Abstract
Aim: This retrospective study aimed to assess the efficacy and safety of ensartinib in Chinese patients with ALK-positive advanced NSCLC in real-world clinical practice. Methods: Clinical data from ALK-positive NSCLC patients treated with ensartinib in China were collected and analyzed. Efficacy end points included objective response rate and progression-free survival. Safety profiles were also evaluated. Results: A total of 682 patients were included in this study. The study demonstrated promising efficacy with an objective response rate of 54.0%, and the median progression-free survival was not estimable. Ensartinib exhibited a manageable safety profile with treatment-related adverse events (TRAEs) consistent with prior clinical trials. The most common TRAE was rash (21.1%) and no TRAE led to death. Conclusion: Ensartinib is active and well tolerated for ALK-positive NSCLC patients in real-world clinical settings.
Plain language summary
Targeted therapies have significantly improved outcomes for patients with ALK-positive NSCLC. Ensartinib, a drug which blocks an enzyme in the body called ALK tyrosine kinase, has shown to be efficient and well tolerated in clinical trials. However, real-world evidence is crucial to confirm its effectiveness and safety in diverse patient populations. We analyzed the real-world outcomes of ensartinib treatment in 682 ALK-positive NSCLC patients in China, by looking at past records. The results showed that ensartinib demonstrated positive effects in most patients, meaning it helped in controlling their cancer progression. Side effects affected approximately one quarter of patients and most reported side effects were mild. Rash was the most reported side effect, accounting for about 21%. This study provides valuable insights into the real-world clinical performance of ensartinib, confirming its effectiveness and safety as a treatment option for ALK-positive NSCLC patients.
Lung cancer stands as one of the leading global causes of cancer-related high mortality and morbidity [Citation1], and NSCLC accounts for approximately 80–85% of lung cancer cases [Citation2]. Unfortunately, a majority of patients presented with advanced stage with local progression and distal metastasis at presentation. Although platinum-based chemotherapy regimen currently present the standard treatment approach for patients with advanced NSCLC, the therapeutic landscape has witnessed a remarkable transformation due to advancements in genetic testing technology and the emergence of targeted therapies. ALK gene was discovered as one of the most important driver oncogenes of NSCLC, with an incidence of positive ALK gene rearrangement at about 9% in advanced NSCLC patients [Citation3]. Randomized trials have demonstrated that ALK inhibitors, such as crizotinib, ceritinib and alectinib, have successfully prolonged the median progression-free survival (PFS) and improved quality of life in advanced patients with ALK-positive NSCLC over conventional chemotherapy [Citation4].
Ensartinib is an oral, highly selective, potent ALK–tyrosine kinase inhibitor (TKI), which has been approved by the National Medical Products Administration (NMPA) as the first-line treatment for the ALK-positive locally advanced or metastatic NSCLC patients in March 2022. Structurally, ensaertinib represents a novel second-generation ALK–TKI, featuring an aminoquinazoline substitution for the aminopyridine found in the chemical structure of crizotinib. The phase I dose-escalation and expansion trials showed that ensartinib exhibited remarkable efficacy with maintain manageable toxicity [Citation5–7]. The phase I trial showed that ensartinib exhibited remarkable efficacy with maintain manageable toxicity across four cohorts (150, 200, 225 and 250 mg per day) in 48 patients with advanced ALK- or ROS-positive NSCLC [Citation7]. In this above study, ensartinib exhibited moderate absorption, with a median time to maximum concentration ranging from 3.00 to 4.00 h, with a mean half-life (T1/2) ranging from 21.0 to 30.2 h. The area under the curve (AUC) of ensartinib approached saturation at doses between 200 and 225 mg. Two dose-limiting toxicities (DLTs) were observed in 250 mg, resulting in the determination of the maximum tolerated dose (MTD) and the recommended phase II dose (RP2D) as 225 mg per day. The phase II study of ensartinib conducted in 160 patients with crizotinib-resistant ALK-positive NSCLC, median PFS was 9.6 months (95% CI: 7.4–11.6), and the overall and intracranial objective response rate (ORR) was 52% (95% CI: 43–60) and 70% (95% CI: 53–83), respectively [Citation8]. Of note, in the phase III eXalt3 study, ensartinib as the first-line treatment significantly extended median PFS with advanced ALK-positive NSCLC patients over crizotinib (25.8 months vs 12.7 months; HR: 0.51; 95% CI: 0.35–0.72; p < 0.001). The confirmed intracranial response rate, as confirmed by the independent review committee, demonstrated a substantial advantage with ensartinib at 63.6%, in stark contrast to the 21.1% observed with crizotinib [Citation9].
Given that ensartinib is a novel and newly available second-generation ALK–TKI, its efficacy and safety in large-scale, real-world patient populations remain uncertain. To address this uncertainty, this retrospective study was conducted to assess the efficacy and safety of ensartinib in ALK-positive locally advanced or metastatic NSCLC patients in actual treatment practice.
Methods
Patients & study design
Data were obtained from the patient record from the hospital electronic database which was analyzed by the Betta Pharmaceuticals Co., Ltd. All patients with unresectable stage IIIB–IV ALK-positive locally advanced or metastatic NSCLC who were scheduled to be treat with ensartinib between 16 December 2020 and 16 December 2021 were eligible for the study. Eligible patients (age ≥18 years) were required to receive ensartinib (225 mg orally, once daily) until disease progression or intolerable toxicity. The primary objective was PFS. The second objective was ORR and safety. The Institutional Review Board (IRB) at China Pharmaceutical Industry Research and Development Association waived the need for approval or informed consent because of the anonymized and retrospectively analyzed data. The study was performed in accordance with the Helsinki Declaration and Good Clinical Practice Guidelines.
Assessment
For each patient, a comprehensive set of demographic and baseline data were collected, encompassing key factors such as age, sex, smoking history, Eastern Cooperative Oncology Group (ECOG) performance status (PS), tumor histology, tumor stage and line of therapy. Efficacy and safety data were recored at 1 month after the first dose of ensartinib, and then at least every 2 months. Physician-based tumor response was evaluated by computerized tomography (CT) imaging according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, which was recorded as complete remission (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Safety assessment was conducted in patients who had received at least one dose of ensartinib. All treatment-related adverse events (TRAEs) were coded following the Medical Dictionary for Regulatory Activities (MedDRA) version 12.1 and graded by the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
Statistical Analysis
Analyses were conducted using Statistical Analysis Software version 9.4 (SAS Institute, Cary, NC) in the study. And analyses were performed using descriptive methods including frequency and percentage. All TRAEs occurred during medication period were coded and complied with MedDRA (version 12.1). Tumor response of ensartinib was correlated with both PFS and ORR. PFS was defined as the time from the start of ensartinib until objective disease progression, and ORR was defined as the percentage of patients achieving CR or PR. Univariate and multivariate Cox proportional hazard regression modeling was used for determining risk factors for PFS. ORR was subjected to a detailed analysis based on predefined subgroups, encompassing variables such as sex, age, smoking history, ECOG PS, tumor histology, tumor stage and line of therapy.
Results
Baseline characteristics
From 16 December 2020 to 16 December 2021, a total of 682 patients were enrolled in this study. All patients were assessable for the efficacy and safety analysis. The median follow-up time was 4.8 months. Baseline demographics were shown in . The median age was 57 years (range: 18–95 years). With regard to age, 515 patients (75.5%) were younger than 65 years and 167 patients (24.5%) were older than 65 years. The majority of patients had no smoking history (89.0%), and had adenocarcinoma (91.3%) and ECOG PS of 0–1 (86.1%). Most patients (85.8%) were diagnosed with stage IV disease. Ensartinib predominantly served as the second-line therapy for most patients (44.9%), while 30.8% and 0.3% of patients initiated treatment with ensartinib as their first-line therapy and third/multiple-line therapy, respectively.
Table 1. Patient demographics and clinical characteristics.
Efficacy
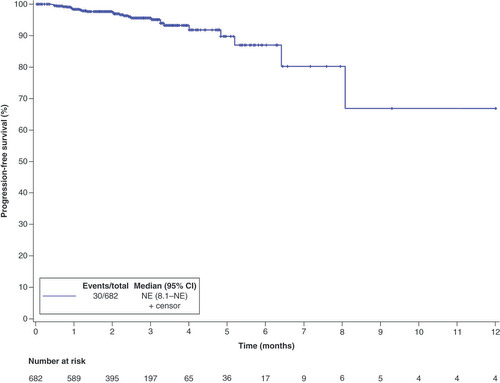
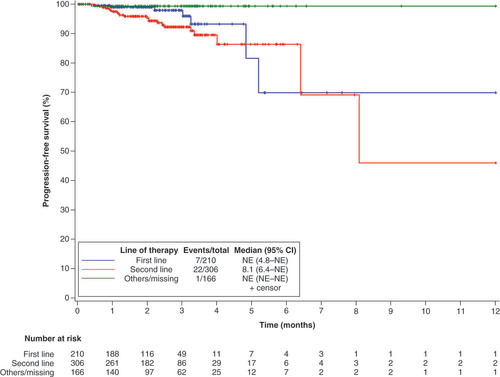
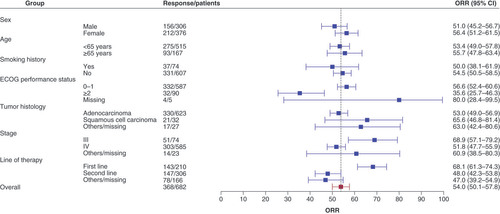
As of the date of data cutoff, a total of 30 of 682 patients (4.4%) patients had PFS events. The overall median PFS was not estimable (NE; 95% CI: 8.1–NE) (). Subgroup analysis showed that the median PFS with the first-line therapy and second-line therapy was NE (95% CI: 4.8–NE) and 8.1 months (95% CI: 6.4–NE), respectively (). Univariate analysis identified that being male (HR: 2.23, 95% CI: 1.06–4.66; p = 0.0295) and having squamous cell carcinoma (HR: 6.95, 95% CI: 2.95–16.37; p < 0.001) were significant predictors with poorer PFS. By multivariate analysis, squamous cell carcinoma was related to a worse PFS (HR: 4.44, 95% CI: 1.75–11.26; p = 0.0017) (). The ORR among the 682 efficacy-evaluable patients was 54.0% (95% CI: 50.1–57.8%) (). In subgroup analysis, ORR was 50.0% (37/74) and 54.5% (331/607) in smoker and nonsmoker subgroups, 56.6% (332/587) and 35.6% (32/90) in ECOG PS of 0–1 and ≥2 subgroups, 53.0% (330/623) and 65.6% (21/32) in adenocarcinoma and squamous cell carcinoma subgroups, 68.9% (51/74) and 51.8% (303/585) in stage III and IV subgroups, 68.1% (143/210) and 48.0% (147/306) in first-line and second-line subgroups.
Safety
TRAEs were recorded in 162 (23.8%; 162/682) patients. The most frequently reported TRAE were rash (21.1%). Other TRAEs, each occurring in at least 1% of patients, including pruritus (3.8%), constipation (2.2%), facial edema (1.3%) and vomiting (1.0%). Grade 3 or higher TRAE was reported in 1.0% of patients. No treatment-related deaths were reported. One patient aged 68 years discontinued due to grade 2 interstitial lung disease (ILD) (). Instances of liver injury, characterized by elevated alanine aminotransferase, were observed in 4 patients (0.6%), while elevated aspartate aminotransferase was noted in two patients (0.3%). Grade 3 increases in alanine aminotransferase occurred in 2 (0.3%) of patients and no grade 3 or higher elevated aspartate aminotransferase reported. The complete TRAEs were summarized in Supplementary Table 1.
Table 2. Treatment-related adverse events reported in ≥1% patients.
Table 3. Uni- and multivariate Cox regression analyses of independent prognostic factors for progression-free survival.
Discussion
In this retrospectively analysis, we examined the real-world efficacy and safety of ensartinib in routine clinical practice. Our findings revealed that ensartinib showed a satisfying efficacy and manageable safety profile in Chinese patients with ALK-positive NSCLC, as well as confirming the results of the phase III eXalt3 study.
At the time of analysis, 30 patients (4.4%) receiving ensartinib treatment had a PFS event, with an overall median PFS that could not be precisely estimated (NE; 95% CI: 8.1–NE). Subgroup analysis showed that the median PFS was NE (95% CI: 4.8–NE) and 8.1 (95% CI: 6.4–NE) for patients receiving first-line or second-line treatment. In contrast, previous trials of ensartinib demonstrated the median PFS of 25.8 months (range: 0.03–44.0) and 9.6 months (95% CI: 7.4–11.6) for first-line and second-line treatments, respectively [Citation8,Citation9]. However, it’s essential to recognize that our study is retrospective in nature and comes with inherent limitations. Unlike rigorous clinical setting, patients may benefit from combination with other therapeutic agents the alongside ensartinib, which should be more reflective of ensartinib efficacy in the real-world. To date, several ALK–TKIs has been developed, including first-generation ALK–TKI (e.g., crizotinib); second-generation ALK–TKIs (eg., alectinib, ceritinib, brigatinib, and ensartinib) third-generation ALK–TKI (e.g., lorlatinib). Among second-generation ALK inhibitor, ceritinib exhibited a median PFS of 16.6 months (95% CI: 12.6–27.2) and brigatinib reported 24 months (95% CI: 18.5–43.2) in the first-line setting [Citation10,Citation11]. Furthermore, median PFS for patients with ceritinib and brigatinib was 5.7 months (95% CI: 5.4–7.6) and 12.9 months (95% CI: 11.1–NE) for second-line studies, respectively [Citation12,Citation13]. Although across-trial comparisons are difficult and unreliable, the median PFS of current available second-generation ALK inhibitors were broadly similar.
In terms of another crucial efficacy parameter, ORR, our study reached an overall rate of 54.0% (95% CI: 50.1–57.8). The ORR in the ALK-positive patients receiving first-line and second-line ensartinib therapy was 68.1% (95% CI: 61.3–74.3) and 48.0% (42.3–53.8), respectively. These findings align similar to the data from previous phase II/III clinical study, where the ORR for ensartinib in first-line and second-line treatments was reported as 74% (95% CI, 66–81) and 52% (95% CI, 43–60), respectively [Citation8,Citation9]. Compared with other ALK inhibitors, ORR in the ALK-positive patients after first-line alectinib treatment was 82.9% (95% CI, 76.0–88.5) [Citation14]. In the second-line setting, ORR with ceritinib and brigatinib was 38.6% (95% CI, 30.5–47.2) and 54% (97.5% CI, 43–65), respectively [Citation12,Citation13]. It’s important to acknowledge that, due to the selection bias of retrospective studies and the relative insufficient follow-up time, our study data exhibited more variability compared with previous trials involving second-generation ALK-TKIs. Nonetheless, the overall ORR of 54% reaffirms the favorable efficacy of ensartinib in the real-world population.
The safety profile of ensartinib in this study remained consistent with previously reported data and exhibited no new safety signals [Citation5–9]. The most common TRAE was rash (21.1%), mainly due to the concentrations of ensartinib in skin are nine-times than those in plasma [Citation5]. The majority of rash was grade 1–2 in severity, with 1.0% of patients having grade 3–4 rash. This proportion was much lower than that of ensartinib in previous phase ii (56%) and phase III (67.8%) trials [Citation8,Citation9]. Importantly, no patients discontinued treatment due to rash, and there were no instances of treatment-related fatalities. In first-line studies [Citation9,Citation10,Citation14,Citation15], the most common AE for alectinib is anemia (20%), while for brigatinib, it is diarrhoea (52%), and for ceritinib, it is diarrhea (85%). In our current study, only 2 (0.3%) patients developed anemia and just 1 (0.2%) patient reported diarrhoea, with none of these cases reaching grade 3 or higher severity. Notably, these four drugs have displayed varying levels of hepatotoxicity in prior studies. While direct comparisons between these studies may be challenging, in the first-line settings, the incidence of grade 3 or higher elevated alanine aminotransferase and elevated alanine aminotransferase for alectinib is 15% and 14%, respectively [Citation14], brigatinib reported rate of 21% and 26% [Citation15], and ceritinib had an incidence of 31% and 17% [Citation10]. In contrast, ensartinib has a low incidence, with only 4.2% and 0.7% for alanine aminotransferase and alanine aminotransferase elevation, respectively [Citation9]. Here, the incidence of alanine aminotransferase and aspartate aminotransferase elevation was 0.6% (grade ≥3, 0.3%) and 0.3% (grade ≥3, 0). Consequently, ensartinib appears to be the least hepatotoxic option for the first-line treatment among ALK-positive NSCLC patients. Furthermore, this study recorded low incidences of other TRAEs, such as pruritus and constipation, which were reported at approximately 3.8% and 2.2%, respectively. In term of serious adverse events, only one patient had grade 2 ILD, and ILD was considered by the investigator to be related to the study medication. Fortunately, this patient showed improvement following the symptomatic treatment.
Some limitations exist in this study, such as the inherent selection bias, and the relatively short duration follow-up. Besides, data was not collected for the ALK-positive patients with brain metastases; the most frequently mutation types via next-generation sequencing were not distinguished, and stringent inclusion criteria were not used in this study. Finally, we cannot exclude that TRAEs were underreported in this study. Regarding the lower occurrence rate of TRAEs with ensartinib in real-world study compared with clinical trials, one such possibility is that clinical trials usually adhere to stricter monitoring and reporting procedures to ensure accurate recording and assessment of TRAEs. In the real world, these procedures may not be as rigorous, potentially resulting in lower reporting rates. In general, ensartinib was well tolerated with a manageable safety profile in the real-world population.
Conclusion
This retrospective study indicated favorable efficacy and safety of ensartinib in Chinese patients with ALK-positive NSCLC. On the practical side, ensartinib exhibits promising overall and intracranial efficacy. Furthermore, the safety profile of ensartinib is not identical to other second generation ALK–TKIs, which may lead to better tolerability in some patients. For those patients who cannot tolerate other ALK–TKIs, especially in liver injury patients, ensartinib may offer a viable alternative. Additionally, clinician formulate treatment plans based on the patient’s specific circumstances and medical history. The choice of ensartinib may be influenced by the patient’s genotype, clinical condition, and resistance mechanisms. In certain situations, ensartinib may be the more suitable treatment option.
This is a retrospective real-world study evaluating the efficacy and safety of ensartinib in Chinese patients with ALK-positive locally advanced or metastatic NSCLC.
A total of 682 patients were included to evaluate the efficacy and safety of ensartinib and the majority of patients had adenocarcinoma (91.3%), Eastern Cooperative Oncology Group performance status of 0–1 (86.1%) and stage IV disease (85.8%).
The median progression-free survival was not reached, and the median progression-free survival with the first-line therapy and second-line therapy was not estimable and 8.1 months.
Overall, the objective response rate was 54.0%, and objective response rate for the first-line and second-line therapy was 68.1% and 48%, respectively.
The most common treatment-related adverse event was rash (21.1%), and grade 3 or higher treatment-related adverse events were occurred in 1.0% of patients.
One case of grade 2 interstitial lung disease, and no treatment-related deaths occurred.
Ensartinib may be the least hepatotoxic ALK–tyrosine kinase inhibitors for first-line treatment in ALK-positive NSCLC patients.
Ensartinib as a reliable treatment option for ALK-positive advanced NSCLC.
Author contributions
Substantial contributions to conception and design: LM Ding, YB Ma, D Ji; acquisition, analysis, or interpretation of data: XB Yuan, Y Wang, M Yang, PX Wu, H Chen, Y Yun, ZL Shen; supervision: LM Ding, YB Ma; writing-original draft preparation: XB Yuan, Y Wang; writing-review and editing: XB Yuan, Y Wang, PX Wu, ZL Shen. All authors approved the final version and agreed to publish the manuscript. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests disclosure
All authors are employees of Betta Pharmaceuticals Co., Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study protocol was reviewed and the need for approval was waived by the China Pharmaceutical Industry Research and Development Association because of the retrospective nature of this research. This study was conducted by the China Pharmaceutical Industry Research and Development Association and does not require approval from the institutional ethics committee. The need for written informed consent from the participants prior to the study was waived by the China Pharmaceutical Industry Research and Development Association. Written informed consent was waived since nonidentified patient data were used. Nevertheless, the study was done in accordance with the Helsinki Declaration and Good Clinical Practice Guidelines.
Acknowledgments
The authors would like to thank all participating physicians and registered patients.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/lmt-2023-0005
Financial disclosure
This research was funded by the Betta Pharmaceuticals Co., Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Data sharing statement
All data generated or analyzed during this study are available upon reasonable request from correspondence author.
Additional information
Funding
References
- Bray F , Ferlay J , Soerjomataram I , Siegel RL , Torre LA , Jemal A . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2018).
- Gridelli C , Peters S , Sgambato A , Casaluce F , Adjei AA , Ciardiello F . ALK inhibitors in the treatment of advanced NSCLC. Cancer Treat. Rev. 40(2), 300–306 (2014).
- Lee JK , Park HS , Kim DW et al. Comparative analyses of overall survival in patients with anaplastic lymphoma kinase-positive and matched wild-type advanced non-small-cell lung cancer. Cancer 118(14), 3579–3586 (2012).
- Peng L , Zhu L , Sun Y et al. Targeting ALK rearrangements in NSCLC: current state of the art. Front. Oncol. 12, 863461 (2022).
- Horn L , Infante JR , Reckamp KL et al. Ensartinib (X-396) in ALK-positive non-small-cell lung cancer: results from a first-in-human Phase I/II, multicenter study. Clin Cancer Res. 24(12), 2771–2779 (2018).
- Fang W , Ma Y , Huang J et al. Ensartinib (X-396), a second-generation ALK TKI, in Chinese ALK-positive non-small-cell lung cancer: a Phase I, dose-escalation study. J. Clin. Oncol. 36(Suppl. 15), e21122 (2018).
- Ma Y , Pan H , Liu Y et al. Ensartinib in advanced ALK-positive non-small-cell lung cancer: a multicenter, open-label, two-staged, phase 1 trial. J Thorac Dis. 14(12), 4751–4762 (2022).
- Yang Y , Zhou J , Zhou J et al. Efficacy, safety, and biomarker analysis of ensartinib in crizotinib-resistant, ALK-positive non-small-cell lung cancer: a multicentre, phase 2 trial. Lancet Respir Med. 8(1), 45–53 (2020).
- Horn L , Wang Z , Wu G et al. Ensartinib vs crizotinib for patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized clinical trial. JAMA Oncol. 7(11), 1617–1625 (2021).
- Soria JC , Tan DSW , Chiari R et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 389(10072), 917–929 (2017).
- Camidge DR , Kim HR , Ahn MJ et al. Brigatinib versus crizotinib in ALK inhibitor-naive advanced ALK-positive NSCLC: final results of phase 3 ALTA-1L trial. J Thorac Oncol. 16(12), 2091–2108 (2021).
- Crinò L , Ahn MJ , De Marinis F et al. Multicenter Phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J. Clin. Oncol. 34(24), 2866–2873 (2016).
- Kim DW , Tiseo M , Ahn MJ et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter Phase II trial. J. Clin. Oncol. 35(22), 2490–2498 (2017).
- Peters S , Camidge DR , Shaw AT et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N. Engl. J. Med. 377(9), 829–838 (2017).
- Camidge DR , Kim HR , Ahn MJ et al. Brigatinib versus crizotinib in advanced ALK inhibitor-naïve ALK-positive non-small-cell lung cancer: second interim analysis of the Phase III ALTA-1L trial. J. Clin. Oncol. 38(31), 3592–3603 (2022).