyperplasia or disappearance of the perirectal vein signal cavity were other potential sources of false-positive MRI results [Citation27,Citation28]. Therefore, on the basis of the current diagnosis, increasing the index of superior rectal vein diameter may increase the accuracy of EMVI diagnosis.
The response to neoadjuvant therapy can be predicted by measuring and comparing the diameter changes of SRV and IMV pre- and post-neoadjuvant therapy [Citation4,Citation29]. Ivan et al. proposed that the IMV diameter may be used to evaluate the response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer and may be a potential surrogate marker. Compared with non-responders, the mean IMV diameter of responders decreased by 17.54%, and that of non-responders increased by 1.39%, p = 0.0001 [Citation4]. Due to the lack of abdominal and pelvic CT data after neoadjuvant therapy, the changes in superior rectal vein diameter pre- and post-neoadjuvant therapy were not compared.
Through neoadjuvant chemoradiotherapy followed by optimized total mesorectal excision, the local recurrence rate of locally advanced rectal cancer was significantly reduced to equal or less than 5% [Citation30–32]. Distant metastasis has become the main pattern of treatment failure for rectal cancer. The study reported that approximately 15–24.4% of patients had metastasis within 5 years after the operation [Citation33,Citation34]. In this study, the 4-year cumulative local recurrence rate was 1.60%, and approximately 16.90% of patients had distant metastasis, which was basically consistent with previous studies. Better control of systemic diseases has become the major challenge in the treatment of LARC.
There are still some limitations in this study. First, as a retrospective study, it only included patients treated at our institution, which may introduce some degree of selection bias and retrospective bias. Secondly, since abdominal CT was not a routine examination after treatment for rectal cancer, there was a lack of comparative information on dSRV before and after treatment. Furthermore, tumor deposits in rectal cancer was independent prognostic factors separate from ypN [Citation35], and the pathology report did not separate tumor deposits from ypN, which was another limitation of this study. Finally, due to the small diameter of the superior mesenteric vein in the rectum, there may be some vascular variations, leading to measurement bias in the diameter of the superior mesenteric vein. However, the study design specifically required precise measurement levels and the average value was obtained through independent measurements by two physicians to minimize measurement bias. Therefore, before applying it to daily practice, further research with larger-scale, multicenter samples and more stages is needed.
Conclusion
The hemodynamic changes in rectal cancer lesions may be reflected by measuring the diameter of the superior rectal vein, which is a potential prognostic indicator of concern.
When the tissue or organ undergoes pathological changes, its blood reflux and the diameter of its draining vein will change.
Previous studys indicated that the diameter of superior rectal vein (dSRV) can not only be used as a predictor of lymphovascular emboli and lymph node metastasis of rectal cancer, but also has certain value in judging liver metastasis and predicting KRAS mutation of rectal cancer. However, survival data were immature.
In this study, patients whose dSRV >3.60 mm had better 3 years metastasis-free survival (MFS; 87.70% vs 68.2%, p < 0.001), 3 years disease-free survival (DFS; 85.50% vs 64.2%, p < 0.001) and 3 years overall survival (OS; 91.90% vs 82.20%, p = 0.005).
The dSRV and extramural venous invasion (EMVI) were independent prognostic factors for OS and the dSRV >3.60 mm had better OS (HR = 1.61, 95% CI: 1.04–2.49).
The dSRV and post-neoadjuvant pathologic node stage (ypN) were independent prognostic factors for DFS and the dSRV >3.60 mm had better DFS (HR = 1.52, 95% CI: 1.09–2.10).
The dSRV, EMVI and ypN were independent prognostic factors for MFS and the dSRV >3.60 mm had better MFS (HR = 1.41, 95% CI: 1.01–1.97).
The hemodynamic changes in rectal cancer lesions may be reflected by measuring the diameter of the superior rectal vein, which is a potential prognostic indicator of concern.
Author contributions
Contributions to the study conception or design: B Xu, A Li. Contribution to the acquisition, analysis, or interpretation of data: Y Hong, R Chen. Drafting of the manuscript or revising it critically for important intellectual content: Y Hong, A Li. Final approval of the version to be published: all authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all authors.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
Although patient consent was not specifically obtained for this analysis, all information was retrospectively extracted in the context of compliance with the ethical standards of the institutional and/or national research committees and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Patient medical records were analyzed retrospectively, with no individual patient identifiable information used. Thus, the Fujian Medical University Union Hospital Ethics Review Board deemed patient consent unnecessary.
Acknowledgments
The authors thank the patients and their families and caregivers for their participation.
Financial disclosure
This work was supported by National Natural Science Foundation of Fujian Province, China (2022J02037) and Fujian provincial health technology project (No. 2021CXA011). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- Siegel RL , Miller KD , Fuchs HE , Jemal A . Cancer Statistics, 2021. CA Cancer J. Clin. 71(1), 7–33 (2021).
- van Gijn W , Marijnen CA , Nagtegaal ID et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 12(6), 575–582 (2011).
- Sauer R , Liersch T , Merkel S et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 30(16), 1926–1933 (2012).
- Ivan CV , Mullineux JH , Verma R et al. Assessment of the inferior mesenteric vein diameter as a surrogate marker to evaluate response to neoadjuvant chemoradiotherapy for locally advanced rectal adenocarcinoma. Colorect. Dis. 19(12), 1076–1080 (2017).
- Dujovny N , Quiros RM , Saclarides TJ . Anorectal anatomy and embryology. Surg. Oncol. Clinic. N. Am. 13(2), 277–293 (2004).
- Wu CC , Lee RC , Chang CY . Prediction of lymphovascular invasion in rectal cancer by preoperative CT. Am. J. Roentgenol. 201(5), 985–992 (2013).
- Li X , Song C , Cai H et al. Correlation between the diameter of superior rectal vein and inferior mesenteric vein and the lymph node metastasis of rectal carcinoma. J. Sun Yat-Sen Univ. (6), 538–544 (2017).
- Kang KA , Jang KM , Kim SH , Kang TW , Cha DI . Risk factor assessment to predict the likelihood of a diagnosis of metastasis for indeterminate hepatic lesions found at computed tomography in patients with rectal cancer. Clin. Radiol. 72(6), 473–481 (2017).
- Song C , Shen B , Dong Z et al. Diameter of superior rectal vein - CT predictor of KRAS mutation in rectal carcinoma. Cancer Manag. Res. 12, 10919–10928 (2020).
- Gursoy Coruh A , Peker E , Elhan A , Erden I , Erden A . Evaluation of extramural venous invasion by diffusion-weighted magnetic resonance imaging and computed tomography in rectal adenocarcinoma. Can. Assoc. Radiol. J. 70(4), 457–465 (2019).
- Bakke KM , Meltzer S , Grøvik E et al. Sex differences and tumor blood flow from dynamic susceptibility contrast MRI are associated with treatment response after chemoradiation and long-term survival in rectal cancer. Radiology 297(2), 352–360 (2020).
- Satoh A , Shuto K , Okazumi S et al. Role of perfusion CT in assessing tumor blood flow and malignancy level of gastric cancer. Dig. Surg. 27(4), 253–260 (2010).
- Hayano K , Shuto K , Koda K , Yanagawa N , Okazumi S , Matsubara H . Quantitative measurement of blood flow using perfusion CT for assessing clinicopathologic features and prognosis in patients with rectal cancer. Dis. Colon Rectum 52(9), 1624–1629 (2009).
- Polacheck WJ , Zervantonakis IK , Kamm RD . Tumor cell migration in complex microenvironments. Cell. Mol. Life Sci. 70(8), 1335–1356 (2013).
- Sorg BS , Hardee ME , Agarwal N , Moeller BJ , Dewhirst MW . Spectral imaging facilitates visualization and measurements of unstable and abnormal microvascular oxygen transport in tumors. J. Biomed. Opt. 13(1), 014026 (2008).
- Vaupel P , Mayer A , Hockel M . Tumor hypoxia and malignant progression. Methods Enzymol. 381, 335–354 (2004).
- Vaupel P . Prognostic potential of the pre-therapeutic tumor oxygenation status. Adv. Exp. Med. Biol. 645, 241–246 (2009).
- Vaupel P , Hockel M , Mayer A . Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal 9(8), 1221–1235 (2007).
- Gilkes DM , Semenza GL , Wirtz D . Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat. Rev. Cancer 14(6), 430–439 (2014).
- Lee KE . Hypoxia as a regulator of tumor stroma and metastasis. Am. J. Physiol. Cell Physiol. 324(1), C10–C13 (2023).
- Gupta GP , Massague J . Cancer metastasis: building a framework. Cell 127(4), 679–695 (2006).
- Yao X , Yang SX , Song XH , Cui YC , Ye YJ , Wang Y . Prognostic significance of computed tomography-detected extramural vascular invasion in colon cancer. World J. Gastroenterol. 22(31), 7157–7165 (2016).
- Dighe S , Swift I , Magill L et al. Accuracy of radiological staging in identifying high-risk colon cancer patients suitable for neoadjuvant chemotherapy: a multicentre experience. Color. Dis. 14(4), 438–444 (2012).
- Chand M , Swift RI , Tekkis PP , Chau I , Brown G . Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer. Br. J. Cancer 110(1), 19–25 (2014).
- Zhang XY , Wang S , Li XT et al. MRI of extramural venous invasion in locally advanced rectal cancer: relationship to tumor recurrence and overall survival. Radiology 289(3), 677–685 (2018).
- Kim TH , Firat C , Thompson HM et al. Extramural venous invasion and tumor deposit at diffusion-weighted MRI in patients after neoadjuvant treatment for rectal cancer. Radiology 308(2), e230079 (2023).
- Ahn JH , Kim SH , Son JH , Jo SJ . Added value of diffusion-weighted imaging for evaluation of extramural venous invasion in patients with primary rectal cancer. Br. J. Radiol. 92(1096), 20180821 (2019).
- Bae JS , Kim SH , Hur BY et al. Prognostic value of MRI in assessing extramural venous invasion in rectal cancer: multi-readers’ diagnostic performance. Eur. Radiol. 29(8), 4379–4388 (2019).
- Zhu H , Wei Z , Huang W , Chen Z . Application value of diameter change of superior rectal vein and inferior mesenteric vein by CT examination in the efficacy evaluation of neoadjuvant therapy for locally advanced rectal cancer. Chinese J. Digest. Surg. 08, 797–802 (2019).
- Sauer R , Becker H , Hohenberger W et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N England J. Med. 351(17), 1731–1740 (2004).
- Gerard JP , Conroy T , Bonnetain F et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J. Clin. Oncol. 24(28), 4620–4625 (2006).
- Rodel C , Graeven U , Fietkau R et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, Phase III trial. Lancet Oncol. 16(8), 979–989 (2015).
- Wang F , Fan W , Peng J et al. Total mesorectal excision with or without preoperative chemoradiotherapy for resectable mid/low rectal cancer: a long-term analysis of a prospective, single-center, randomized trial. Cancer Commun (London). 38(1), 73 (2018).
- Tang J , Wu X , Bai Y et al. Long-term outcome of oxaliplatin and capecitabine (XELOX) concomitant with neoadjuvant radiotherapy and extended to the resting period in high risk locally advanced rectal cancer. Journal of Cancer 9(8), 1365–1370 (2018).
- Agger E , Jorgren F , Joud A , Lydrup ML , Buchwald P . Negative prognostic impact of tumor deposits in rectal cancer: A National Study Cohort. Ann. Surg. 278(3), e526–e533 (2023).