Abstract
Aim: To assess real-world management of patients diagnosed with hepatocellular carcinoma (HCC) within an integrated delivery network. Materials & methods: A retrospective cohort analysis of adults newly diagnosed with HCC from January 2014 to March 2019. Overall survival and treatment journey were assessed over the entire available follow-up period per patient. Results: Of the 462 patients, 85% had ≥1 treatment. The 24-month overall survival rate (95% CI) from first treatment was 77% (72–82%). Majority of Child-Pugh class A (71%) and B (60%) patients received locoregional therapy first. Half (53.6%) of the patients with liver transplantation first were Child-Pugh class C patients. Sorafenib was the predominant systemic therapy. Conclusion: This integrated delivery network data analysis offers a comprehensive insight into the real-world management of HCC.
Hepatocellular carcinoma (HCC) is the most common form of liver cancer and accounts for 80–90% of cases [Citation1]. Worldwide, liver cancer is the sixth most prevalent cancer and the fourth leading cause of cancer related deaths [Citation2]. In the USA, HCC is designated as an orphan disease, but the incidence of liver cancer and thus HCC has more than tripled since 1980 to approximately nine per 100,000 people; it continues to rise an average of 1.7% per year, principally in relation to the spread of the HCV [Citation1,Citation3–5]. Notably, despite a relatively low incidence, the morbidity associated with HCC is significant, and it is the sixth leading cause of cancer related death in the US [Citation5].
The management of patients with HCC is often challenging due to underlying liver disease and the complex anatomy of the liver. The overall prognosis of patients is poor, with 5-year relative survival rates ranging from 34.2% with early-stage disease to 2.5% with advanced or metastatic disease [Citation5]. Clinical practice guidelines recommend that patients with early-stage disease and moderate to severe cirrhosis/liver dysfunction, be evaluated for orthotopic liver transplantation as initial treatment, as it addresses the malignancy and removes the underlying diseased liver, although this approach is frequently limited by organ availability [Citation6,Citation7]. The most recent European Association for the Study of the Liver (EASL) guidelines note that liver function should be evaluated beyond the conventional Child-Pugh staging [Citation7]. Additional curative treatment options include surgical resection and local tumor ablation and are reserved for patients with Child-Pugh class A and tumors amenable to these modalities [Citation8]. Because the majority of patients with HCC will not be surgical candidates for curative-intent treatment, locoregional (LR) therapies, such as radiofrequency ablation, transarterial chemoembolization (TACE) and transarterial radioembolization, have gained increasing significance. LR therapies are employed in a variety of curative- and palliative-intent methods, including bridging the waiting time to transplantation and downstaging advanced tumors to fulfill criteria of resection or transplantation [Citation6,Citation8].
For untreated patients with advanced-stage HCC, the median overall survival (OS) is approximately 6 months [Citation6]. Historically, no systemic standard of care therapy was available for patients with advanced HCC. In 2007, sorafenib, a multikinase inhibitor, became the first US FDA-approved systemic agent for the treatment of unresectable HCC based on results from the phase III SHARP trial, which demonstrated a 31% reduction in the risk of death with sorafenib compared with placebo (hazard ratio [HR]: 0.69; p < 0.01) and a median OS of 10.7 versus 7.9 months, respectively [Citation9,Citation10]. Since then, five other therapies have been FDA approved for advanced HCC based on phase III trial data, including atezolizumab + bevacizumab, lenvatinib, regorafenib, cabozantinib and ramucirumab and three have received accelerated approval (nivolumab, pembrolizumab and nivolumab + ipilimumab) [Citation11–18]. However, in July 2021, nivolumab was voluntarily withdrawn for the indication from the US market following the FDA’s evaluation of confirmatory data for checkpoint inhibitors that have not met their post-marketing requirements.
Because of the complex treatment paradigm associated with HCC, including multiple surgical, LR and systemic options, it is important to characterize the recent treatment landscape. In this study, we assessed the characteristics and real-world management of patients in the USA diagnosed with HCC within an integrated delivery network (IDN).
Materials & methods
Study design & data source
This was a retrospective cohort study in adult patients newly diagnosed with HCC. The study used clinical and administrative data sourced from the Henry Ford Health System (HFHS), a US-based comprehensive, integrated, nonprofit, managed care healthcare organization. HFHS is a large, vertically integrated healthcare system located in the greater Detroit, Michigan region. As a nonprofit corporation consisting of five hospitals, over 30 medical centers and over 1200 physicians in 40 medical specialties, HFHS provides care for approximately 800,000 southeastern Michigan residents. The HFHS Virtual Data Warehouse is the standard, decentralized data model developed for research and used by HFHS with its HealthCare Systems Research Network partners and with other research collaborations. The HFHS Enterprise Data Warehouse contains additional patient data from the electronic medical record that is mined for research [Citation19].
Patient & cohort identification
The study period was from 1 July 2013 through 30 June 2019 (Supplementary Figure 1). Adult patients aged ≥18 years newly diagnosed with HCC (identified using International Classification of Diseases for Oncology, 3rd Edition [ICD-O-3] histology codes: 8170, 8171, 8172, 8173, 8174, 8175) between 1 January 2014 and 31 March 2019 (identification period) were included. The earliest recorded HCC diagnosis date during the identification period was deemed the index diagnosis date; the corresponding year was deemed the index year. Patients were excluded if they were diagnosed with other primary cancers (identified using International Classification of Diseases, 9/10th Revision, Clinical Modification [ICD-9/10-CM] codes) during the study period, were treated for HCC prior to the index diagnosis date, had <60 days of follow-up data (patients who died within 60 days were not excluded) or were likely referred to HFHS for advanced care (defined as those having >30 days between their index diagnosis date and their HFHS medical record number assignment date and/or first encounter date at the IDN) (Supplementary Figure 2). The follow-up period spanned from the index diagnosis date through the earliest date of death, loss to follow-up (maximum of last activity date and enrollment date) or study end date (30 June 2019). Patients who died within 14 days after their last activity/enrollment date were followed until the date of death.
Patient demographics, including age, gender, race, ethnicity, follow-up time (in days) and study index year, were defined as of the index diagnosis date. Additional clinical characteristics assessed included vitals (as of closest valid record within ±30 days of the index diagnosis date) including height (in inches), weight (in pounds) and BMI; specific and Elixhauser comorbidities defined using ICD-9/10-CM diagnosis codes captured in the 6 months prior to and 2 months after the index diagnosis date; tumor-specific characteristics including stage at diagnosis as recorded on the index diagnosis date per the American Joint Committee on Cancer (AJCC) tumor node metastasis (TNM) classification system; laboratory procedures and values, including total bilirubin, total albumin, prothrombin time/international normalized ratio, as of the closest valid record within the 60 days before or after the index diagnosis date; and Child-Pugh classification using an algorithm described in Supplementary Table 1.
Outcomes
Non-systemic treatment modalities identified during follow-up included liver transplantation, liver resection/excision and LR therapy (Supplementary Table 2). LR therapies were composed of liver ablation; chemoembolization, including TACE or drug-eluting bead chemoembolization (DEB-TACE); radioembolization (including brachytherapy); transarterial embolization without chemotherapy or radiation therapy; and external beam radiation therapy. Systemic therapies recorded included tyrosine kinase inhibitors, immuno-oncology therapies, other targeted therapies and chemotherapy. Everolimus was not deemed a systemic therapy for this population (but instead as an immunosuppressant used after transplantation). The ICD-9/10 Procedure Coding System, Current Procedural Terminology, 4th Edition, Healthcare Common Procedure Coding System and other codes used to identify treatments (codes in Supplementary Table 2) and the treatment journey for each patient were reviewed by a board-certified oncology nurse for validity before finalization.
OS in days was measured from both the index diagnosis date and treatment initiation date to the date of death.
Statistical analysis
This study was exploratory; all objectives were assessed using descriptive methods and standard summary statistics. For continuous measures, means ± standard deviations (SDs), medians and 25th and 75th percentiles are provided, and for categorical measures, the number and percent of patients are presented. All analyses were conducted using SAS Enterprise Guide v.9.1 (SAS Institute, Inc, NC, USA.). Median survival time and survival rates at 18 and 24 months from diagnosis and treatment initiation, respectively, were computed using Kaplan–Meier methods. Patients who were alive until the end of follow-up were censored at the earliest date of loss to follow-up or the study end date.
Results
Patient characteristics
Of 637 patients diagnosed with HCC, a total of 462 (72.5%) were included in the final sample for analysis (Supplementary Figure 2) after applying all the inclusion/exclusion criteria. Most patients were excluded due to evidence of other primary malignant cancers (15.5%). The mean age was 65.2 years (SD: 9.7) and the majority (71.0%) of patients were >61 years old (). The population was composed of predominantly male (76.2%), White patients (56.3%) who were overweight (mean BMI: 29.2 [SD: 6.2]). The mean follow-up time was 18.8 months (SD: 15.8; median = 14.1).
Table 1. Baseline demographic and clinical characteristics.
Of the 462 patients, 67.8% (n = 313) had ≥6 months of data available prior to the index diagnosis date; Elixhauser comorbidity information was only reported for this subset of patients. The mean total Elixhauser comorbidities per patient was 3.7 (SD: 2.4), with 59.7% of the sample having evidence of ≥3 comorbidities at index HCC diagnosis. The top five comorbidities (apart from liver disease) were hypertension (44.1%), alcohol abuse (32.6%), diabetes (29.1%), fluid and electrolyte disorders (20.8%) and depression (20.8%). Of the other specific comorbidities evaluated among the entire sample (N = 462), hepatitis C (53.7%), ascites (28.4%), and non alcoholic steatohepatitis (10.4%) were recorded in >10% of the sample.
The majority of the patients were diagnosed with stage I or II HCC (64.9%), with 19% of patients having an unknown/unspecified AJCC TNM stage at diagnosis. As per the computed Child-Pugh Classification, 33.6% of patients were classified as Class A, 18.0% as Class B, 13.4% as Class C and 35.1% as unknown (due to unavailability of a valid albumin, bilirubin and/or prothrombin laboratory result within ±60 days of index diagnosis date).
Treatment journey & patterns
In total, 84.6% of patients (n = 391/462) had a recorded HCC related treatment during the follow-up period. The first recorded treatments are summarized in (A–D). LR therapy was the most frequently used first treatment in this sample in approximately three-fourths of patients (74.7%; n = 292) (A). Of the patients who had LR therapy first, chemoembolization was the most frequently used LR therapy in 56.8% of patients (B).
(A) First recorded treatment. (B) First recorded locoregional therapy. (C) Child-Pugh classification at diagnosis by first recorded treatment. (D) First recorded treatment by Child-Pugh classification at diagnosis.
EBRT: External beam radiation therapy; LR: Locoregional; TAE: Transarterial embolization.
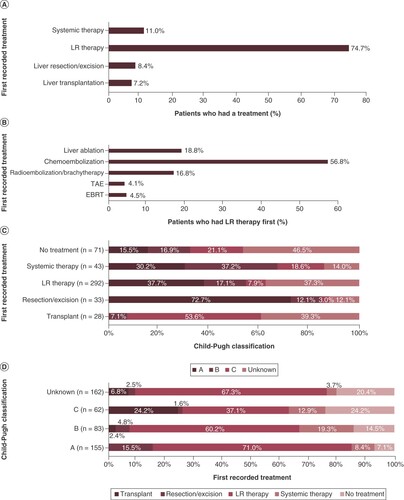
Approximately three-fourths (72.7%) of patients who underwent a liver resection/excision first were Child-Pugh Class A patients, and 37.7% of the patients who had LR therapy first were Class A patients (C). Higher proportions of patients who underwent a liver resection/excision and LR therapy first were Class A and/or AJCC TNM Stage I or II (data not shown) compared with patients who had systemic therapy first. A majority of the patients who underwent liver transplantation first were Child-Pugh Class C (53.6%) and/or AJCC TNM Stage I or II (57.2%) patients. Conversely, a majority of the patients deemed as Child-Pugh Class A (71.0%) and B (60.2%) had LR therapy first (D). The predominant subsequent therapy following LR therapy varied by Child-Pugh Class at diagnosis. Among Class A patients who received LR therapy first and had a recorded subsequent therapy, 68% received LR therapy again; similarly, among Class B patients, 44% underwent a liver transplantation subsequent to the LR therapy; and among Class C patients, 44% had systemic treatment subsequent to the LR therapy (percentages reported are out of patients with known subsequent therapy following first LR therapy).
The mean time to first recorded treatment was 65.7 days (SD: 76.9; median: 44) following the index HCC diagnosis date but varied by treatment modality. The mean time to a liver resection/excision was 33.1 days (SD: 24.0; median: 28) following diagnosis, whereas the mean time for a transplantation was 128.4 (SD: 155.3; median: 56.5) days following diagnosis.
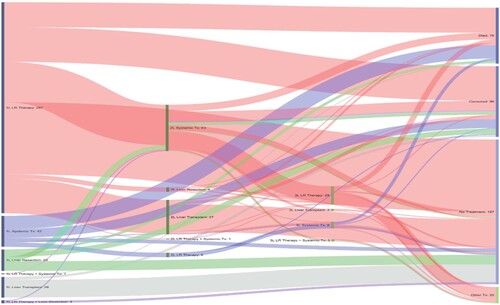
The treatment journey of all treated patients (n = 391) is detailed in . A third of the patients who received LR therapy first had no subsequent treatment for ≥6 months since last receipt of LR therapy (patients were alive and had data for ≥6 months from last recorded treatment) and 13% underwent a subsequent transplantation followed by no additional treatments for ≥6 months; the rest were either lost to follow-up or progressed to second-line systemic therapy. The majority of patients who received liver transplantation as first treatment or subsequent to initial LR therapy had no subsequent treatment documented during the follow-up period even though they were alive, and their data were available for ≥6 months after the procedure. Patients who received second-line systemic therapy had additional rounds of LR therapy and other subsequent treatment. Most patients who received systemic therapy in first-line treatment, likely indicating advanced disease, died during the study period.
Patients who were alive but had <6 months of follow-up from last treatment were censored. Patients who were alive and had data for ≥6 months from last treatment, but no recorded treatments were classified as ‘no treatment’.
1L: First line; 2L: Second line; 3L: Third line; LR: Locoregional; TX: Treatment.
Overall survival
Of the 462 patients, 118 (25.5%) died during follow-up. The median OS, from both diagnosis and treatment initiation, was not reached. The 18- and 24 month OS rates from diagnosis were 74.3% (95% CI: 69.6–78.3) and 72.1% (95% CI: 67.2–76.4), respectively. The 18 and 24-month OS rates from treatment initiation were 79.0% (95% CI: 74.2–83.1) and 77.3% (95% CI: 72.2–81.7), respectively. The 24-month OS rates varied by known Child-Pugh Class at diagnosis: Class A = 84.5% (95% CI: 76.4–89.9), Class B = 65.3% (95% CI: 53.0–75.1) and Class C = 47.9% (95% CI: 34.1–60.5). It is important to note that most of the patients in this sample were diagnosed with early-stage disease, which may affect the OS results.
Discussion
This retrospective, observational cohort study was conducted using clinical and administrative data sourced from a US-based comprehensive, integrated, nonprofit managed care organization to assess the characteristics and real-world management of patients diagnosed with HCC. The overall goal was to summarize clinically rich information on patients’ treatment journeys from initial diagnosis of HCC. The manuscript does not aim to provide interventional radiologists any guidance/best practices for performing chemoembolization as this is outside of the intended scope.
Our findings demonstrated that LR therapy, specifically chemoembolization, was the most frequently used first treatment modality. Only a small number of patients received systemic therapy as first treatment. These results are not surprising considering the majority of patients in our sample were diagnosed with early-stage disease.
The HCC BRIDGE study was a retrospective chart review that analyzed patients with HCC from January 2005 to September 2012 globally at 42 sites in 14 countries [Citation20]. A total of 2326 patients were included from four sites in North America (two sites in the US). Child-Pugh distribution in the North American BRIDGE study population was 63% Class A, 20% Class B, 5% Class C and 12% unknown compared with 34% Class A, 18% Class B, 13% Class C and 35% unknown in our study. The differences in the distribution may be attributable to a combination of factors; our study did not curate data from patient charts, possibly leading to higher missingness and the North American BRIDGE study population included patients from two Canadian sites in addition to the two US sites.
Similar to our results, the most frequent first recorded treatment in the North American BRIDGE population was chemoembolization, specifically TACE (33% in BRIDGE study vs 42.5% of the treated population in our study). The most common second therapy following TACE in the North American BRIDGE population was liver transplantation (32% in BRIDGE study vs 22% of patients with a known second therapy following TACE in our study); both studies were unable to distinguish if TACE was being utilized as a neoadjuvant therapy or preoperative therapy prior to transplant. These results highlight the continued utility of LR therapy for early-stage disease in patients amenable to these modalities and the continued importance of attempting potentially curative therapies.
The BRIDGE study authors also reported that the proportion of patients who received transplant with Barcelona Clinic Liver Cancer stage D disease was surprisingly high, potentially reflective of liver transplant in patients with Child-Pugh C disease who otherwise have liver disease meeting Milan criteria for transplantation. While we did not classify our population into Barcelona Clinic Liver Cancer stages and cannot directly compare to the BRIDGE study, we did observe that the majority of patients who received liver transplantation as first therapy in our study had Child-Pugh Class C disease (53.6%). This is aligned with recommendations from practice guidelines but reflects the limited utility of the Child-Pugh score alone to determine eligibility for liver transplantation [Citation21]. The 2022 EASL guidelines highlight the importance of insight- and knowledge-based continued personalized clinical management integrating baseline patient profiles and evolutionary events [Citation7].
Treatment guidelines for advanced HCC were updated in 2020 and replicating this analysis using more recent data may highlight this shift in the treatment paradigm and, subsequently, in outcomes. During the timeframe of this study (2013–2019), sorafenib and lenvatinib were the only FDA approved therapies for first-line treatment of advanced HCC [Citation22]. Of the patients who received systemic therapy in our population first (presumed to have advanced HCC), 95% were treated with sorafenib and 47% died within 6 months of their last treatment. This may be reflective of several factors such as the majority of the study sample having early-stage disease leading to a small sample size that received systemic therapy, treatment approvals occurring during our treatment period or slow uptake of novel therapies.
However, this is an important consideration as lenvatinib and atezolizumab + bevacizumab have shown noninferior and superior outcomes, respectively, when compared with sorafenib in first-line treatment and are likely to be increasingly utilized in advanced HCC [Citation14,Citation15]. In a 2020 update to the American Society of Clinical Oncology (ASCO) guidelines for systemic therapy for advanced HCC, recommendations for first-line therapy included atezolizumab + bevacizumab in patients with Child-Pugh class A, Eastern Cooperative Oncology Group performance status of 0–1, and managed esophageal varices, and tyrosine kinase inhibitors such as sorafenib or lenvatinib in patients with contraindications to atezolizumab and/or bevacizumab [Citation23].
The addition of novel therapies in the first line has also had an impact on second-line treatment options and the timing/sequencing of switches. While our study population primarily received LR therapies first, likely due to early-stage disease, it will be important to continue to assess real-world populations of HCC patients to evaluate patterns of use and the impact of novel systemic therapies on outcomes of patients with advanced HCC.
Limitations
This was a retrospective analysis and is limited by the availability of data in the HFHS database. There are inherent limitations in such studies including misclassifications resulting from inaccurate or missing data. For example, data for patient care received outside of the IDN are not available, including survival data for patients who leave the practice. Further, reasons for discontinuation were not available and therefore it is unknown if the observed treatment changes were due to unsuccessful treatment or relapse of disease.
The HFHS database is limited to providers and practices that are included in the HFHS network and, as such, our results may not be generalizable to all patients with HCC treated in the US. Similarly, the treatment patterns and outcomes of patients outside this study may differ in settings with mandated treatment pathways such as academic or international settings. Treatment sequences are influenced by the follow-up time available for each patient; thus, while all patients were followed for a minimum of 60 days (unless they died), they had variable follow-up times (median: 14.1 months; interquartile range: 5.5, 26.4). This may have influenced whether or not a patient received a specific intervention, such as resection or liver transplant, during the observed follow-up time.
Finally, this study only included patients with data up to 30 June 2019. Treatment guidelines have been updated since this time period and; therefore, the results may not reflect the current treatment paradigm but are nonetheless important for comparison with future research to document the shift in the treatment paradigm.
Conclusion
HCC in the US is a complex, heterogenous disease state that includes a high prevalence of patients with cirrhosis in addition to HCC. This creates, in effect, two disease states that must be considered when treating these patients. The real-world population presented here was highly comorbid and highlights the importance of baseline population characteristics when comparing health outcomes between studies.
IDN data from organizations such as HFHS have the possibility to offer comprehensive insights into the real-world treatment patterns and management of HCC from diagnosis. This will be imperative to consider as the HCC treatment pathways continue to evolve with the addition of more granularity and sub classifications in staging systems and as novel therapies are introduced to market.
The complex treatment paradigm associated with hepatocellular carcinoma (HCC), including multiple surgical, locoregional and systemic options, necessitates characterization of the recent real-world treatment landscape.
This retrospective cohort analysis used integrated delivery network data to offer comprehensive insights into the routine clinical management of HCC from initial diagnosis by stage and Child-Pugh classification.
The majority (65%) of the patients in the integrated delivery network were diagnosed with stage I or II HCC. Per the computed Child-Pugh classification, 34% of patients were classified as Class A, 18% as Class B, 13% as Class C and 35% as unknown.
The 24-month overall survival rate (95% CI) from first treatment was 77% (72%, 82%).
Most patients (85%) had ≥1 HCC-related treatment recorded post diagnosis during follow-up; mean (median) time to first treatment was 65.7 (44) days.
Locoregional therapy, specifically chemoembolization, was the most frequently used first treatment modality.
Only a small number of patients (n = 43) received systemic therapy as first treatment (likely advanced HCC); 95% were treated with sorafenib and 47% died within 6 months of last treatment.
Treatment guidelines for advanced HCC were updated in 2020. Replicating this analysis using more recent data will help stakeholders highlight this shift in the treatment paradigm and, subsequently, in outcomes.
Author contributions
Authors T Lokhandwala, A Aly, E Farrelly, JP Willey, AD Coutinho and BS Seal were responsible for study concept and design; authors T Lokhandwala, A Aly, E Farrelly, LE Lamerato, AD Coutinho and BS Seal were responsible for data acquisition, authors T Lokhandwala, E Farrelly and JP Willey were responsible for data analysis; all authors were responsible for data interpretation and drafting and revisions of the manuscript.
Ethical conduct of research
The Henry Ford Health System (HFHS) Institutional Review Board approved this study and granted a waiver of the requirements to obtain informed consent.
Supplementary Figure 1. Study Design Schematic
Download MS Word (95.8 KB)Acknowledgments
This study was funded by AstraZeneca PLC. We would like to acknowledge Bridgette Kanz Schroader for her assistance with medical writing.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.tandfonline.com/doi/suppl/10.2217/hep-2021-0011
Financial & competing interests disclosure
This work was funded by AstraZeneca, PLC (AZ). Authors A Aly, M Healey and B Seal were employees of AZ during study conduct and may own company stock. Authors T Lokhandwala, E Farrelly, JP Willey and AD Coutinho were employees of Xcenda, LLC during study conduct and may own company stock. Xcenda LLC received consultancy fees from AZ for study design, analysis and medical writing. LE Lamerato is employed by the Henry Ford Health System. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Medical writing and editorial support were provided by BK Schroader of Xcenda, LLC and were funded by AZ. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- National Organization for Rare Disorders (NORD). Hepatocellular carcinoma (2017). https://rarediseases.org/rare-diseases/hepatocellular-carcinoma/
- BrayF, FerlayJ, SoerjomataramI, SiegelR, TorreL, JemalA. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2004).
- American Cancer Society (ACS). Liver and intrahepatic bile duct (2020). https://cancerstatisticscenter.cancer.org/#!/cancer-site/Liver%20and%20intrahepatic%20bile%20duct
- YangJ, HainutP, GoresGet al. A global view of hepatocellular carcinoma: trends, risks, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 16(10), 589–604 (2019).
- Surveillance, Epidemiology, and End Results (SEER). SEER cancer stat facts: liver and intrahepatic bile duct cancer. (2020). https://seer.cancer.gov/statfacts/html/livibd.html
- LlovetJM, KelleyRK, VillanuevaAet al. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 7(1), 6 (2021).
- ReigM, FornerA, RimolaJet al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J. Hepatol. 76, 681–693 (2022).
- LurjeI, CziganyZ, BednarschJet al. Treatment strategies for hepatocellular carcinoma – a multidisciplinary approach. Int. J. Mol. Sci. 20(6), 1465 (2019).
- Nexavar prescribing information. Bayer HealthCare (2018). www.accessdata.fda.gov/drugsatfda_docs/label/2018/021923s020lbl.pdf
- LlovetJM, RicciS, MazzaferroVet al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 3359(4), 378–390 (2008).
- Abou-AlfaGK, MeyerT, ChengALet al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379, 54–63 (2018).
- BruixJ, QinS, MerlePet al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, Phase III trial. Lancet 389, 56–66 (2017).
- El-KhoueiryAB, SangroB, YauTet al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase I/II dose escalation and expansion trial. Lancet 389(10088), 2492–2502 (2017).
- FinnRS, QinS, IkedaMet al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
- KudoM, FinnRS, QinSet al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase III non-inferiority trial. Lancet 391, 1163–1173 (2018).
- YauT, KangYK, KimTYet al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 6(11), e204564 (2020).
- ZhuAX, KangYK, YenCJet al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, Phase III trial. Lancet Oncol. 20(2), 282–296 (2019).
- ZhuAX, FinnRS, EdelineJet al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase II trial. Lancet Oncol. 19(7), 940–952 (2018).
- Henry Ford Health Systems. 2019 Fact Sheet (2019). www.henryford.com/newsroom/facts
- ParkJW, ChenM, ColomboMet al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 35, 2155–2166 (2015).
- TsorisA, MarlarCA. Use of the Child Pugh score in liver disease (2022). www.ncbi.nlm.nih.gov/books/NBK542308/
- AichesonG, PillaiA, DahmanB, JohnBV. Recent advances in systemic therapies for advanced hepatocellular carcinoma. Curr. Hepatol. Rep. 20, 23–33 (2021).
- GordanJD, KennedyEB, Abou-AlfaGKet al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J. Clin. Oncol. 38, 4317–4345 (2020).

