Abstract
Aim: To summarize current knowledge, gaps, quality of the evidence and show main results related to the role of the autonomic nervous system in lung cancer. Methods: Studies were identified through electronic databases (PubMed, Scopus, Embase and Cochrane Library) in October 2023, and a descriptive analysis was performed. Twenty-four studies were included, and most were observational. Results: Our data indicated an increased expression of β-2-adrenergic receptors in lung cancer, which was associated with poor prognosis. However, the use of β-blockers as an add-on to standard treatment promoted enhanced overall survival, recurrence-free survival and reduced metastasis occurrence. Conclusion: Although the results herein seem promising, future research using high-quality prospective clinical trials is required to draw directions to guide clinical interventions.
Plain language summary
Lung cancer is one of the most common causes of cancer-related deaths in the world, which often goes undiagnosed until it is in an advanced stage. Recently, the autonomic nervous system (sympathetic and parasympathetic nervous systems) has been identified as a regulator of cancer growth and spread, including lung cancer. In fact, preclinical studies have demonstrated that autonomic innervation in lung cancer can trigger tumor progression, metastasis, and resistance to treatment, worsening the prognosis. In this sense, add-on strategies to standard cancer treatments have been investigating and one of them has stood out: the incidental use of β-blockers (patients who used β-blockers for the treatment of hypertension and/or cardiovascular diseases or anxiety) before surgeries or during chemotherapy, which has been associated with improved clinical outcomes. Thus, a scoping review was conducted to summarizing the current knowledge about the quality of evidence, gaps and main results related to the role of the autonomic nervous system in human lung cancer. Data from this review indicated an increase in sympathetic nervous system receptors associated with a worse prognosis in patients with lung cancer. Indeed, those patients who took β-blockers along with lung cancer treatment showed an increase in survival and a reduction in the occurrence of metastases. Although the results herein seem promising, further prospective clinical studies are needed to investigate the effect of the intentional and controlled use of β-blockers as an add-on strategy on the treatment of different types and stages of lung cancer.
Tweetable abstract
An increased expression of β-2-adrenergic receptors is linked to a poor prognosis in lung cancer. Adding β-blockers to treatment improved survival and reduced metastasis. See more in our study.
The autonomic nervous system (ANS), divided mainly into sympathetic (SNS) and parasympathetic (PNS) nervous systems, has a widespread innervation that extends to most organs, allowing for rapid adjustments in important body functions to maintain the stability of the internal environment [Citation1]. In this sense, changes in ANS activation may modulate several cellular processes such as the cell cycle, gene expression, angiogenesis, apoptosis and cellular immune responses, which can contribute to the initiation and progression of cancer through tumor innervation. Currently, it is known from preclinical studies that solid tumors, including lung tumors, are physically innervated by ANS, which can densely infiltrate them [Citation2–5] through recruitment of pre-existing nerves, formation of new nerves, or direct invasion of tumor cells along the nerves [Citation2–4,Citation6–9]. Interestingly, this tumor-nerve connection can establish a bidirectional interaction, transmitting informations and modulating functions, thus, favoring tumor growth and metastases, in addition to making the tumor more resistant to treatment [Citation2,Citation6,Citation10]). Regarding the branches of ANS, the SNS has been related to tumor angiogenesis and an enhanced expression of genes involved in metastasis and inflammation in several animal models of solid tumors, such as prostate, breast and colon cancer [Citation11,Citation12]. In contrast, the PNS has been associated with the activation and proliferation of cancer stem cells [Citation12–15].
In humans, dysautonomy (increased sympathetic/parasympathetic balance) has been reported in prostate, colon and lung cancer patients, which has been correlated with worse prognosis and disease severity [Citation3,Citation4,Citation11,Citation13,Citation16]. These findings drive the attention for the ANS influencing tumor microenvironment in a clinical setting. Based on these preclinical and clinical findings, tumor innervation has been recently proposed as a hallmark of cancer [Citation6,Citation7]. However, further investigation is needed to demonstrate that tumor innervation by ANS is also present in human solid tumors.
Lungs receive sympathetic innervation from the paravertebral chain (fourth thoracic ganglion) and parasympathetic innervation from the vagus nerve (X), which normally control tone of bronchi and bronchioles. Therefore, growth and spread of lung cancer might be related to the extend of lung innervation by SNS and PNS fibers.
Lung cancer has a high prevalence and is among the most lethal cancers worldwide [Citation17]. It can be divided in two main types: small cell cancer (SCLC) which accounts for less than 20% of cases and is almost always associated with tobacco use, and non-small-cell lung cancer (NSCLC) which is the most common [Citation18] and originates in larger cells of the lung, such as epithelial cells [Citation5]. It can be subdivided into four categories: adenocarcinoma, which is more common in non-smokers; squamous cell carcinoma; large-cell carcinoma and bronchial carcinoid tumor [Citation17,Citation19].
Preclinical studies have been demonstrated the existence and pattern of lung tumor innervation [Citation20–22]. Indeed, tumor autonomic denervation inhibits lung tumor growth and proliferation [Citation4,Citation8,Citation23–25]. Yet, in clinical studies, the incidental use of β-blockers before surgeries or during chemotherapy was associated with improved clinical outcomes for lung cancer patients [Citation15,Citation20,Citation26,Citation27]. In this sense, understanding the stage of investigation into the relationship between lung cancer and the autonomic nervous system in clinical trials is essential for establishing directions and guiding clinical practice. Nevertheless, due to scarcity of prospective clinical studies focused on ANS influence in lung cancer clinical outcomes, we conducted a scoping review aimed to summarize the current knowledge about the quality of evidence, gaps and main results related to the role of the autonomic nervous system, particularly the sympathetic nervous system in lung cancer in humans. This review also intends to provide information that will contribute to future research planning.
Methods
Protocol & search strategy
The present scoping review follows the recommendations of the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) extension for scoping reviews (PRISMA-ScR), Cochrane Guideline [Citation28], Tricco et al. [Citation29] and Peters et al. [Citation30]. The PROSPERO database does not accept records for scoping reviews. Thus, we published our protocol in an alternative database: Open Science Framework (OSF), which accepts different types of studies. Our protocol is accessible at the following link: https://osf.io/kevjf/?view_only=e392a74b947d4b4ea9f78ff4a527fb3a
A literature search was performed in October 2023 to identify potentially relevant articles from inception up to October 2023. It was conducted in four electronic databases: PubMed, Scopus, Embase and Cochrane’s Library. The search strategy combined the following terms: Lung Neoplasms OR Lung Cancer OR non-small-cell Lung Cancer OR Small Cell Lung Cancer AND Autonomic Nervous System OR Sympathetic Nervous System OR Parasympathetic Nervous System OR Adrenergic OR Cholinergic. The MeSH Search Tags were used on Pubmed when available. After the study selection, a backward tracking process on the reference lists of the articles included in this review was performed to identify potentially eligible articles.
Eligibility criteria
We framed our selection criteria based on the PICOS strategy: participants, intervention (or exposure), comparator, outcomes, and study design. In addition, the studies were included in this scoping review if they met the following criteria: i) a sample composed of people diagnosed with lung cancer regardless of the disease stage; ii) an evaluation of the effect of the autonomic nervous system on lung cancer as the primary outcome; iii) an assessment of the activation and inactivation of the autonomic nervous system; and iv) English as the language used in the article. Reviews, case reports, in vitro studies, or those that assessed animal models or other types of cancers were excluded from this scoping review. Studies that examined lung cancer alongside other cancers were included, but only lung cancer data were used.
Study selection & data extraction
Two investigators (F.G. and T.F.C.) independently assessed the studies’ eligibility for the scoping review. First, titles and abstracts were evaluated, and then the full text of the selected articles was analyzed. In cases where titles and abstracts did not provide enough information for eligibility, the full text was assessed. In case of disagreements, a consensus was adopted, or, if necessary, a third researcher examined the article (C.U.).
Relevant data regarding information on the studies, such as the authors’ names, publication year, country, outcomes, study design, sample characteristics, intervention/exposure, comparison/control group, author’s conclusion, and conflict of interest, were extracted. Two researchers (F.G. and T.F.C.) performed the data extraction independently to reduce the potential for selection bias. A third researcher (C.U.) randomly selected 20% of the included studies to determine the correctness of data extraction.
Critical appraisal of individual sources of evidence
Two reviewers (F.G. and T.C.) used the Downs and Black Checklist to assess the methodological quality of the included articles. Downs and Black Checklist is a reliable tool that allows appraising the methodological strengths and weaknesses of both randomized controlled trials and nonrandomized studies [Citation31,Citation32]. It is ranked as a quality assessment tool suitable for systematic reviews [Citation33]. The checklist consists of 27 items that address the following methodological components: reporting, external validity, internal validity (bias and confounding), and power. Twenty-six items were rated either as yes (=1) or no/unable to determine (=0), and one item was rated on a 3-point scale (yes = 2, partial = 1, and no = 0). The highest possible score for the checklist is 28. Thus, the methodological quality of articles was categorized as follows: excellent (26–28), good (20–25), fair (15–19), and poor (≤14) [Citation32].
Data synthesis
Due to the exploratory nature of the research question and the heterogeneity among the studies, a qualitative analysis was performed to summarize the quantity and quality of evidence, and the gaps in autonomic dysfunction in lung cancer. Thus, the authors’ name, publication year, country, study design, aim, sample characteristics, lung cancer type, outcomes, intervention, and authors’ conclusions were analyzed.
Results
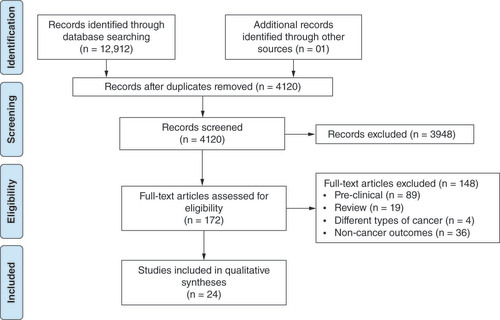
In the database search, 12912 articles were found, of which 2222 were retrieved from PubMed, 7178 from Embase, 3097 from Scopus, 415 from Cochrane, and one from the reference lists of the included articles. After excluding duplicate entries, 4120 studies remained. Of these, 3948 were excluded after screening the titles and abstracts, 89 were excluded as were preclinical studies, 19 reviews, four included samples of different types of cancer without presenting subanalyses by the type of cancer and 36 for analyzing noncancer-related outcomes. At the end of the selection process, only 24 studies met the eligibility criteria ().
Quality assessment
Based on the Downs and Black checklist, we assessed the methodological quality of the 24 studies included in the present scoping review (). The Downs and Black checklist score showed that 22 studies were of fair quality [Citation34–55], and two were of poor quality [Citation16,Citation56] (Supplementary Table A1). This tool evaluated four methodological components: reporting, external validity, internal validity (bias and confounding), and power. Only two studies, in the reporting [Citation35,Citation49] and external validity [Citation36,Citation41], achieved the maximum score (11 and 3, respectively). No study reached the maximum score in internal validity (bias and selection bias) and power items.
Table 1. Summary of methodological quality.
Study characteristics
According to the design of the 24 studies included in this review, 18 were observational and retrospective (14 cohorts and four case-control), and six were quasiexperimental studies. Considering the type of lung cancer, 15 studies evaluated NSCLC, one study evaluated both types of lung cancer (i.e., NSCLC and small-cell lung cancer [SCLC]), and eight studies did not distinguish the types of lung cancer assessed (NSCLC or SCLC). In addition, five studies analyzed more than one type of cancer (these studies analyzed, in addition to lung cancer, other types of cancer such as breast cancer). However, only the results related to lung cancer were included in this scoping review.
The 24 studies encompassed 72132 participants of both sexes, mostly from developed countries. Regarding the age of the participants, most studies evaluated older individuals (). Additionally, the number of men and women, the presence of comorbidities (e.g., hypertension, diabetes, cardiovascular diseases), and smoking were also different among studies. However, these factors are commonly observed in the 18 observational design studies included in this review. In the observational studies, data were collected only from local registries (hospitals) or national databases.
Table 2. Summary of included studies.
The duration of the disease, type, number of treatment sessions, and dose of medication were not shown in most of the studies. Of the 17 studies that examined the use of some type of medication, only 6 [Citation36,Citation41,Citation44,Citation46,Citation49,Citation53] mentioned the dose used, and three [Citation37,Citation51,Citation52] reported only the dose administered in the radiotherapy treatment.
Outcomes
Almost all studies included in this scoping review (23 studies) evaluated the role of β-adrenergic signaling in lung cancer in different outcomes: prognosis, tumor malignancy, overall and recurrence-free survival, specific cancer mortality, cancer incidence/risk, and metastasis occurrence. In addition, some of these studies assessed more than one outcome ().
Table 3. β-adrenergic outcomes.
β-adrenergic receptors
Six studies evaluated the relationship between the expression of β-adrenergic receptors and lung cancer on different outcomes: the expression of β-adrenergic receptors in two different types of NSCLC [Citation38]; the relationship between the expression of β 2-adrenergic receptors and poor prognosis in recurrence-free survival and overall survival [Citation16,Citation40,Citation55]; expression of β-adrenergic receptors related to tumor cell proliferation, angiogenesis and metastasis [Citation16]; and genetic variants of the β-2 adrenergic receptor and risk of developing lung cancer [Citation42,Citation50].
In one of the studies [Citation38], a decrease in the expression of β1-adrenergic receptors in adenocarcinoma and squamous cell carcinoma (types of NSCLC) and an increase in the expression of β2-adrenergic receptors in adenocarcinoma tumor cells were observed. Interestingly, the increased expression of β2-adrenergic receptors was associated with poor prognosis in recurrence-free survival and overall survival [Citation16,Citation40]. Additionally, the expression of β-adrenergic receptors was also related to tumor cell proliferation, angiogenesis, and metastasis [Citation16]. Conversely, one study [Citation55] found a decrease in the expression of β2-adrenergic receptors in adenocarcinoma tumor cells, which was associated with low levels of immune cells infiltration, leading to a poor prognosis and overall survival.
Two studies [Citation42,Citation50] verified the genetic variants of the β2-adrenergic receptor and their influence on the risk of developing lung cancer, finding contrasting results. In Wang´s study [Citation50], no significant differences in the β 2-adrenergic receptor genotype were found between the healthy Chinese population and those with adenocarcinoma. In contrast, Mei´s study [Citation42] showed that the polymorphism in the minor allele of ADRB2, which encodes the β2-adrenergic receptor, was associated with an increased risk of lung cancer.
Collectively, most studies suggest that increased expression of β 2-adrenergic receptors in lung tumors is related to poor prognosis and tumor malignancy. However, contradictory results were observed regarding the polymorphism of the genes that encode the β2-adrenergic receptor.
β-blockers
Seventeen studies analyzed the effect of β-blockers using distinct approaches: 1) evaluation of the effect of β-blockers without considering other cancer treatments [Citation39,Citation41,Citation44,Citation47,Citation49,Citation53,Citation54,Citation56]; 2) the use of β-blockers associated with current cancer treatments [Citation34,Citation36,Citation37,Citation45,Citation51,Citation52]; and 3) the use of β-blockers in the perioperative period [Citation35,Citation43,Citation46]. The effect of each approach on overall and recurrence-free survival, specific cancer mortality, and cancer incidence are described below.
Effect of incidental use of β-blockers
Eight studies assessed the incidental use of β-blockers disassociated from other cancer treatments. Five evaluated the effect of β-blockers on overall survival [Citation39,Citation47,Citation53,Citation54,Citation56] and one study [Citation56] also assessed recurrence-free survival. No differences were observed in patient survival in any of the studies. In addition, another study also analyzed cancer-specific mortality [Citation49], but the incidental use of β-blockers showed no effect. Two studies also investigated the influence of β-blockers on cancer incidence [Citation41,Citation44]. In one of the studies, no effect was observed with the use of alpha or β-blockers in preventing lung cancer [Citation44]. In contrast, another study [Citation41] showed that long-term treatment with carvedilol (a non-selective β-blocker with additional intrinsic alpha1-adrenergic receptor blocking effects) was associated with a reduction in lung cancer risk [Citation41].
Considering these results, the incidental use of β-blockers dissociated from other cancer treatments showed no effects on overall survival, recurrence-free survival, and cancer-specific mortality. In contrast, controversial results were observed for lung cancer incidence.
Use of β-blockers as an add-on to standard cancer treatments
The effect of incidental use of β-blockers as an ad-om to cancer treatments on overall survival was assessed by six studies [Citation34,Citation36,Citation37,Citation45,Citation51,Citation52]. In addition, four studies also evaluated recurrence-free survival [Citation37,Citation45,Citation51,Citation52], and three assessed metastasis occurrence [Citation37,Citation51,Citation52]. Interestingly, all studies showed a greater overall and recurrence-free survival and a reduction in the occurrence of metastases when β-blockers were associated with cancer treatments. These results suggest a potential additive effect of β-blockers when combined with conventional anticancer therapy.
Use of β-blockers during the perioperative period
The use of β-blockers in the perioperative period was analyzed in three studies. Of these, all three assessed overall survival [Citation35,Citation43,Citation46], and two [Citation35,Citation46] also verified recurrence-free survival, but only one study observed differences in overall and recurrence-free survival [Citation46]. Furthermore, one study [Citation43] evaluated specific cancer mortality, but no difference was found. Thus, using β-blockers in the perioperative period is still controversial in lung cancer.
Autonomic nerve density
Only one study [Citation48] assessed the parasympathetic and sympathetic nerve density of lung cancer. In this study, Shao and coworkers found increased autonomic nervous system infiltration (both sympathetic and parasympathetic), which was related to pathological risk grading and poor prognosis. Furthermore, sympathetic nervous fibers were more highly expressed in the paratumor area (surrounding the tumor), while parasympathetic nervous fibers were more highly expressed in the tumor area.
Discussion
This scoping review summarized the current knowledge about the main results, quality of evidence, and gaps related to the role of the autonomic nervous system, particularly the sympathetic nervous system. The primary studies investigated the activation or inhibition of SNS, as assessed through the expression of β-adrenergic receptors, isolated or associated with the β-blocker effect and nerve density.
β-adrenergic receptors, and consequently sympathetic activation, has been associated with cancer development in several types of tumors [Citation4]. Most tumors seem to exhibit elevated catecholamine levels and β-adrenergic receptor density [Citation5,Citation57,Citation58], which upregulate some growth factors and cytokines, promoting protumorigenic effects [Citation59]. In this sense, preclinical studies have shown that β-adrenergic signaling may stimulate lung cancer progression [Citation15,Citation59], primarily through β2-adrenergic receptor activation [Citation58]. Our data showed that the expression of β-2-adrenergic receptors (i.e., a proxy of activity) was increased in lung cancer, which was associated with poor prognosis in recurrence-free survival and overall survival. Interestingly, only one study observed an inverse correlation between β-adrenergic receptors and prognosis, showing that reduced expression of these receptors in adenocarcinoma worsens patients’ prognosis. The authors associate this response with the type of tumor and the stage of lung cancer progression. It is worth mentioning that this study evaluated different databases related to ADBR2 gene expression. In addition, the upregulation of β-2-adrenergic receptors seems to differ depending on the type of lung cancer. Coelho and coworkers [Citation38] evaluated the expression of β-2-adrenergic receptors in two subtypes of NSCLC, adenocarcinoma and squamous cell carcinoma. They observed that receptor expression increased in both histological subtypes but was highly expressed in adenocarcinoma compared with squamous cell carcinoma.
Two studies [Citation42,Citation50] investigated the relationship between genetic susceptibility (e.g., polymorphisms) and lung cancer risk. One study observed an increased risk of lung cancer in people with specific single nucleotide polymorphisms (SNPs) (rs1042711 and rs1560642). However, the other study did not find an increased risk. Although both studies evaluated SNPs in the Chinese population, Wang et al. [Citation50] and Mei et al. [Citation42] investigated different polymorphisms. Furthermore, Mei et al. [Citation42] did not describe the type of lung cancer that the investigated population had. In this sense, the relationship between genetic susceptibility and increased expression of β-2-adrenergic receptors requires further scrutiny [Citation60]. Thus, data related to β-adrenergic receptors, in general, indicate an important role of these receptors in lung cancer development, although the small number of studies and their observational nature produce a fair quality of evidence. In this sense, the contribution of β-2, β-1, and β-3-adrenergic receptors to lung cancer development still requires well-designed clinical trials. In addition, the influence of these receptors on disease progression in different types and stages of lung cancer remains to be elucidated.
Although the role of β-adrenergic receptors in lung cancer is not fully elucidated, several retrospective studies have investigated the correlation of incidental inhibition (i.e., patients who used β-blockers for the treatment of hypertension and/or cardiovascular diseases or anxiety) of these receptors with clinical outcomes in lung cancer. The potential of β-blockers to inhibit the growth of different types of tumors and, consequently, metastasis has been supported by in vitro and in vivo studies [Citation24,Citation61,Citation62]. Additionally, the non-small-cell lung cancer (NSCLC) cell lines HT-29 and A549 treated with norepinephrine showed increased cellular migration, indicating a more invasive and metastatic phenotype, which was abrogated by propranolol [Citation63]. Nevertheless, the studies analyzed in this scoping review [Citation39,Citation41,Citation44,Citation47,Citation49,Citation53,Citation54,Citation56] that evaluated the effect of β-blockers showed no improvements in overall survival, recurrence-free survival, and cancer-specific mortality, even when β-blocker selectivity (nonselective, selective for β-1 or β-2-adrenergic receptor) was considered. Two studies [Citation34] evaluated the cancer risk and only one [Citation34] observed a positive effect of a selective β-blocker (carvedilol) treatment, reducing lung cancer risk. These contradictory results related to risk of lung cancer may be associated with the different β-blockers used (Lin et al. [Citation41] – carvedilol; Numbere et al. [Citation44] – β or alpha blockers) and statistical analyses performed (Lin et al. [Citation41] – Kaplan–Meier method; Numbere et al. [Citation44] - multivariate logistic regression analyses). On the other hand, the studies that evaluated the effect of β-blockers as an add-on to standard treatment (e.g., chemotherapy, radiotherapy, and immune checkpoint inhibitors) demonstrated enhanced overall survival, improved recurrence-free survival, and reduced occurrence of metastases in lung cancer patients. Regarding β-blocker selectivity, two studies [Citation45,Citation51] observed that selective β-blockers (for β-2-adrenergic receptors in Oh’s study [Citation45] and unspecified in Wang’s study [Citation51]) were more effective in improving overall survival and recurrence-free survival than nonselective β-blockers when combined with standard treatments. It is noteworthy that β-blocker posology and its association with other drugs (e.g., COX-2 or ACE inhibitors) were not clearly described in the studies, which is an important source of bias. Indeed, Cole and Sood [Citation64] highlighted that the absence of treatment parameters (e.g., type, dosage, and duration), information on influential risk factors, and the primary β-blocker indication (e.g., cardiovascular disease) are common biases in observational studies evaluating the use of β-blockers in cancer treatment. β-blockers are known to be safe, inexpensive, easy to use, and used in cancer treatment, but high-quality evidence is needed to understand when, how and which β-blockers should be used in the treatment of lung cancer.
β-blockers have also been used in the perioperative period to mitigate the stress response associated with high levels of anxiety [Citation65] and/or surgery-induced inflammatory response [Citation66,Citation67]. Three studies [Citation35,Citation43,Citation46] presented equivocal survival results. In this sense, our data corroborate Yap et al.‘s (2018) [Citation68] results, which evaluated the overall effect of β-blockers in the perioperative period in different types of cancer and observed no apparent effect on disease-free survival and overall survival.
Nerve density in the tumor microenvironment is directly related to autonomic activation. Preclinical [Citation69–71] and clinical [Citation11] studies have shown that high autonomic innervation density contributes to tumor development in several types of cancer. Nonetheless, autonomic innervation density in the tumor microenvironment has not been extensively studied in lung cancer. Only one study [Citation48] included in this review showed that increased innervation of both the SNS and PNS worsens the prognosis in lung cancer [Citation13]. In this regard, as clinical data are scarce, further studies are needed to determine whether autonomic nervous system density, especially that of the parasympathetic nervous system, harms the clinical outcomes of lung cancer. At this time, it is not possible to establish clinical guidelines with the available data.
This scoping review has some limitations. Although the total number of studies included in this review is high, the number of studies that investigated nerve density was small, making it difficult to draw conclusions. In addition, high heterogeneity among the studies regarding the type and stage of lung cancer, the intervention and study designs was observed.
Lung cancer diagnosis is another aspect that may interfere with this area of research, as it usually occurs in the later stages of the disease. A later diagnosis may significantly hamper β-blocker effects on cancer development research. Additionally, the methodological approach is not simple, often requiring invasive techniques to assess cancer-related mechanisms. Thus, high-quality translational studies are required to 1) clearly describe the associated mechanisms and 2) modulate the activity of these mechanisms in clinical trials.
Conclusion
Autonomic nerve density has been deemed a regulator of cancer growth and spread. However, the knowledge produced on the topic is mainly based on preclinical studies and retrospective observational clinical studies. Therefore, the results presented herein seem promising, but future research using high-quality prospective clinical trials is required to draw directions to guide future clinical interventions.
Future perspective
β-blockers are a safe and low-cost strategy that is generally well tolerated by patients. Its use, albeit incidental, has brought a new perspective to treating lung cancer, especially to control disease progression and metastasis and when used as an add-on to standard treatments.
In the Grading of Recommendations Assessment, Development and Evaluation (GRADE) [Citation72], some aspects are noted as important to increase the level of evidence of a study, for example: 1) the potential benefits are greater than the risks; 2) the intervention is cheap and viable; 3) there is high acceptability of the intervention; and 4) there are residual effects from confounding factors that must be estimated. In this sense, β-blockers can be considered a potential add-on therapeutic strategy for lung cancer. However, more prospective studies with a high level of evidence are needed to confirm this potential.
Alternatively, exercise training seems to produce similar effects to β-blockers modulating autonomic nervous system activity, which has been considered a protective tool against some types of cancer, such as breast and colon cancer [Citation73,Citation74]. In fact, exercise training reduces sympathetic nervous activity, improving parasympathetic/sympathetic balance [Citation75–79]. Indeed, exercise training decreases resting heart rate because of an increase in cardiac vagal tone and a decline in intrinsic heart rate [Citation78]. Additionally, exercise training is a safe and low-cost strategy that increases quality of life, functionality, and survival rates with minimal or no side effects [Citation75]. Furthermore, exercise training also appears to have an impact on several hallmarks of cancer [Citation6,Citation7] such as resistance to cell death, sustained proliferative signaling, invasion and metastasis; contributing to increased angiogenesis in the tumor region, reprogramming energy metabolism (from glycolytic to oxidative), preventing the destruction of immune system cells and stimulating apoptosis of tumor cells [Citation80]. Specifically, increased angiogenesis may assist in another regulatory mechanism of tumor proliferation: the production of myokines. Myokines are proteins, lipids and nucleic acids (DNA, miRNAs), which are produced and released into the bloodstream by skeletal muscle during and immediately after physical exercise, stimulating internal signaling and/or communication with other organs and tissues, such as the liver, adipose tissue and heart [Citation81–84] and which can mitigate the proliferation of tumor cells. Indeed, myokines can produce diverse local and distant antineoplastic effects [Citation80]. Hojman and colleagues [Citation85] showed that breast cancer cells treated with serum collected from animals after physical exercise (with myokines) caused both a significant decrease in proliferation and an increase in apoptosis of tumor cells, leading to inhibition of tumor growth. Therefore, some effects of exercise training resemble the effects of β blockers which may be beneficial, for example, before surgery or in association with standard treatments. Furthermore, physical training promotes benefits that can minimize other effects of cancer, and is recommended for all phases of treatment (before, during and after treatment), regardless of the type and stage of the disease [Citation86]. In this sense, exercise training can be prescribed according to the capabilities of patients due to the side effects of cancer. Thus, supervised exercise training may contribute to improving the response to treatment, survival and, mainly, the quality of life of patients with lung cancer.
Preclinical studies suggest autonomic innervation in lung cancer can trigger tumor progression, metastasis, and resistance to treatment.
Increased expression of β2-adrenergic receptors has been associated with poor prognosis in recurrence-free survival and overall survival in lung cancer patients.
The incidental use of β-blockers as an add-on to standard lung cancer treatments has led to an increase in overall survival and recurrence-free survival, in addition to reducing the occurrence of metastasis.
However, when considering the isolated use of β-blockers, no significant effects on overall survival, recurrence-free survival or cancer-specific mortality have been observed.
The impact of incidental β-blocker use on the perioperative period remains inconclusive, showing the need for further research in this area.
Author contributions
FT Garramona and TF Cunha conducted the literature search, assigned, and evaluated and ex- or included the literature according to the methodological guidelines and were the major contributors in writing of the manuscript with equal contributions; JS Vieira, G Borges and G Santos accompanied the literature research and participated in writing of the manuscript; G Castro Junior has revised the specific topic of cancer in the manuscript; C Ugrinowitsch evaluated and ex-/included the literature according to the methodological guidelines, revised the methodological design and the writing of the manuscript; PC Brum revised and supervised all the manuscript. All authors have read and agreed to the published version of the manuscript.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/lmt-2023-0006
Financial disclosure
TF Cunha held a scholarship from Conselho Nacional de Pesquisa e Desenvolvimento (CNPq #2021/11800-4). JS Vieira held a scholarship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP #). G Borges held a scholarship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP #2021/12279-3). C Ugrinowitsch is supported by CNPq (#303047/2022-4) and FAPESP (#2020/06032-5). PC Brum is supported by CNPq (#308071/2021-2). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Gibbons CH . Basics of autonomic nervous system function. Handbook Clin. Neurol. 160, 407–418 (2019).
- Hutchings C , Phillips JA , Djamgoz MB . Nerve input to tumours: pathophysiological consequences of a dynamic relationship. Biochim. Biophys. Acta Rev. Cancer 1874(2), 188411 (2020).
- Servick K . War of nerves. Science 365(6458), 1071–1073 (2019).
- Zahalka AH , Frenette PS . Nerves in cancer. Nat. Rev. Cancer 20(3), 143–157 (2020).
- Entschladen F , Palm D , Lang K , Drell TL IV , Zaenker KS . Neoneurogenesis: tumors may initiate their own innervation by the release of neurotrophic factors in analogy to lymphangiogenesis and neoangiogenesis. Med. Hypotheses 67(1), 33–35 (2006).
- Hanahan D . Hallmarks of cancer: new dimensions. Cancer Disc. 12(1), 31–46 (2022).
- Hanahan D , Weinberg RA . Hallmarks of cancer: the next generation. Cell 144(5), 646–674 (2011).
- Kappos EA , Engels PE , Tremp M et al. Denervation leads to volume regression in breast cancer. J. Plasti. Reconstr. Aesthet. Surg. 71(6), 833–839 (2018).
- Makale MT , Kesari S , Wrasidlo W . The autonomic nervous system and cancer. Biocybern. Biomed. Engin. 37(3), 443–452 (2017).
- Senga SS , Grose RP . Hallmarks of cancer—the new testament. Open Biol. 11(1), 200358 (2021).
- Magnon C . Role of the autonomic nervous system in tumorigenesis and metastasis. Mol. Cell. Oncol. 2(2), e975643 (2015).
- March B , Faulkner S , Jobling P et al. Tumor Innervation and Neurosignalling in Prostate Cancer. Nat. Rev. Urol. 17(2), 119–130 (2020).
- Hutchings C , Phillips J , Djamgoz MB . Nerve input to tumors: pathophysiological consequences of a dynamic relationship. Biochim. Biophys. Acta Rev. Cancer 1874(2), 188411 (2020).
- Faulkner S , Jobling P , March B , Jiang CC , Hondermarck H . Tumor neurobiology and the war of nerves in cancer. Cancer Discov. 9(6), 702–710 (2019).
- Nilsson MB , Sun H , Diao L et al. Stress hormones promote EGFR inhibitor resistance in NSCLC: implications for combinations with β-blockers. Sci. Tansl. Med. 9(415), 1–22 (2017).
- Yazawa T , Kaira K , Shimizu K et al. Prognostic significance of β2-adrenergic receptor expression in non-small-cell lung cancer. Am. J. Transl. Res. 8(11), 5059 (2016).
- Torre LA , Siegel RL , Ward EM , Jemal A . Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol. Biomarkers Prev. 25(1), 16–27 (2016).
- Travis WD . Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small-cell carcinomas. Mod. Pathol. 25(1), S18–S30 (2012).
- Nilsson MB , Le X , Heymach JV . Beta-adrenergic signaling in lung cancer: a potential role for β-blockers. J. Neuroimmune Pharmacol. 15, 27–36 (2020).
- Al-Wadei HA , Al-Wadei MH , Schuller HM . Cooperative regulation of non-small-cell lung carcinoma by nicotinic and beta-adrenergic receptors: a novel target for intervention. PLOS ONE 7(1), e29915 (2012).
- Collisson E , Campbell J , Brooks A et al. Comprehensive molecular profiling of lung adenocarcinoma: the cancer genome atlas research network. Nature 511(7511), 543–550 (2014).
- Nilsson MB , Le X , Heymach JV . β-Adrenergic signaling in lung cancer: a potential role for β-blockers. J. Neuroimmune Pharmacol. 15(1), 27–36 (2020).
- Zoucas E , Nilsson C , Alm P , Ihse I . Selective microsurgical sympathetic denervation of the rat pancreas. Eur. Surg. Res. 28(5), 367–373 (1996).
- Kamiya A , Hayama Y , Kato S et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 22(8), 1289–1305 (2019).
- Horvathova L , Padova A , Tillinger A , Osacka J , Bizik J , Mravec B . Sympathectomy reduces tumor weight and affects expression of tumor-related genes in melanoma tissue in the mouse. Stress 19(5), 528–534 (2016).
- Neeman E , Zmora O , Ben-Eliyahu S . A new approach to reducing postsurgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clin. Cancer Res. 18(18), 4895–4902 (2012).
- Plummer HK , Dhar MS , Cekanova M , Schuller HM . Expression of G-protein inwardly rectifying potassium channels (GIRKs) in lung cancer cell lines. BMC Cancer 5(1), 1–10 (2005).
- Higgins JP , Thomas J , Chandler J et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons., NJ, USA, 2 (2019).
- Tricco AC , Lillie E , Zarin W et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 169(7), 467–473 (2018).
- Peters MD , Godfrey CM , Khalil H , McInerney P , Parker D , Soares CB . Guidance for conducting systematic scoping reviews. JBI Evidence Implement. 13(3), 141–146 (2015).
- Downs SH , Black N . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Comm. Health 52(6), 377–384 (1998).
- Hooper P , Jutai JW , Strong G , Russell-Minda E . Age-related macular degeneration and low-vision rehabilitation: a systematic review. Can. J. Ophthalmol. 43(2), 180–187 (2008).
- Deeks JJ , Dinnes J , D’Amico R et al. Evaluating non-randomised intervention studies. Health Technol. Assess. 7(27), iii–173 (2003).
- Aydiner A , Ciftci R , Karabulut S , Kilic L . Does beta-blocker therapy improve the survival of patients with metastatic non-small-cell lung cancer? Asian Pacific J. Cancer Preven. 14(10), 6109–6114 (2013).
- Cata JP , Villarreal J , Keerty D et al. Perioperative beta-blocker use and survival in lung cancer patients. J. Clin. Anesth. 26(2), 106–117 (2014).
- Chang C-H , Lee C-H , Ko J-C et al. Effect of β-blocker in treatment-naïve patients with advanced lung adenocarcinoma receiving first-generation EGFR-TKIs. Front. Oncol. 10, 583529 (2020).
- Chaudhary KR , Yan SX , Heilbroner SP et al. Effects of β-Adrenergic antagonists on chemoradiation therapy for locally advanced non-small-cell lung cancer. J. Clin. Med. 8(5), 575 (2019).
- Coelho M , Imperatori A , Chiaravalli AM et al. Beta1-and beta2-adrenoceptors expression patterns in human non-small-cell lung cancer: relationship with cancer histology. J. Neuroimm. Pharmacol. 14(4), 697–708 (2019).
- Holmes S , Griffith EJ , Musto G , Minuk GY . Antihypertensive medications and survival in patients with cancer: a population-based retrospective cohort study. Cancer Epidemiol. 37(6), 881–885 (2013).
- Kaira K , Kamiyoshihara M , Kawashima O et al. Prognostic impact of β2 adrenergic receptor expression in surgically resected pulmonary pleomorphic carcinoma. Anticancer Res. 39(1), 395–403 (2019).
- Lin C-S , Lin W-S , Lin C-L , Kao C-H . Carvedilol use is associated with reduced cancer risk: a nationwide population-based cohort study. Int. J. Cardiol. 184, 9–13 (2015).
- Mei L , Huang C , Wang A , Zhang X . Association between ADRB2, IL33, and IL2RB gene polymorphisms and lung cancer risk in a Chinese Han population. Internation. Immunopharmacol. 77, 105930 (2019).
- Musselman RP , Bennett S , Li W et al. Association between perioperative beta blocker use and cancer survival following surgical resection. Eur. J. Surg. Oncol. 44(8), 1164–1169 (2018).
- Numbere B , Fleming KM , Walker A , Card TR . Adrenergic blockers and the risk for common solid cancers: a case–control study. Eur. J. Cancer Prev. 26(1), 86–93 (2017).
- Oh MS , Guzner A , Wainwright DA et al. The impact of beta blockers on survival outcomes in patients with non–small-cell lung cancer treated with immune checkpoint inhibitors. Clin. Lung Cancer 22(1), e57–e62 (2021).
- Sakamoto A , Yagi K , Okamura T , Harada T , Usuda J . Perioperative administration of an intravenous beta-blocker landiolol hydrochloride in patients with lung cancer: a Japanese retrospective exploratory clinical study. Scient. Rep. 9(1), 1–6 (2019).
- Shah SM , Carey IM , Owen CG , Harris T , Dewilde S , Cook DG . Does β-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br. J. Clin. Pharmacol. 72(1), 157–161 (2011).
- Shao JX , Wang B , Yao YN , Pan ZJ , Shen Q , Zhou JY . Autonomic nervous infiltration positively correlates with pathological risk grading and poor prognosis in patients with lung adenocarcinoma. Thor. Cancer 7(5), 588–598 (2016).
- Udumyan R , Montgomery S , Fang F et al. β-Blocker Use and Lung Cancer Mortality in a Nationwide Cohort Study of Patients with Primary Non–Small Cell Lung Cancer. Cancer Epidemiol. Biomark. Preven. 29(1), 119–126 (2020).
- Wang H , Hao B , Chen X et al. Beta-2 adrenergic receptor gene (ADRB2) polymorphism and risk for lung adenocarcinoma: a case–control study in a Chinese population. Cancer Lett. 240(2), 297–305 (2006).
- Wang H , Liao Z , Komaki R et al. Improved survival outcomes with the incidental use of β-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann. Oncol. 24(5), 1312–1319 (2013).
- Wang H , Liao Z , Zhuang Y et al. Incidental receipt of cardiac medications and survival outcomes among patients with stage III non–small-cell lung cancer after definitive radiotherapy. Clin. Lung Cancer 16(2), 128–136 (2015).
- Weberpals J , Jansen L , Haefeli WE et al. Pre-and post-diagnostic β-blocker use and lung cancer survival: a population-based cohort study. Scient. Reports 7(1), 1–11 (2017).
- Yang P , Deng W , Han Y et al. Analysis of the correlation among hypertension, the intake of β-blockers, and overall survival outcome in patients undergoing chemoradiotherapy with inoperable stage III non-small-cell lung cancer. Am. J. Cancer Res. 7(4), 946 (2017).
- Ji L , Xu F , Zhang J et al. ADRB2 expression predicts the clinical outcomes and is associated with immune cells infiltration in lung adenocarcinoma. Scien. Reports 12(1), 15994 (2022).
- Cui Y , Wen W , Zheng T et al. Use of antihypertensive medications and survival rates for breast, colorectal, lung, or stomach cancer. Am. J. Epidemiol. 188(8), 1512–1528 (2019).
- Entschladen F , Palm D , Niggemann B , Zaenker KS . The cancer’s nervous tooth: considering the neuronal crosstalk within tumors. Semin Cancer Biol. 18(3), 171–5 (2008).
- Schuller HM , Cekanova M . NNK-induced hamster lung adenocarcinomas over-express β2-adrenergic and EGFR signaling pathways. Lung Cancer 49(1), 35–45 (2005).
- Kondratenko T , Zacharova I , Kuzina N et al. Alterations in human lung adrenergic receptors in cancer. Biochem. Mol. Biol. Int. 29(1), 123–130 (1993).
- Daly C , McGrath J . Previously unsuspected widespread cellular and tissue distribution of β-adrenoceptors and its relevance to drug action. Trends Pharmacol. Sci. 32(4), 219–226 (2011).
- Zahalka AH , Fram E , Lin W et al. Use of beta-blocker types and risk of incident prostate cancer in a multiethnic population. Urol. Oncol. 38(10), 794.e11–794.e16 (2020).
- Fjæstad KY , Rømer AMA , Goitea V et al. Blockade of beta-adrenergic receptors reduces cancer growth and enhances the response to anti-CTLA4 therapy by modulating the tumor microenvironment. Oncogene 41(9), 1364–1375 (2022).
- Zhang J , Deng Y-T , Liu J et al. Norepinephrine induced epithelial–mesenchymal transition in HT-29 and A549 cells in vitro . J. Cancer Res. Clin. Oncol. 142(2), 423–435 (2016).
- Cole SW , Sood AK . Molecular pathways: beta-adrenergic signaling in cancer. Clinical Cancer Res. 18(5), 1201–1206 (2012).
- Cserép Z , Losoncz E , Balog P et al. The impact of preoperative anxiety and education level on long-term mortality after cardiac surgery. J. Cardiothor. Surg. 7(1), 1–8 (2012).
- Shaashua L , Shabat-Simon M , Haldar R et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin. Cancer Res. 23(16), 4651–4661 (2017).
- Haldar R , Ricon-Becker I , Radin A et al. Perioperative COX2 and β-adrenergic blockade improves biomarkers of tumor metastasis, immunity, and inflammation in colorectal cancer: a randomized controlled trial. Cancer 126(17), 3991–4001 (2020).
- Yap A , Lopez-Olivo M , Dubowitz J et al. Effect of beta-blockers on cancer recurrence and survival: a meta-analysis of epidemiological and perioperative studies. Br. J. Anaesth. 121(1), 45–57 (2018).
- Hayakawa Y , Sakitani K , Konishi M et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer cell 31(1), 21–34 (2017).
- Romeo HE , Colombo LL , Esquifino AI , Rosenstein RE , Chuluyan HE , Cardinali DP . Slower growth of tumors in sympathetically denervated murine skin. J. Auton. Nerv. Syst. 32(2), 159–164 (1991).
- Zhao C-M , Hayakawa Y , Kodama Y et al. Denervation suppresses gastric tumorigenesis. Sci. Translat. Med. 6(250), 250ra115 (2014).
- Guyatt GH , Oxman AD , Schünemann HJ , Tugwell P , Knottnerus A . GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 64(4), 380–382 (2011).
- Hardman AE . Physical activity and cancer risk. Proc. Nutr. Soc. 60(1), 107–113 (2001).
- Khan N , Afaq F , Mukhtar H . Lifestyle as risk factor for cancer: evidence from human studies. Cancer Lett. 293(2), 133–143 (2010).
- Fu Q , Levine BD . Exercise and the autonomic nervous system. Handbook Clinical Neurol. 117, 147–160 (2013).
- Negrao CE , Middlekauff HR . Exercise training in heart failure: reduction in angiotensin II, sympathetic nerve activity, and baroreflex control. J. Appl. Physiol. 104(3), 577–8 (2008).
- Rondon E , Brasileiro-Santos MS , Moreira ED et al. Exercise training improves aortic depressor nerve sensitivity in rats with ischemia-induced heart failure. Am. J. Physiol. Heart Circulat. Physiol. 291(6), H2801–H2806 (2006).
- Rosenwinkel ET , Bloomfield DM , Arwady MA , Goldsmith RL . Exercise and autonomic function in health and cardiovascular disease. Cardiol. Clin. 19(3), 369–387 (2001).
- Brum P , Bacurau A , Medeiros A , Ferreira J , Vanzelli A , Negrão C . Aerobic exercise training in heart failure: impact on sympathetic hyperactivity and cardiac and skeletal muscle function. Braz. J. Med. Biol. Res. 44, 827–835 (2011).
- Ruiz-Casado A , Martín-Ruiz A , Pérez LM , Provencio M , Fiuza-Luces C , Lucia A . Exercise and the hallmarks of cancer. Trends Cancer 3(6), 423–441 (2017).
- Steensberg A , van Hall G , Osada T , Sacchetti M , Saltin B , Pedersen BK . Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 529(Pt 1), 237–42 (2000).
- Pedersen B , Steensberg A , Fischer C et al. Searching for the exercise factor: is IL-6 a candidate? J. Muscle Res. Cell Motility 24, 113–119 (2003).
- Ouchi N , Oshima Y , Ohashi K et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J. Biol. Chem. 283(47), 32802–32811 (2008).
- Izumiya Y , Hopkins T , Morris C et al. Fast/glycolytic muscle fiber growth reduces fat mass and improves metabolic parameters in obese mice. Cell Metab. 7(2), 159–172 (2008).
- Hojman P , Gehl J , Christensen JF , Pedersen BK . Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 27(1), 10–21 (2018).
- Deminice R . Exercício físico para o tratamento do câncer: evidências científicas eo contexto brasileiro. J. Phys. Educ. 33(1), e3301 (2022).