Abstract
Aim: To evaluate the safety and efficacy of uncooled TATO microwave ablation (MWA) for primary and metastatic liver cancer. Materials & methods: This was a retrospective study on percutaneous liver ablations performed with TATO MWA. Twenty-five ablations were performed; 11 (44%) were performed for hepatocellular carcinoma, 14 (56%) for colorectal carcinoma, gastric and pancreatic metastases. Results: Adverse events were reported only in one (4%) ablation: an abscess that was observed in the ablated area and was resolved with a percutaneous drainage and antibiotic therapy. Local tumor control rate was 92% at the 3-month follow-up. Conclusion: TATO MWA was safe and effective with high reproducibility in treating primary and secondary liver cancer with satisfactory technical and clinical outcomes.
Plain language summary
The removal of cancer from the liver or liver metastases from cancers in distant sites can be performed safely and effectively with a system that uses a microwave generator (TATO) that allows a good visibility of ablation procedure under intraprocedural real-time ultrasound imaging.
Tweetable abstract
Microwave ablations with the TATO system were safe, reproducible and effective for the treatment of primary and secondary liver cancer and allowed real time visualization of the procedure.
Liver cancer is one of the most common cause of cancer death worldwide and hepatocellular carcinoma (HCC) is the most frequent (85–90%) primary liver cancer [Citation1,Citation2]. The liver is also a common target for metastases both from the liver and from other organs (colon-rectum, stomach and pancreas) [Citation3]. Surgical treatment (resection or liver transplantation) has been considered as first-line treatment of HCC and liver metastases by clinical guidelines, but only 10–20% of liver cancers are resectable and tumor recurrence is observed in 70% of cases at 5 years [Citation4]. HCC most often is diagnosed at the late stage of disease and its prognosis is generally poor, since, limited treatment options are available and have only marginal clinical benefits. Systemic therapy, in particular, is generally ineffective, with the exception of molecular targeted therapies that seem to be a favorable approach for HCC management [Citation5,Citation6].
Recent clinical guidelines recommend local ablation therapy of unresectable HCC or metastatic liver lesions, since it is minimally invasive, repeatable, safe and effective [Citation6]. The most common thermal ablation technologies actually used in clinical settings are radiofrequency ablation and microwave ablation (MWA) that are equally safe and effective for very early and early-stage HCC [Citation7]. MWA is widely applied for the treatment of liver primary tumors and metastases alone or in association with other oncologic treatment [Citation8,Citation9]. There are several different MWA systems available worldwide for the treatment of liver cancer, offering a variety of features and capabilities [Citation8–14].
A recent meta-analysis compares single-antenna and multiple-antenna MWA for the treatment of very early, early HCC and ≤5 cm liver metastases, showing that they have similar effectiveness for local treatment of liver tumors. However, synchronous multi-antenna MWA have better local tumor progression free, complete ablation, 3 year overall survival and major complications than other MWA approaches, particularly for larger (≥2 cm) liver cancers [Citation9]. The use of fine needle-assisted puncture positioning technique for CT-guided MWA may improve outcomes in terms of local tumor progression-free survival and procedure related complications for HCC in comparison to the conventional puncture [Citation14].
Different cooling system (water or CO2) can influence ablation zone volume and applied energy of different MWA devices in HCC and colorectal liver metastases treatment [Citation15]. This issue may be overcome using uncooled systems. One of the latest multiple antenna systems for MWA is represented by TATO (Biomedical, Italy). This is a 2,45 Ghz MW system that is characterized by the absence of a cooling circuit and allows a good visibility of ablation procedure under intraprocedural ultrasound (US) imaging, that is useful to evaluate in real-time the results as well as personalize the parameters in each lesion to achieve a complete treatment. This uncooled system with fine needle has also been effective and safe for the treatment of benign thyroid nodules, preserving patients thyroid function [Citation16]. The primary objective of this study is to assess safety and efficacy of non cooled MWA with TATO system that allows the assessment of ablation result in real time, for the ablation of primary and metastatic liver cancer. Secondary objective was to assess if there is difference in efficacy between primary and metastatic treatment with uncooled MWA.
Materials & methods
Study design & patients selection
This was a retrospective single center study on MWA for primary or secondary liver cancers performed with the novel TATO uncooled system (Biomedical, Florence, Italy) between March 2018 and March 2019 and received approval from our institutional review board. Informed consent was obtained from all the patients. A total of 19 patients were included in the study and a total of 25 MWAs with the TATOPro antenna were performed.
Inclusion criteria: diagnosis of primary or secondary liver cancer, treatment with TATO microwave system. Exclusion criteria: patients who underwent a combined treatment with another technique such as chemoembolization or bland embolization.
Ablation procedures
The MWA system used in this study included a 2.45 GHz microwave generator (TATO, Biomedical, Italy) and an uncooled dedicated probe antenna. The MWA antenna was placed into the nodule along its long axis. Patients were under moderate sedation or preferably general anesthesia. MWA were performed using the following image-guidance: US in 14 (76%) ablations, contrast-enhanced US in five (20%) and ExpertCT with the Philips Angiosuit in six (24%) MWA.
A 2,45 Ghz microwave generator (TATO, Biomedical, Italy) and uncooled 14G or 17G dedicated probe antenna were used for MWA, one to two antennas were used depending on the size of the desired ablative area. In tumors whose maximum diameter is less than 2 cm we use a 17G antenna since it is thinner and achieves an ablative area of sufficient size for these cases. In the rest of the tumors, whose maximum diameter is greater than 2 cm, we used one or two 14G antennas. in particular, 14G was used in 10 (40%) MWA and 17G in 15 (60%) MWA. The energy cable was detachable, this was useful in handling the antenna during positioning. Maximum power applied was 40W/single antenna for 5–15 min depending on the size of the nodule.
Total ablation time was 5 min in six (24%) MWA and 9–15 min in 19 (76%) MWA corresponding to greater tumor size and presence of multiple lesions in both HCC and liver metastases groups ( & ). The schematic representation of MWA was reported in Box 1.
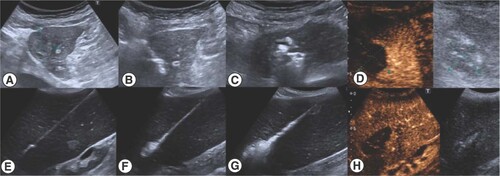
Contrast enhanced ultrasound was performed just a few minutes after the procedure and before it was finished to certify a complete ablation in the same act (A–D) HCC in close relationship with the heart. (E–H) Spleen diffuse ablation in malignant splenomegaly.
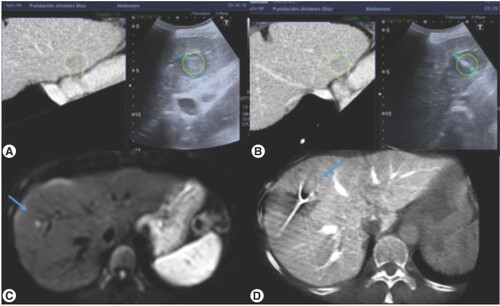
(A & B) Ultrasound CT fusion allowed a real-time synchronization of a recent imaging test where the target was clearly identified with the intraoperative ultrasound (C & D) Expert CT used for the approach of the lesion as well as for the final check of the procedure.
Schematic description of uncooled microwave ablation (MWA) ablation.
Ablation procedures were performed under moderate/deep sedation or preferably general anesthesia.
Different image guidance techniques used: ultrasound (US), contrast-enhanced US and expert computed tomography.
One to two antennas were used depending on the size of the desired ablative area.
In tumors whose maximum diameter is less than 2 cm we usually use a 17 G antenna since it is thinner and achieves an ablative area of sufficient size for these cases. In the rest of the tumors, whose maximum diameter is greater than 2 cm, we used one or two 14G antennas.
Good real-time visibility under US of ablation progression and surrounding structures was achieved due to low power (maximum 40 W per antenna) and low vapor formation.
When the liver lesion could not be visualized with US or contrast-enhanced US method, MWA was guided with expert computed tomography, follow the reference tables of diameters provided by the commercial company (always seeking an ablative area with a safety margin of 1 cm).
When the liver lesion could be visualized with US method, MWA was confirmed at the end of the procedure with the use of US contrast.
Tracking ablation was performed for all lesions treated.
Outcome measures
For the assessment of primary objective: safety, the intensity and type of adverse events were recorded and classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Target tumor size, volume and echogenicity were established with preprocedure imaging with MRI. For the assessment of primary objective efficacy: tumor response was assessed with modified response evaluation criteria (mRECIST) in solid tumors from MRI at 3 months after ablation and was compared with pre procedure imaging. Complete response (CR) was obtained when all target lesions disappeared; partial response (PR) was obtained when the sum of index lesions diameter was reduced ≥30%, otherwise it was considered as stable disease (SD). Progressive disease (PD) was considered when target lesions increased ≥20%, or one/more new lesions appeared. For the assessment of the secondary objective the number of CR, PR, SD and PD of HCC and liver metastases were compared.
Statistical analysis
Data collected were analyzed with descriptive statistical methods. Continuous variables were reported as the mean ± standard deviation (SD), range, frequency and percentage. Chi-square was used for comparison of tumor response of HCC and liver metastases, p < 0.05 was used for statistical significance. Statical analysis was performed with using STATA 17.0 software.
Results
The sample
Nineteen patients were included in the study, 16 (84%) were men and three (16%) female. Their mean age was 71 years, ECOG status of patients was 0 for each patient. A total of 25 MWAs with the TATO system were performed: 11 (44%) for HCC, 12 (48%) for colorectal carcinoma metastases, one (4%) for gastric adenocarcinoma metastases and one (4%) for pancreatic adenocarcinoma metastases (). Tumor size was ≤20 mm in 20 (80%) patients and >20 mm in five (20%) patients.
Table 1. Sample description and tumor response.
Assessment of primary objective: safety
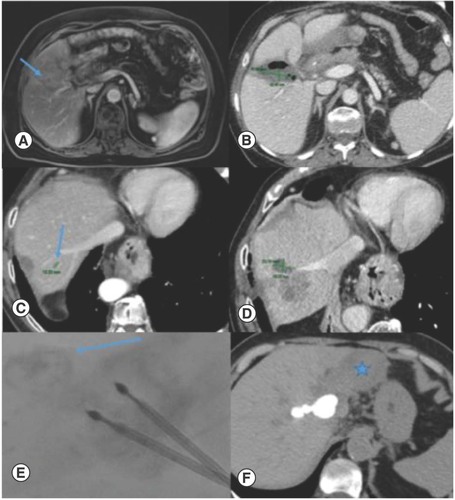
Only one (4%) adverse event was observed out of 25 ablations. It was an abscess that was observed in the ablated area after the procedure and was close to the hepatic hilum (). This situation was resolved with a percutaneous drainage and antibiotic therapy. No other adverse events were observed.
(A & B) Liver abscess after ablation of a small metastases near the hepatic hilum that was resolved with percutaneous drainage. (C & D) Tumor progression of metastases adjacent to hepatic dome vein. (E & F) Tomography performed after the introduction of lipiodol with a small amount of alcohol through the internal hole of the ablation electrode. There was a small leakage (arrow) that conditioned marked atrophy of the left lobe (star).
Assessment of primary objective: efficacy
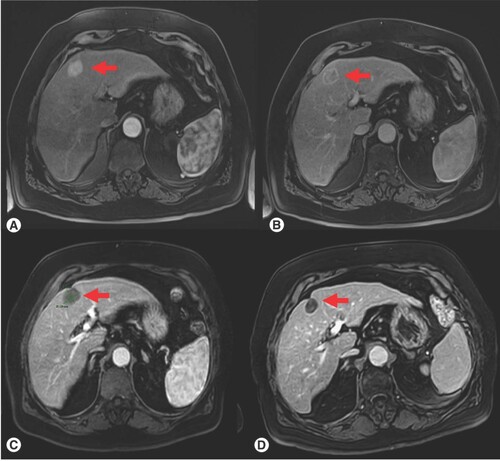
As concerning tumor response, 22 (88%) complete response, one (4%) partial response and two (8%) progressions were observed 3 months after ablation with TATO system (Box 1). Local tumor control rate was 92% (). An example of tumor response was reported in .
Table 2. Tumor response in primary and metastatic liver cancer.
(A) Pretreatment, arterial phase on dynamic MRI, (B) Pretreatment, portal phase on dynamic MRI; (C) Post-treatment, 3-month follow-up; (D) Post-treatment, 6-month follow-up, there is a clear progressive reduction in size of the lesion as well as absence of contrast enhancement. Red arrow indicates the target liver lesion.
A vascular structure greater than 3 mm (supra-hepatic vein) was observed adjacent to index tumor in one of the progression cases (n = 13) and in the case of partial response (n = 11) at the 3-month follow-up. This could have influenced the ablation and the failure of the technique (). The other case of progression (n = 6) corresponded to a subcentimeter lesion located in the hepatic dome that had previously received multiple chemotherapy cycle and was not possible to visualize under US imaging during the treatment.
Secondary objective: comparison between HCC & metastases treatment efficacy
There was no statistical difference between tumor response in HCC and metastases treatment (). CR was 82% for HCC and 93% for liver metastases; however, the difference was not statistically different (p = 0.399). No PR was observed in liver metastases, whereas there was one in HCC. One case of PD was observe in both HCC and liver metastases group.
Discussion
MWA with TATO system allowed the real time visualization of the ablation. The procedure was safe (4% adverse events), reproducible and effective (92% local tumor control rate) for the treatment of both primary and secondary liver cancer, confirming previously reported results of its use for the thyroid [Citation8]. These results are in agreement with the clinical outcomes for the various MWA devices in current use for hepatic malignancies treatment. A recent review shows that in a total of 29 studies reporting data on 3250 patients and 4500 tumors (mostly HCC 55% and colorectal liver metastases 10%) treated with different MWA devices, the majority of them report technical success (range: 91.6–100%), ablation success (range: 73.1–100%) and few major procedural complications (range: 0–9.1%) [Citation17].
A study on colorectal liver metastases shows that US-guided percutaneous MWA allows to achieve complete ablation in 99.27% (408/411) of lesions and 97.81% (134/137) of patients with a major complication rate of 3.65%, a minor complication rate of 8.03% [Citation18]. Another study on colorectal liver metastases describes complete ablation in 100% of US-guided MWA with 4% major complication rate and an overall complication rate of 8% [Citation11]. Other studies show local tumor control rate of 83.3% and complete radiological response in 30/36 nodules (83.3%) in 24/30 patients (80%) at imaging performed one-month after MWA of HCC [Citation19,Citation20]. Our results are within these ranges, suggesting that this new device has comparable safety and efficacy to available MWA devices in both primary and metastatic liver cancer.
Image guidance techniques represent a critical role in MWA. In US-guided ablation, there are blind spots and vaporization interferences due to the cooling system used, limiting tumor visibility, which may result in incomplete intraoperative treatment or increased risk of tissue damage [Citation14]. CT scanning can overcome gas interference of US-guiding; however, it cannot achieve real-time localization of the ablation [Citation18]. These disadvantages can be overcome by uncooled MWA.
The main advantages of uncooled MWA with TATO system are the good US visibility of the ablation progression, the small diameters of the antennas used and the ease of transport and installation of the equipment since it does not require refrigeration. These characteristics also make it a great option for intraoperative ablations of small lesions. In our current daily practice we used contrast-enhanced US if the lesion was visible ultrasonographically. The US contrast is accessible, inexpensive and coupled with this novel microwave system allows an objective confirmation of the necrosis obtained just about 5 min after the ablation, when the vapor generated by the procedure has practically disappeared. We also noticed a good visibility of the antenna under US, with low vapor formation, which allowed a good realtime visibility of the ablation progression and of the structures surrounding the lesions. This may be explained by the use of a lower power (maximum 40 W per antenna) compared with other MW systems.
In our experience, before concluding the intervention it is important to verify if complete intraoperative ablation is achieved. Navigation technology and multimodality image fusion is very important for improving, optimizing and refining interventional radiology workflow, especially to perform complex and difficult biopsies and ablations [Citation21,Citation22].
The main limitations of this study are: retrospective data collection lacking comparative sample and no randomization, short time of observation, inclusion of only one treating center with small number of patients in observation. Nevertheless, this was a pilot study with as main aim to show the feasibility and safety of the new TATO MWA device. Future prospective studies with larger patient populations, longer follow-up and different types of tumor will provide further evidence on the effectiveness and safety of this novel MW system.
Conclusion
Findings from this retrospective study of 25 liver ablations with the microwave TATO system confirm safety, technical and clinical effectiveness of this novel device that allows for real time visualization of the ablation in both primary and metastatic liver cancer.
Microwave ablations with the non cooled TATO system was safe and highly reproducible in both primary and secondary liver cancer treatment.
This new technique was also effective with a disease response of 92% (88% complete and 4% partial response).
Microwave ablations with the non cooled TATO system had the same efficacy for primary and secondary liver cancer treatment.
This method allowed the real time visualization under ultrasound of ablation.
Author contributions
E Crespo, A Hermosín, Á Villalba, E Daguer, J Flores, J Periañez, M Martínez-Galdámez, E Santos substantially contributed to the conception and design of the work and interpretation of data for the work; revising the work critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- SiegelRL, MillerKD, JemalA. Cancer statistics, 2020. CA Cancer J. Clin. 70(1), 7–30 (2020).
- SiaD, VillanuevaA, FriedmanSL, LlovetJM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology 152(4), 745–761 (2017).
- HornSR, StoltzfusKC, LehrerEJet al. Epidemiology of liver metastases. Cancer Epidemiol. 67, 101760 (2020).
- European Association for the Study of the Liver. Electronic address: [email protected]; European Association for the study of the liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma [published correction appears in J Hepatol. 2019 Apr;70(4):817]. J. Hepatol. 69(1), 182–236 (2018).
- AlqahtaniA, KhanZ, AlloghbiAet al. Hepatocellular carcinoma: molecular mechanisms and targeted therapies. Medicina (Kaunas) 55(9), 526 (2019).
- RaeesA, KamranM, ÖzkanH, JafriW. Updates on the diagnosis and management of hepatocellular carcinoma. Euroasian J. Hepatogastroenterol. 11(1), 32–40 (2021).
- YuQ, LiuC, NavuluriR, AhmedO. Percutaneous microwave ablation versus radiofrequency ablation of hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Abdom. Radiol. (NY). 46(9), 4467–4475 (2021).
- WellsSA, HinshawJL, LubnerMGet al. Liver ablation: best practice. Radiol. Clin. North Am. 53(5), 933–971 (2015).
- HanY, ZhaoW, WuM, QianY. Efficacy and safety of single- and multiple-antenna microwave ablation for the treatment of hepatocellular carcinoma and liver metastases: a systematic review and network meta-analysis. Medicine (Baltimore) 101(51), e32304 (2022).
- DouJ, YuJ, ChengWet al. Learning curve of microwave ablation for liver cancers. Eur. J. Radiol. 158, 110613 (2023).
- KnottEA, ZiemlewiczTJ, LubnerSJet al. Microwave ablation for colorectal cancer metastasis to the liver: a single-center retrospective analysis. J. Gastrointest. Oncol. 12(4), 1454–1469 (2021).
- TomitaK, MatsuiY, UkaMet al. Evidence on percutaneous radiofrequency and microwave ablation for liver metastases over the last decade. Jpn J. Radiol. 40(10), 1035–1045 (2022).
- HaoMZ, HuYB, ChenQZ, ChenZX, LinHL. Efficacy and safety of computed tomography-guided microwave ablation with fine needle-assisted puncture positioning technique for hepatocellular carcinoma. World J Gastrointest Oncol. 14(9), 1727–1738 (2022).
- RuiterSJS, de JongJE, PenningsJP, de HaasRJ, de JongKP. Comparison of two 2.45 GHz microwave ablation devices with respect to ablation zone volume in relation to applied energy in patients with malignant liver tumours. Cancers (Basel) 14(22), 5570 (2022).
- YildirimG, KarakasHM. Uncooled microwave ablation as a treatment option to preserve thyroid function in patients with benign thyroid nodules. J. Belg. Soc. Radiol. 106(1), 50 (2022).
- BarrowB, MartinIi RCG. Microwave ablation for hepatic malignancies: a systematic review of the technology and differences in devices. Surg. Endosc. 37(2), 817–834 (2022).
- LiZ, WangC, SiGet al. Image-guided microwave ablation of hepatocellular carcinoma (≤5.0 cm): is MR guidance more effective than CT guidance?BMC Cancer 21(1), 366 (2021).
- CrocettiL, de BaéreT, PereiraPL, TarantinoFP. CIRSE standards of practice on thermal ablation of liver tumors. Cardiovasc. Intervent. Radiol. 43(7), 951–962 (2020).
- CrocettiL, ScaliseP, BozziEet al. Microwave ablation of very-early- and early-stage HCC: efficacy evaluation by correlation with histology after liver transplantation. Cancers (Basel) 13(14), 3420 (2021).
- QinS, LiuGJ, HuangMet al. The local efficacy and influencing factors of ultrasound-guided percutaneous microwave ablation in colorectal liver metastases: a review of a 4-year experience at a single center. Int. J. Hyperthermia 36(1), 36–43 (2019).
- AppelbaumL, MahgereftehSY, SosnaJ, GoldbergSN. Image-guided fusion and navigation: applications in tumor ablation. Tech. Vasc. Interv. Radiol. 16(4), 287–295 (2013).
- LyonsGR, PuaBB. Ablation planning software for optimizing treatment: challenges, techniques, and applications. Tech. Vasc. Interv. Radiol. 22(1), 21–25 (2019).

