Abstract
Background. The plasma level of cystatin C is a better marker than plasma creatinine for successful aging. It has been assumed that the advantage of cystatin C is not only due to it being a better marker for glomerular filtration rate (GFR) than creatinine, but also because an inflammatory state of a patient induces a raised cystatin C level. However, the observations of an association between cystatin C level and inflammation stem from large cohort studies. The present work concerns the cystatin C levels and degree of inflammation in longitudinal studies of individual subjects without inflammation, who undergo elective surgery. Methods. Cystatin C, creatinine, and the inflammatory markers CRP, serum amyloid A (SAA), haptoglobin and orosomucoid were measured in plasma samples from 35 patients the day before elective surgery and subsequently during seven consecutive days. Results. Twenty patients had CRP-levels below 1 mg/L before surgery and low levels of the additional inflammatory markers. Surgery caused marked inflammation with high peak values of CRP and SAA on the second day after the operation. The cystatin C level did not change significantly during the observation period and did not correlate significantly with the level of any of the four inflammatory markers. The creatinine level was significantly reduced on the first postoperative day but reached the preoperative level towards the end of the observation period. Conclusion. The inflammatory status of a patient does not influence the role of cystatin C as a marker of successful aging, nor of GFR.
Key Words::
Introduction
Increased levels of cystatin C have been shown to be associated with less successful aging than normal levels of cystatin C and cystatin C has been described to have a better diagnostic performance than creatinine to predict successful aging [Citation1–6]. It is known that a decrease in GFR is associated with less successful aging [Citation7,Citation8] and it has therefore been speculated that the advantage of cystatin C as a marker of successful aging is caused by it being a better marker for GFR than creatinine [Citation1,Citation2,Citation9]. However, studies of large patient cohorts have revealed a statistically significant correlation between cystatin C level and degree of inflammation and it has therefore been suggested that cystatin C is a marker not only of GFR but also of the degree of inflammation, thus explaining its superiority as a marker of successful aging [Citation5,Citation10–13]. But, although significant statistical correlations between levels of different components in cohort studies suggest, they do not demonstrate, causal relationships between the levels of the components. The present study was undertaken to verify or refute a causal relationship between the levels of cystatin C or creatinine and the degree of inflammation. It concerns modification of the inflammatory status of patients and its influence upon their plasma levels of cystatin C and creatinine.
Materials and methods
Study population
Thirty-five patients scheduled for elective surgery, with normal body temperatures and able to provide written informed consent, without the need for a proxy respondent, were considered for inclusion in the study. The study complied with the Declaration of Helsinki as revised in 1983. Tissue trauma during surgical procedures varied from minor to medium.
Determination of protein and creatinine levels
Venous blood samples were collected in EDTA- containing tubes on the day before surgery (Day 0) and daily postoperatively for seven days (Day 1 to 7).
Plasma CRP, orosomucoid (alpha-1-acid glycoprotein) and haptoglobin were determined by immunoturbidimetry using a Roche-Hitachi cobas 6000 analysis platform and with a calibrator based upon the international calibrator CRM 470 [Citation14]. Reference ranges for orosomucoid: 0.5–1.2 g/L, and for haptoglobin: 0.3–2.0 g/L [Citation14]. The median CRP-levels in the third and seventh decades of healthy individuals in the general adult population have been given as 1 and 2 mg/L, respectively [Citation15]. Plasma cystatin C was measured by an automated particle-enhanced immunoturbidimetric assay [Citation16] using the same Roche-Hitachi cobas 6000 analysis platform and a calibrator of isolated non-truncated recombinant human cystatin C [Citation16]. Reference range: 20–50 years of age: 0.70–1.21 mg/L and, above 50 years of age: 0.84–1.55 mg/L [Citation17]. Plasma SAA was measured by an automated particle-enhanced immunonephelometric assay [Citation18] using a Siemens BN ProSpec analysis system and reagents from Siemens. The SAA-values were calibrated against the 1st international standard 1997 serum amyloid A protein lot 92/680 [Citation19]. Reference range: 0.84–11.4 mg/L [Citation18]. Plasma creatinine was determined by a creatininase-based procedure using the same Roche-Hitachi cobas 6000 analysis platform as described above. The creatinine calibrator was traceable to primary reference material with values assigned by isotope dilution mass spectrometry [Citation20]. Reference ranges for males 20–70 years of age: 56–103 μmol/L and for females 20–70 years of age: 42–82 μmol/L [Citation21].
Statistical analysis
All statistical procedures were performed using SPSS release 15.0.0 (SPSS Inc., Chicago, US). To limit the problem of multiple comparisons, the time course of the levels of each inflammatory marker (CRP, SAA, haptoglobin and orosomucoid), as well as of creatinine and cystatin C, were evaluated by two distinct statistical tests only: (i) Differences between preoperative levels and the first (CRP, SAA, creatinine and cystatin C) or second (haptoglobin and orosomucoid) postoperative day were tested using Wilcoxon signed rank test; and (ii) for CRP, SAA, creatinine and cystatin C, log-linear trends in the changes in levels on postoperative day 1 through 7 were tested overall, using a linear mixed model with the logarithm of the marker day 1 through 7 as dependent variable, day as a fixed continuous covariate and with no random effect but a first-order autoregressive model for the residuals. To assess the correlation between inflammatory markers and creatinine and cystatin C, the mixed models with inflammatory markers as dependent variables were extended by including the natural logarithm of creatinine and cystatin C, respectively, as fixed covariates. Due to missing data at several time points, mixed models were preferred to methods requiring complete data at all time points. p-values < 0.05 were considered significant.
Results
The presence of systemic inflammation in 35 patients scheduled for elective surgery was investigated by analysis of the plasma levels of four commonly used markers for inflammation: CRP, SAA, haptoglobin and orosomucoid. The preoperative CRP levels in 20 of the patients were below 1 mg/L, which is below the median CRP level in the third decade of a general adult population [Citation15] indicating absence of systemic inflammation in these 20 patients, which therefore were selected for further study. The preoperative levels of orosomucoid and haptoglobin were below the upper reference levels for all or 18, respectively, of the 20 patients, supporting the absence of pronounced inflammation. However, the preoperative level of SAA was slightly above the upper reference level for 8 of the 20 patients with a maximal level of 22.5 mg/L, indicating that a low-degree inflammation might have been present in some of the patients. The levels of cystatin C, 0.72–1.45 mg/L, and creatinine, 49–115 μmol/L, were below, or just above, the upper reference limits.
The levels of CRP and SAA increased markedly on the first postoperative day (p < 0.001 and p = 0.001, respectively) and reached maximal values on the second postoperative day with median levels corresponding to 46 and 32 times the preoperative levels of CRP and SAA, respectively (). Both the SAA and CRP levels decreased after the second day but were still above the upper reference levels on the seventh postoperative day (). The levels of haptoglobin and orosomucoid were significantly increased on the second postoperative day and did not decrease significantly during the following five postoperative days ().
Figure 1. Changes in plasma levels of (A) CRP, SAA, creatinine and cystatin C and of (B) orosomucoid, haptoglobin, creatinine and cystatin C during seven consecutive days after elective surgery. Day zero denotes the day before surgery. The ordinates represent the median level of each analyte in multiples of the preoperative level.
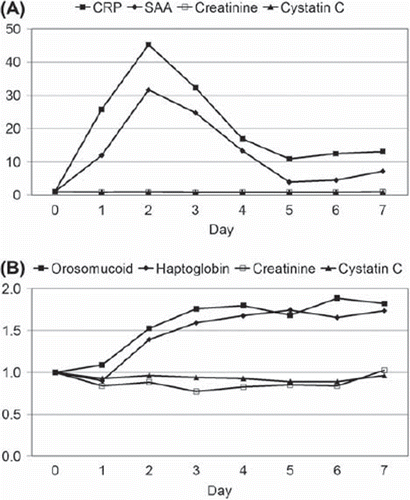
In contrast to the levels of the inflammatory markers, the cystatin C level did not change significantly on the first postoperative day (p = 0.20; preoperative median = 1.08 mg/L, first postoperative day median = 1.00 mg/L), and remained stable during day 1–7 (p = 0.49). Creatinine decreased from 81–65 μmol/L in median on the first postoperative day (p = 0.009), but tended to increase again during day 1–7 (p = 0.19). The median values and the corresponding 1st and 3rd quartiles of the CRP, SAA, haptoglobin, orosomucoid, creatinine and cystatin C levels are given in .
Figure 2. Changes in plasma levels of (A) CRP, (B) SAA, (C) haptoglobin, (D) orosomucoid, (E) creatinine and (F) cystatin C during seven consecutive days after elective surgery. Day zero denotes the day before surgery. The ordinates represent the median and the 1st and 3rd quartiles of the level of each analyte.
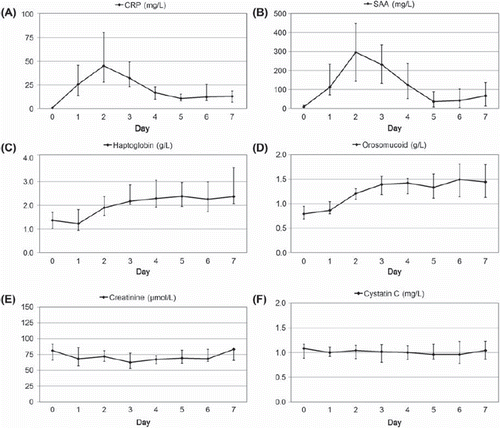
The cystatin C level showed no statistically significant correlation with the levels of any of the inflammatory markers (SAA: p = 0.10, CRP: p = 0.65, Haptoglobin: p = 0.72, Orosomucoid: p = 0.58). The creatinine level displayed a significant correlation with the SAA level (p = 0.019), but not with the levels of any of the other inflammatory markers (CRP: p = 0.13, Haptoglobin: p = 0.70, Orosomucoid: p = 0.80).
Discussion
It has been suggested that cystatin C is a better marker than creatinine for survival success not only because it is a better marker for GFR but also because the presence of an inflammatory reaction in an individual produces a raised cystatin C level [Citation5,Citation10–13]. This suggestion has been based upon demonstration of a statistically significant correlation between the levels of CRP, a marker for inflammation, and cystatin C in large cohort studies. However, correlations in cohort studies can suggest, but not demonstrate, causal relationships between the levels of the components. The present investigation comprised testing of the inflammatory status of 20 patients scheduled for elective surgery by studying their plasma levels of four inflammatory markers – CRP, SAA, haptoglobin and orosomucoid before surgery and during seven consecutive postoperative days. CRP and SAA were selected because their plasma levels are known to increase rapidly and extensively after initiation of an inflammatory process and swiftly decrease after disappearance of the cause of the inflammation. Haptoglobin and orosomucoid were selected because inflammation produces a more sustained increase of their plasma levels than those of CRP and SAA. The 20 patients were selected from a larger cohort of patients scheduled for elective surgery, because their CRP levels were below 1 mg/L, thus indicating the absence of inflammation in these 20 patients. The levels of cystatin C and creatinine in 19 of the 20 patients were below the upper reference limits. As expected, surgery caused a marked increase in the plasma levels of all four markers of inflammation. In contrast, no change in the cystatin C levels could be observed during the seven postoperative days. Neither did the level of cystatin C show any significant statistical correlation with the levels of any of the four markers of inflammation. We believe that these data strongly argue against the suggestion that systemic inflammation causes increased plasma levels of cystatin C. However, if inflammatory conditions without alterations in the levels of any of the four inflammatory markers used in this work exist, such conditions might theoretically increase the cystatin C level.
Although the results of the present study might seem to be in conflict with the previous observations of significant correlations between the plasma levels of CRP and cystatin C in cohort studies [Citation5,Citation10–13], we do not think this is the case. For the cohort studies concerned the risk of cardiovascular events and death in elderly populations and demonstrated that higher levels of both cystatin C and CRP were significantly correlated with higher incidence of cardiovascular events and premature death. This means that even without a causal relationship between the levels of CRP (‘inflammation’) and cystatin C, a statistical significant correlation will be found because raised levels of both components indicate increased risk of cardiovascular events and premature death.
The present study indicates that the advantage of cystatin C compared to creatinine as a marker of successful aging is not due to the fact that an increased level of cystatin C signals the presence of inflammation in addition to a decrease in GFR, as previously suggested [Citation5,Citation10–13]. The advantage might, of course, solely be related to the fact that cystatin C is a better marker for a decrease in GFR than creatinine, but additional causes for its superiority as a marker for successful aging might be suggested. For example, cystatin C might identify the alteration in the composition of the glomerular filtrate with a reduced filtration of large molecules, e.g. cystatin C (13 343 Da), but an unaltered filtration of small molecules, e.g. water (18 Da) and creatinine (113 Da), which often occurs before a decrease in GFR can be demonstrated [Citation22–25]. The capacity of cystatin C to early identify an abnormal filtration quality might also explain the observation that an elevated cystatin C level indicates higher incidence of cardiovascular events and death even in the presence of a normal GFR [Citation4,Citation8]. The observations reported in two recent studies of cystatin C in hypertensive patients and in patients on hemodialysis agree with the result of the present study that there is no correlation between cystatin C and CRP in important patient groups [Citation26,Citation27].
Acknowledgements
The investigation was supported by grants from the Swedish Science Research Council (Project No. 5196) from the Alfred Österlund Foundation, Greta and Johan Kock Foundation, Medical Faculty of the University of Lund and from Region Skåne.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of the article.
References
- Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005;352:2049–60.
- Stevens LA, Levey AS. Chronic kidney disease in the elderly – how to assess risk. N Engl J Med 2005;352:2122–4.
- Zethelius B, Berglund L, Sundström J, Ingelsson E, Basu S, Larsson A, Venge P, Ärnlöv J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med 2008;358:2107–16.
- Sarnak MJ, Katz R, Fried LF, Siscovick D, Kestenbaum B, Seliger S, Rifkin D, Tracy R, Newman AB, Shlipak MG. Cystatin C and aging success. Arch Intern Med 2008;168:147–53.
- Taglieri N, Koenig W, Kaski JC. Cystatin C and cardiovascular risk. Clin Chem 2009;55:1932–43.
- Jernberg T, Lindahl B, James S, Larsson A, Hansson LO, Wallentin L. Cystatin C: a novel predictor of outcome in suspected or confirmed non-ST-elevation acute coronary syndrome. Circulation 2004;110:2342–8.
- Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function – measured and estimated glomerular filtration rate. N Engl J Med 2006;354:2473–83.
- Rifkin DE, Katz R, Chonchol M, Fried LF, Cao J, deBoer IH, Siscovick DS, Shlipak MG, Sarnak MJ. Albuminuria, impaired kidney function and cardiovascular outcomes or mortality in the elderly. Nephrol Dial Transplant 2010;25:1560–7.
- Perkins BA, Nelson RG, Krolewski AS. Cystatin C and the risk of death. N Engl J Med 2005;353:843.
- Koenig W, Twardella D, Brenner H, Rothenbacher D. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rate. Clin Chem 2005;51:321–7.
- Singh D, Whooley MA, Ix JH, Ali S, Shlipak MG. Association of cystatin C and estimated GFR with inflammatory biomarkers: the Heart and Soul study. Nephrol Dial Transplant 2007;22:1087–92.
- Keller CR, Odden MC, Fried LF, Newman AB, Angleman S, Green CA, Cummings SR, Harris TB, Shlipak MG. Kidney function and markers of inflammation in elderly persons without chronic kidney disease: the health, aging, and body composition study. Kidney Int 2007;71:239–44.
- Knight EL, Verhave JC, Spiegelman D, Hillege HL, deZeeuw D, Curhan GC, deJong PE. Factors influencing cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int 2004;65:1416–21.
- Dati F, Schumann G, Thomas L, Aguzzi F, Baudner S, Bienvenu J, Blaabjerg O, Blirup-Jensen S, Carlström A, Hyltoft-Petersen P, Johnson AM, Milfor-Ward A, Ritchie R, Just Svendsen P, Whicher J. Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP reference material (CRM 470). Eur J Clin Chem Clin Biochem 1996;34:517–20.
- Hutchinson WL, Koenig W, Fröhlich M, Sund M, Lowe GDO, Pepys MB. Immunoradiometric assay of circulating C-reactive protein: age-related values in the adult general population. Clin Chem 2000;46:934–8.
- Kyhse-Andersen J, Schmidt C, Nordin G, Andersson B, Nilsson-Ehle P, Lindström V, Grubb A. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem 1994;40:1921–6.
- Norlund L, Fex G, Lanke J, von Schenck H, Nilsson JE, Leksell H, Grubb A. Reference intervals for the glomerular filtration and cell-proliferation markers: serum cystatin C and serum beta-2-microglobulin/cystatin C ratio. Scand J Clin Lab Invest 1997;57:463–70.
- Rothkrantz-Kos S, Schmitz MP, van Dieijen-Visser MP. Analytical evaluation and reference values of serum amyloid-A on the BN ProSpec. Clin Chem Lab Med 2004;42:973–5.
- Poole S, Walker D, Gaines Das RE, Gallimore JR, Pepys MB. The first international standard for serum amyloid A protein (SAA). Evaluation in an international collaborative study. J Immunol Methods 1998;214:1–10.
- Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–54.
- Pottel H, Vrydags N, Mahieu B, Vandewynckele E, Croes K, Martens F. Establishing age/sex related serum creatinine reference intervals from hospital laboratory data based on different statistical methods. Clin Chim Acta 2008;396:49–55.
- Grubb A. Non-invasive estimation of glomerular filtration rate (GFR). The Lund model: simultaneous use of cystatin C- and creatinine-based GFR-prediction equations, clinical data and an internal quality check. Scand J Clin Lab Invest 2010;70:65–70.
- Grubb A, Lindström V, Kristensen K, Christensson A, Wide-Swensson D, Strevens H, Schmidt C, Blirup-Jensen S. Filtration quality: a new measure of renal disease. Clin Chem Lab Med 2007;45:(Suppl. S2):73–4.
- Strevens H, Wide-Swensson D, Torffvit O, Grubb A. Serum cystatin C for assessment of glomerular filtration rate in pregnant and non-pregnant women. Indications of altered filtration process in pregnancy. Scand J Clin Lab Invest 2002;62:141–8.
- Roberts M, Lindheimer MD, Davison JM. Altered glomerular permselectivity to neutral dextrans and heteroporous membrane modeling in human pregnancy. Am J Physiol 1996;270:F338–43.
- Okura T, Jotoku M, Irita J, Enomoto D, Nagao T, Desilva VR, Yamane S, Pei Z, Kojima S, Hamano Y, Mashiba S, Kurata M, Miyoshi K, Higaki J. Association between cystatin C and inflammation in patients with essential hypertension. Clin Exp Nephrol 2010;14:358–63.
- Al-Maliki N, Heidenheim PA, Filler G, Yasin A, Lindsay RM. Cystatin C levels in functionally anephric patients undergoing dialysis: the effect of different methods and intensities. Clin J Am Soc Nephrol 2009;4:1606–10.
