Abstract
Congenital cytomegalovirus (CMV) infection is the leading non-genetic cause of sensori-neural hearing loss and neurodevelopmental sequelae. Despite these alarming facts, the general public healthcare system is often not aware of CMV and not enough is done to prevent congenital CMV infection.We describe the clinical and laboratory monitoring of a case with primary CMV infection occurring before the first trimester of gestation. Specific literature review is included in order to point out major goals achieved in the diagnosis and prognosis of congenital CMV infection and the many questions still unanswered. Serological diagnosis of primary CMV infection was performed based on serum-CMV specific-IgM antibodies, combined with low avidity anti-CMV IgG antibodies. The maternal infection was asymptomatic, as it is for most infections in immunocompetent patients. Therefore, disclosing primary infection depended on specific serological tests during the initial period of pregnancy (before weeks 12–16 of gestation). The invasive (amniocentesis) and non-invasive (ultrasonographic examination) prenatal tests, carried out at 21 weeks gestation, revealed a severe CMV infection in a fetus small for gestational age with ventriculomegaly. The presence of overt ultrasound abnormalities combined with high viral load in the amniotic fluid sampled at the appropriate times was highly suggestive of an unfavourable prognosis. The autopsy performed on the fetus confirmed severe disseminated CMV infection with histological brain damage.
Introduction
The overall birth prevalence of human cytomegalovirus (CMV) congenital infection was 0.64 %, but varied considerably among different study populations [Citation1].
Only 10–15 % of congenitally infected babies show symptoms of infection at birth and these infants have a perinatal mortality rate of around 10 % with 70–80 % of surviving babies presenting major neurological sequelae [Citation2]. Despite infection, 85–90 % of babies have no symptoms at birth however 8–15 % of them will suffer delayed injury [Citation2].
Mother-child transmission is mainly the result of primary maternal CMV infection which carries a risk of transmission of about 40 % whereas the transmission rate is higher in the last weeks of gestation (about 78 %). Cases of CMV transmission due to nonprimary infection have been reported in 1–2.2 % of cases [Citation1]. The extent of foetal-newborn injury, namely severe brain damage, is correlated to primary maternal infection contracted in the first trimester of pregnancy [Citation2].
Congenital CMV infection can lead to a wide spectrum of brain abnormalities, including microcephaly, periventricular calcifications, hydrocephalus, cerebellar atrophy and polymicrogyria [Citation2]
CMV is the leading non genetic cause of deafness in children; more than half the babies born with symptomatic infection and 10 % of asymptomatic newborns will develop mild to severe neurosensory hearing loss which is progressive in 50 % of cases. Hearing loss is bilateral in 50 % of cases leading to language impairment and learning delay the severity of which is directly proportional to the delay in diagnosis precluding prompt rehabilitation [Citation2].
Less than 25 % of pregnant women with primary infection are reported to be symptomatic, most CMV infections encountered are asymptomatic even during the acute stage [Citation4]. Clinical diagnosis of CMV infection is unreliable, thus laboratory tests are the best means of establishing diagnosis of CMV infection.
This paper describes the clinical and laboratory monitoring of a case involving a primarily CMV- infected woman during her second pregnancy; a literature review is also included.
Methods
Serologic tests
Anti CMV IgG was evaluated with Elecsys® CMV IgG assay (Roche, Penzberg, Germany). This assay measures the concentration of CMV-specific IgG in serum and plasma samples and results < 0.5 AU/mL (arbitrary units/mL) are considered negative and > 1.0 AU/mL positive. Cut-off indexes between 0.5 and 1.0 AU/mL are considered equivocal. Anti-CMV IgM was evaluated using an anti-CMV IgM qualitative kit (Elecsys®, Roche) and sample results (s/co) < 0.7 are considered negative and > 1.0 positive. Cut-off indexes between 0.7 and 1.0 are considered equivocal. Both kits were used as suggested by the manufacturer.
IgG avidity was determined using an Elecsys® CMV IgG Avidity (Roche, Penzberg, Germany). The results were interpreted as suggested by the manufacturer; in particular, an avidity index < 45 % was considered low, > 55 % was considered high, and between 45 % and 55 % was considered moderate.
Anti CMV IgM was confirmed using a home-made immunoblot for the detection of CMV-specific IgM. The test contains four viral proteins (vp150, vp82, vp65 and vp28) purified from viral particles and four recombinant proteins (rp150, rp130, rp52, and rp38) purified from Escherichia coli. The results were interpreted as described previously [Citation5].
Virological tests
CMV isolation. The shell vial procedure was used for CMV isolation from AF. The cells were fixed 24 hours after inoculation and were stained by an indirect immunofluorescence assay with a monoclonal antibody reacting with the CMV IE1 and EA gene product (clones E13-2A2; Argene, Varhiles, France).
Quantitative (Polymerase Chain Reaction) PCR. DNA was extracted from AF (1 mL eluted in 25 μL of elution buffer) and saliva (500 μL eluted in 55 μL of elution buffer) with the NucliSens easyMAG System (bioMerieux, Marcy l’Etoile, France) and from whole blood (200 μL eluted in 90 μL of elution buffer) and urine (500 μL eluted in 90 μL of elution buffer) with the QIAsymphony SP/AS System (QIAGEN GmbH, Hilden, Germany), according to the manufacturer's package insert. A total of 20 μL of each sample of body fluids extracted was processed for CMV quantification.
CMV-DNA was quantified with a real-time PCR assay (CMV ELITe MGB kit, ELITechGroup, Turin, Italy), according to the manufacturer's package insert. Amplification, detection, and analysis were performed with the ABI PRISM 7300 platform (PE Applied Biosystems, Foster City, California, USA). The detection limit was 11 copies/reaction and viral load was reported as number of copies/mL for all body fluids examined.
Morphologic and histologic examination
Foetal tissues were fixed in buffered 4 % formaldehyde, haematoxylin and eosin standard sections were obtained from paraffin-embedded blocks of the placenta, pancreas, kidney, lung, liver, brain, and heart. Immunohistochemical staining for CMV early (ppUL44) antigen (EA) was performed to identify CMV-positive cells (clones CCH2 and DDG9; Dako, Glostrup, Denmark).
Organs function, immune response and placenta extramedullary haematopoiesis were studied as described previously [Citation6].
Ethics
The foetal tissues were analyzed after the informed consent had been obtained from the parents and according to the policies of the Ethical Committee of St. Orsola-Malpighi University Hospital, Bologna, Italy as well as the regulations of the Italian Ministry of Health, Rome, Italy.
Case report
A 35-year-old woman during her second pregnancy experienced no complication until 10 weeks of gestation, when routine CMV serologic tests revealed suspected CMV infection. At the end of the first pregnancy, 2 years before, she was seroimmune-negative for CMV.
She had no clinical symptoms and/or abnormal laboratory findings related to an active CMV-infection and further investigations for CMV were performed at 14 weeks of gestation. CMV-immunoglobulin (IgG) with low avidity and CMV-IgM were demonstrated in both samples obtained during 10 and 14 weeks of gestation. Positive IgM results were confirmed with immunoblot testing [Citation8]. The detection of viral DNA in urine and saliva was positive with very low viral load (both less than 500 copies/mL) and CMV-DNAemia was negative. Taking into consideration the overall results obtained with laboratory tests, primary CMV infection acquired within the first 12 weeks of gestation was diagnosed ().
Table I. Interpretation of results of maternal and foetal laboratory findings at critical weeks of gestation.
At 21 weeks gestation, amniotic fluid (AF) samples were collected and CMV foetal infection was demonstrated by positive DNA detection (1.3 × 107 copies/mL of AF) and positive virus isolation (). At the time of amniocentesis, maternal blood was CMV-negative. Ultrasound examination showed an intrauterine growth retardation, ventriculomegaly, hyperechogenic bowel and hepatomegaly. The diagnostic protocol, both invasive (amniocentesis) and non-invasive (ultrasonographic examination) prenatal tests, revealed a CMV-infected foetus, small for gestational age with ventriculomegaly. The laboratory and instrumental findings were highly suggestive of CMV infection complicated by severe cerebral compromission.
On the basis of severe foetal damage, the couple opted to terminate the pregnancy within the time frame currently provided by Italian legislation that does not allow late termination.
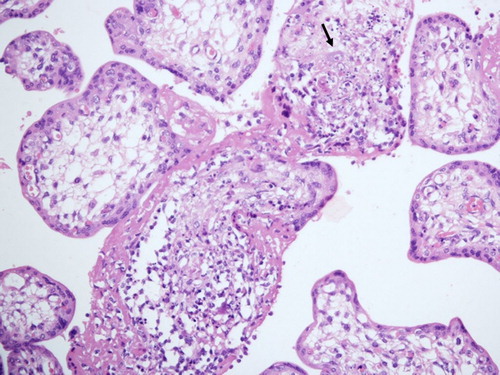
At autopsy, CMV-positive cells were detected in the placenta, pancreas, lungs, kidneys and liver. The foetus had CMV-positive brain with severe histological brain damage and necrosis of cerebral tissue. Microscopic examination disclosed enlarged, inclusion-bearing cells in the lungs, liver, and kidneys. In the placenta, most of the villi (80 %) were hydropic or necrotic. The areas of necrosis always showed cytomegalic cells and a prominent surrounding inflammatory infiltrate (). The overall features were characteristic of generalized cytomegalic inclusion disease associated with impaired placental function.
Discussion
Diagnosis of maternal compartment
Currently, congenital CMV infection is the most prevalent infection-related cause of congenital neurological handicap since the introduction of a universal rubella vaccination in developed countries.
Despite these alarming facts, the general public healthcare system is not often aware of CMV and not enough is done to prevent congenital CMV infection.
In this clinical case, the woman was experiencing her second pregnancy and 2 years before, at the end of the first pregnancy, she was seroimmune-negative for CMV. However, before this gestation she did not receive any information about the risks of CMV infection during pregnancy.
For pregnant women, the saliva and urine of infected children are the main sources of CMV infection, a secondary source is sexual contact. Pregnant women can become infected from their children and no actions can eliminate all risks of catching CMV from them, however there are some hygiene and behavioural measures that may be taken in order to reduce the viral spread. Prevention behaviours (i.e. hand washing whenever there is contact with a child's saliva or urine, not sharing drinking glasses or eating utensils with young children, and not kissing young children on the mouth or cheek) appear to be generally acceptable.
All women who are CMV seronegative and are planning to become pregnant or are pregnant should be informed about the risks of foetus infection and transmission, in particular those with young children or those exposed to paediatric environments. Although in the last few years many publications have stressed this issue, there is still a low level of awareness. In a cohort of women of childbearing age, only 14 % had heard of congenital CMV infection and few women are aware of CMV or preventative behavioural measures against CMV contraction [Citation7]. On the other hand, the literature highlights several gaps in the knowledge of CMV infection among obstetricians and gynaecologists who do not have a comprehensive understanding of CMV transmission and possible preventative measures.
Going back to our clinical case, only the first and second level serological tests, were able to make a correct diagnosis of primary CMV infection occurring in the first trimester of this pregnancy.
The gold standard of serological diagnosis of primary CMV infection is maternal seroconversion or the presence of serum anti-CMV specific IgM antibodies combined with low avidity anti-CMV IgG antibodies. Testing for anti-CMV IgM antibodies is the most widely used and appropriate procedure for screening pregnant women [Citation8], however the detection of IgM in the serum of pregnant women may simply be a starting point for further advanced diagnostic investigation, in particular anti-CMV IgG avidity testing and CMV IgM confirmation by immunoblotting [Citation9].
Immunoblotting with purified native viral proteins and purified recombinant proteins (structural and non structural) has repeatedly been shown to be an effective method to confirm the presence of CMV IgM antibodies in serum with a high sensitivity (100 %) and specificity (98.6 %) [Citation8].
Low avidity CMV-IgG antibodies are found after primary antigenic stimulation and usually last over a period of approximately 16–18 weeks after the onset of CMV infection [Citation10]. The determination of anti CMV antibody avidity carried out before weeks 12–16 of gestation is therefore a helpful tool to identify pregnant women who may give birth to an infected child (100 % sensitivity). If the determination of IgG avidity index is carried out for the first time later on during pregnancy, the sensitivity is drastically reduced (62.5 %) [Citation10]. A low avidity index indicates the presence of low-avidity IgG antibodies in serum during an acute or recent primary CMV infection. A high avidity index during the first 12–16 weeks of gestation could be considered a good indicator of past infection [Citation11].
Interpreting test results of IgG avidity is critical because serological tests vary from one laboratory to another, thus the method used and its reference values must be carefully assessed. The value of CMV IgG avidity helps to interpret a positive result for CMV IgM while the limits are that the kinetics of IgG avidity depend on the kit used; with some of these kits, the results may depend on CMV IgG concentration [Citation12].
The best algorithm for the diagnosis of primary CMV infection in pregnant women was to determine the anti-CMV antibody avidity only in serum samples positive for IgM antibodies [Citation13] and it is recommended to perform CMV IgG avidity measurements generally only when CMV IgM antibodies are positive (C. Vauloup-Fellous, personal communication).
During the first trimester, the woman in the present case did not show any of the clinical features usually found in immmunocompetent subjects [Citation4], therefore the clinical diagnosis of maternal primary CMV infection was not reliable. Virological tests had a secondary role in identifying the primary infection since the virus was not detected in maternal blood and only traces of viral-DNA in maternal samples of saliva and urine were documented. The virological diagnosis must be considered carefully since it is very often unreliable. Virus isolation and/or CMV-DNA detection in body fluids can only support serological diagnosis and furthermore, any positive result is not associated with a greater risk of infection and/or foetal/neonatal injury [Citation9].
In conclusion, proper timing for serological tests is within 16 weeks of gestation. In particular, serological diagnosis is reliable if pregnant women are screened before 12 weeks gestation and the advanced diagnosis is performed before 16 weeks of gestation.
Diagnosis of foetal compartment
The foetal compartment can be studied by invasive prenatal (amniocentesis) and non-invasive (ultrasound findings) diagnostic investigation.
The prenatal diagnosis should be performed no earlier than 6 weeks after presumed maternal infection and after 20 weeks of gestation. This period has been chosen for the following reasons: (1) CMV is a slow replication virus and 6–9 weeks are required after maternal infection for the virus to be eliminated in the foetus's urine in amounts large enough to be detected in the AF, and (2) the foetus excretes CMV via urine into the AF and a sufficient amount of foetal diuresis is produced only after 20 weeks of gestation [Citation9].
The AF is the most appropriate material for the diagnosis of foetal CMV infection obviating the need for cordocentesis, an invasive technique with a twofold higher risk to the foetus (1–3 % vs 0.5–1 %) [Citation14].
The risks linked to invasive testing are counterbalanced by certain diagnosis of foetal infection. Ultrasound has the advantage of not being invasive and will disclose any structural (ventriculomegaly, cerebral periventricular echogenicity, brain calcifications, microcephaly, hyperechogenic bowel, hydrops, oligohydramnios, hepatosplenomegaly, pleural effusion, and placental enlargement) and/or growth abnormalities caused by CMV infection, but its sensitivity (at 20–21 weeks of gestation) is poor and it correctly identifies no more than 20 % of infected babies, even in a selected population [Citation3,Citation14]. In addition, a structural abnormality may be disclosed a long time after initially negative tests and borderline structural changes detected early in pregnancy could be temporary [Citation3].
In a clinical case, the presence of high viral loads in the AF, sampled at the appropriate time, combined with overt observation of ultrasound abnormalities indicates that the foetus is small for gestational age and findings are thus highly suggestive of CMV foetal infection with severe brain damage [Citation3,Citation14].
The literature reports that about 50 % of children born with symptomatic infection will develop hearing loss, mental retardation with IQs < 70 and microcephaly [Citation2].
Amniocentesis provides the optimal means for diagnosing foetal infection. The specificity is usually very good (100 %), the sensitivity is about 80 %, depending on the technique and the number of techniques used. Currently, the Real Time PCR represents the gold standard of molecular tests. Since prenatal diagnosis is a very critical moment of pregnancy, the results should be evaluated carefully, at least two virological tests are required to be performed on AF.
It is very important to always offer a comprehensive (invasive and non-invasive) prenatal diagnosis because when the status of infection is not known in foetuses exposed to maternal CMV infection, ultrasound abnormalities predict symptomatic congenital infection in only a third of cases. Abnormal ultrasound findings combined with high viral load AF increases the diagnostic and prognostic capacity to identify symptomatic congenital CMV infection [Citation3]. However, a normal ultrasound finding combined with high viral load in the AF may be associated with histological brain damage in only 50 % of foetuses [Citation6].
Literature data of the diagnosis of foetal CMV infection have not described any assay for the detection of virus in foetal blood that proved sensitive enough to significantly improve prenatal diagnosis of intrauterine transmission of the virus. Foetal blood sample for foetal platelet count determination could be a useful adjunct to prenatal ultrasound examination in fetuses that have been diagnosed with CMV infection. Although the prognostic value of foetal DNAemia/viral load and/or level of specific IgM has been evoked, it remains controversial [Citation14,Citation15]. Equally controversial remains the determination of haematological and biochemical markers (i.e. ß-microglobulin) [Citation15]. At present, further studies in a larger number of symptomatic cases are required to be carried out in order to verify the prognostic efficacy of determination of different parameters in foetal blood.
In the present clinical case, the prenatal diagnosis identified severe foetal congenital CMV infection with cerebral involvement and the autopsy confirmed disseminated CMV infection with histological brain damage. Specifically, histological examination revealed cytomegalic cells and extensive laminar necrosis in the third layer of the cortex which is the most metabolically active region of the brain. Moreover, some areas of the cortex had the appearance of early polymicrogyria. These findings suggest superimposed hypoxic damage. The placenta showed severe tissue damage, suggesting an impaired placental function.
Brain anomalies induced by congenital CMV infection are likely to result from a direct effect of viral replication in the brain and an indirect effect occurring in the placenta where the infection can cause placental insufficiency and consequently hypoxic brain damage.
Currently, there are limited antenatal interventions to prevent congenital CMV infection or reduce morbidity and mortality in the offspring after primary maternal CMV infection [Citation16].
No official therapeutic options during pregnancy are available except for clinical trials.
Recent literature data have focused on the efficacy of preventive/therapeutic administration of CMV immunoglobulins [Citation17,Citation18] or antiviral drug (valacyclovir) [Citation19] to pregnant women with primary CMV infection to reduce the rate of vertical transmission and improve neonatal outcome however, these findings await confirmation in randomized studies on large patient cohorts.
Three international randomized clinical studies are currently in progress (NICHD study NCT01376778, Biotest study and, CYMEVAL2 study NCT01651585), while the CHIP (Congenital HCMV Infection Prevention) study (NCT 00881517) has just recently been published. The final results of this randomized, double-blind, placebo-controlled, prospective trial (phase II) showed that the treatment with hyperimmune globulin did not significantly modify the course of primary CMV infection during pregnancy [Citation20].
No vaccine is currently available for use prior to conception in order to prevent congenital CMV infection. The development of a CMV vaccine is one of the highest health priorities, however a phase 3 clinical trial is needed to confirm recent results obtained in a randomized, double-blind, placebo-controlled study of a recombinant CMV gB vaccine [Citation21].
Conclusion
CMV remains the major infectious cause of congenital abnormalities in the central nervous system due to intrauterine infection in humans. More children may be affected by congenital CMV than by any other better known childhood disorder, such as foetal alcohol syndrome, Down Syndrome and Spina bifida.
Although the diagnosis of congenital CMV infection remains complex, major goals have been achieved in recent years including maternal diagnosis with serological tests and foetal diagnosis with instrumental and virological tests.
Nevertheless, many questions remain unanswered in this particular field of prenatal medicine that requires the support of further clinical trials. Future studies are needed in order to identify novel prognostic markers in AF and foetal blood and investigate the prognostic significance of known markers of foetal blood.
In addition, further studies are required to evaluate the effectiveness of the treatment for pregnant women with primary CMV infection performed with the administration of purified immunoglobulin for the prevention of maternal-foetal transmission and evaluate the efficacy of the therapeutic treatment for infected and compromised foetuses with the administration of purified specific immunoglobulins or drugs.
Questions and answers
Q (Young): Why do you think there are no screening programs for CMV?
A (Lazzarotto): Actually, we do not have any vaccine or an official treatment during pregnancy. Congenital CMV infection can happen after primary and non-primary infection. Today, it is impossible to offer a vaccination that only prevents the primary maternal infection.
Q (Young): Are there clinical trials for treatment of CMV infection during pregnancy?
A (Lazzarotto): There are three international randomized clinical studies currently in progress: Biotest study, American and French randomized studies. A few days ago, we published the final results of the CHIP study; a randomized, double-blinded, placebo-controlled, prospective trial (phase II) (Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 2014;370:1316–26). The report showed that the treatment with hyperimmune globulin did not significantly modify the course of primary CMV infection during pregnancy.
Acknowledgements
We are grateful to Lucy Scioscia for editing the English text.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was partially supported by grants [SA43GABR] from St. Orsola-Malpighi University Hospital Bologna (Italy), Ricerca Finalizzata 2006 from Health Care Ministry, Rome (Italy), Ricerca Finalizzata 2010 from the Health Care Ministry, Rome (Italy).
References
- Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007;17:253–76.
- Boppana SB, Ross SA, Fowler KB. Congenital cytomegalovirus infection: clinical outcome. Clin Infect Dis 2013; 57:S178–81.
- Guerra B, Simonazzi G, Puccetti C, et al. Ultrasound prediction of symptomatic congenital cytomegalovirus infection. Am J Obstet Gynecol 2008;198:380.e1–7.
- Picone O, Vauloup-Fellous C, Cordier AG, et al. A series of 238 cytomegalovirus primary infections during pregnancy: description and outcome. Prenat Diagn 2013; 33:751–8.
- Lazzarotto T, Ripalti A, Bergamini G, et al. Development of a new cytomegalovirus (CMV) immunoglobulin M (IgM) immunoblot for detection of CMV-specific IgM. J Clin Microbiol 1998;36:3337–41.
- Gabrielli L, Bonasoni MP, Santini D, et al. Congenital cytomegalovirus infection: patterns of fetal brain damage. Clin Microbiol Infect 2012;18:e419–27.
- Ross DS, Victor M, Sumartojo E, et al. Women's knowledge of congenital cytomegalovirus: results from the 2005 HealthStyles survey. J Women's Health (Larchmt) 2008; 17:849–58.
- De Paschale M, Agrappi C, Manco MT, et al. Positive predictive value of anti-HCMV IgM as an index of primary infection. J Virol Meth 2010;168:121–5.
- Lazzarotto T, Varani S, Spezzacatena P, et al. Maternal IgG avidity and IgM detected by blot as diagnostic tools to identify pregnant women at risk of transmitting cytomegalovirus. Viral Immunol 2000;13:137–41.
- Lazzarotto T, Guerra B, Gabrielli L, et al. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clin Microbiol Infect 2011; 17:1285–93.
- Enders G, Daiminger A, Bäder U, et al. The value of CMV IgG avidity and immunoblot for timing the onset of primary CMV infection in pregnancy. J Clin Virol 2013; 56:102–7.
- Revello MG, Genini E, Gorini G, et al. Comparative evaluation of eight commercial human cytomegalovirus IgG avidity assays. J Clin Virol 2010;48:255–9.
- Macé M, Sissoeff L, Rudent A, et al. A serological testing algorithm for the diagnosis of primary CMV infection in pregnant women. Prenat Diagn 2004;24:861–3.
- Benoist G, Salomon LJ, Mohlo M, et al. The prognostic value of ultrasound abnormalities and biological parameters in blood of fetuses infected with cytomegalovirus. BJOG 2008;115:823–9.
- Fabbri E, Revello MG, Furione M, et al.Prognostic markers of symptomatic congenital human cytomegalovirus infection in fetal blood. BJOG 2011;118:448–56.
- McCarthy FP, Giles ML, Rowlands S, et al. Antenatal interventions for preventing the transmission of cytomegalovirus (CMV) from the mother to fetus during pregnancy and adverse outcomes in the congenitally infected infant. Cochrane Database Syst Rev 2011;16:CD008371.
- Nigro G, Adler SP, La Torre R, et al. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 2005 29;353:1350–62.
- Visentin S, Manara R, Milanese L, et al. Early primary cytomegalovirus infection in pregnancy: maternal hyperimmunoglobulin therapy improves outcomes among infants at 1 year of age. Clin Infect Dis 2012;55:497–503.
- Jacquemard F, Yamamoto M, Costa JM, et al. Maternal administration of valaciclovir in symptomatic intrauterine cytomegalovirus infection. BJOG 2007;114:1113–21.
- Revello MG, Lazzarotto T, Guerra B, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 2014;370:1316–26.
- Pass RF, Zhan C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 2009;360:1191–9.