Abstract
Background. Obesity has been identified as a key risk factor for the development of endometrial cancer (EC), the most common gynecologic malignancy in the US. We hypothesized that adipose tissue from EC patients secretes higher levels of cancer-promoting factors than healthy adipose tissue and promotes tumor cell growth. Methods. In this study, we generated conditioned media from adipose-derived stem cells (ASCs), an important regenerative cell population within adipose tissue. ASCs were isolated from adipose tissue from two EC patients undergoing hysterectomies and four cancer free control patients undergoing elective abdominoplasties. Ishikawa cells were then cultured for 48 hours in ASC-conditioned media (ASC-CM). Study outcomes included cancer cell proliferation rates and angiogenic factor secretion from cancer cells. Results. Our results indicate that ASC-conditioned media significantly increased Ishikawa cell proliferation rate when compared to control Ishikawa culture conditions (p = 0.002). Though not significant, Ishikawa proliferation with conditioned media from EC ASCs was higher than proliferation in conditioned media from control ASCs. Additionally, we found that Ishikawa cells secreted almost 10 % more vascular endothelial growth factor (VEGF) when cultured in EC ASC-CM as compared to Ishikawa cells cultured in healthy (cancer free control) ASC-CM. These results indicate that ASC paracrine factors may positively increase cancer cell growth rate and potentially enhance tumor angiogenesis. Conclusions. Our findings support the hypothesis that adipose tissue is an important source of secreted factors, which increase the rate of EC cell growth. This study provided preliminary evidence that ASCs may be an important parameter to evaluate in relation to EC development.
Introduction
Endometrial cancer (EC) is the most common gynecologic cancer in the United States [Citation1] and is closely associated with obesity. Previous research has demonstrated that the relative risk of EC in severely obese women compared to those with a normal BMI is 4/11 [Citation2]. The ‘unopposed estrogen hypothesis’ is a theoretical framework used to explain the relationship between obesity, endogenous steroid hormones, and EC risk [Citation3–6]; however, it does not explain whole population effects since not all obese women develop endometrial abnormalities. Identifying biomarker(s) in obese women that indicate an increased risk for EC development or relapse would be useful for reducing EC morbidity and mortality. Since adipose tissue is the largest endocrine organ in the human body, we hypothesized that adipose tissue in EC patients may increase tumorigenesis through paracrine factors, which could be measured and used as biomarkers associated with EC development. To our knowledge, adipose tissue biomarker panels have not been explored in the context of EC development.
Adipose tissue is an abundant source of multipotent cells, similar to mesenchymal stem cells, which are present within the stromal vascular fraction of adipose tissue. These adipose-derived stem cells (ASCs) are multipotent and have significant proliferative and differential capacity. Many types of mesenchymal stem cells (MSC), including ASC, have been shown to reside in a perivascular location, and increasing evidence shows that both MSC and ASC may in fact be vascular stem cells. Locally, these cells differentiate into smooth muscle and endothelial cells that are assembled into newly formed blood vessels during angiogenesis and neovasculogenesis [Citation7]. ASC's are known to express/secrete multiple growth factors, including vascular endothelial growth factor (VEGF), which is the key mediator of angiogenesis. The production of VEGF and other growth factors by tumors results in an ‘angiogenic switch’, where new vasculature is formed in and around tumors, allowing exponential tumor growth [Citation8]. Increased angiogenesis is related to poor EC prognosis [Citation9], therefore it is particularly interesting to investigate angiogenic factors in the adipose tissue of EC patients and those patients at high risk.
In breast cancer, it has been suggested that ASCs may play a crucial role in breast tumor growth and progression by inducing regulatory molecules and promoting an anti-inflammatory reaction within the tumor microenvironment [Citation10]. In the field of prostate cancer, it has been reported that ASCs subcutaneously co-injected with prostate cancer cells engraft and promote tumor progression [Citation11]. The role of ASCs has not been explored in experiments relating to EC development and progression, the gap that our research is aiming to fill.
It is possible that the risk of hormone-sensitive cancers may be predicted by evaluating ASC functional characteristics and biologic factors they release, as preliminary evidence suggests changes in mesenchymal stem cells occur with aging and chronic diseases [Citation12]. We hypothesized that biomarkers released by ASCs alter the expression profile of Ishikawa cells, which causes changes in its growth/metastatic potential. The overall goal of this study was to explore functional characteristics of ASCs of EC patients in comparison with adipose tissue of cancer free controls and to characterize Ishikawa EC cells when grown in ASCs medium.
Materials and methods
Adipose tissue collection
Discarded adipose tissue was collected from six overweight and obese patients aged 45–70, during elective surgical procedures at Magee-Women's Hospital of the University of Pittsburgh Medical Center. Of these six patients, Adipose tissue was harvested from two EC patients undergoing hysterectomy and four control patients undergoing elective abdominoplasty. Samples were immediately transported to the laboratory and processed on receipt. Participants were selected on the basis of being overweight and obese. The process for tissue collection was done under the guidelines of the Institutional Review Board of the University of Pittsburgh.
Cell culture media
DMEM plating media consisted of Dulbecco's Modified Eagle Medium (DMEM/DMEM F12)(Cat#11965092/11320082), supplemented with Fetal Bovine Serum (FBS)-heat inactivated (Cat#16140-071), 2.5 % Penicillin and Streptomycin (Pen-Strep)(Cat#15140-122) and 0.5 % Fungizone (Cat#15290-018).
Ishikawa cell line
EC tumor cells were modeled with Ishikawa cells, a well characterized human uterine-derived endometrioid epithelial cancer cell line with both estrogen and progesterone receptors [Citation13]. For this study, Ishikawa cells were generously provided by Dr Anda Vlad's laboratory (University of Pittsburgh, USA) and used at passage number 11.
Adipose-derived stem cell isolation
ASCs were isolated from at least 10 g of subcutaneous adipose tissue. Adipose tissue was minced and digested for 30–60 min in a 37°C water bath in Hank's Balanced Salt Solution (Gibco, Cat#14175-095) containing 3.5 % Fatty Acid Free-Bovine Serum Albumin (BSA) (Cat#82-002-4) (Celliance) and 1 g/L Collagenase Worthington Type 2 [14LS004177]. The digestate was then gauze filtered and centrifuged at 1000 revolutions per minute (rpm) (g force = 167.7 g) for 10 minutes at ambient temperature. The supernatant was discarded and the cellular pellet was resuspended in erythrocyte lysing buffer, which included NH4Cl (0.154 mol/L) KHCO3 (0.01 mol/L) and EDTA (0.001 mol/L) and centrifuged at 1000 rpm for 10 min. The resulting pellet was plated and cultured on tissue culture-treated flasks in ASC plating medium with 10 % FBS. After overnight incubation, the cells that adhered to the flask surface were considered ASCs and were further cultured and expanded. ASCs (passage number 2–5) from EC patients were used to generate EC-conditioned media (EC-ASC CM) and ASCs from control patients were used to generate control ASC-conditioned media (ctrl-ASC CM).
ASC-conditioned media and Ishikawa culture
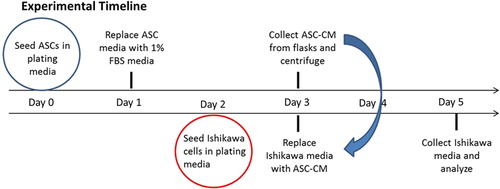
The experimental timeline for the conditioned media study as well as study groups and controls are described in . ASCs were seeded at 10,000 cells per cm2 in tissue culture-treated T-75/175 flasks in DMEM plating media containing 10 % FBS. After 12 h (or overnight), the culture media was replaced with DMEM plating media containing 1 % FBS. ASCs where then cultured for 48 h, the ASC-CM (conditioned media) was then collected and centrifuged. In parallel, Ishikawa cells were seeded in 96-well plates at 6000 cells per cm2 in 10 % FBS plating media. After 24 h, the plating media was replaced with ASC-CM or (-) control media (DMEM with 1 % FBS) and cells were incubated for an additional 48 h. At the completion of the study, Ishikawa media was collected and analyzed for secreted angiogenic factors as described below. Baseline levels of factors of interest in DMEM with 1 % FBS and all ASC-CM were measured.
Cell proliferation assay
CyQUANT® Cell Proliferation Assay kit (Invitrogen, Grand Island, NY, USA) was used to determine if the growth rate (proliferation) of the Ishikawa cell changed after being subjected to ASC condition media. Cell proliferation assay was carried out on Ishikawa cells grown on ECASC- CM, Ctrl ASC-CM and unconditioned media.
After incubating Ishikawa cells in the previously described conditioned media, the cells were washed with phosphate-buffered saline (PBS) and frozen at –80°C. Cell proliferation rates were determined by measuring DNA content following the manufacturer's instructions. Relative Fluorescence Units were plotted for each sample.
ELISA immunoassay
Identification of VEGF through enzyme-linked immunosorbent assay (ELISA) was carried out. ELISA kits were obtained from Quantikine, R&D Systems and used following the manufacturer's guidelines. ELISA was carried out on media collected from Ishikawa cells grown on EC-ASC conditioned media, control-ASC conditioned media and unconditioned media. To control for VEGF secretion by ASC's, VEGF secretion in ASC-conditioned media was measured prior to and after Ishikawa culture.
Statistical analysis
T-tests were used to test significance of differences between groups and confirmed with non-parametric tests. All statistical analyses were performed using Microsoft Excel 2010.
Results
Human Tissue Collection: Adipose tissue was collected from two post menopausal obese EC patients undergoing hysterectomies. Adipose tissue was also collected from four cancer-free control patients undergoing elective abdominoplasties. The respective ages and BMIs of the control patients were: 62 y, BMI = 31.0 kg/m2; 65 y, BMI = 27.8 kg/m2; 43 y, BMI = 36.7 kg/m2 and 43 y, BMI = 36.7 kg/m2. The two groups were not significantly different with regard to age (p = 0.17), or BMI (p = 0.3).
Proliferation of Ishikawa cells
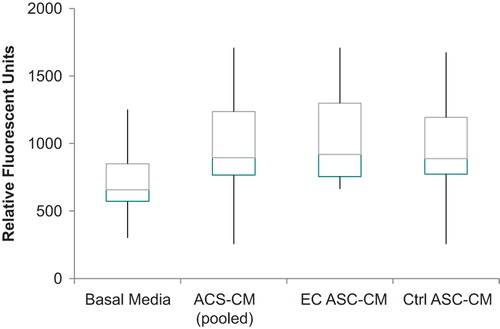
Ishikawa cells from all subpopulations were able to proliferate, however Ishikawa cells grown in ASC-conditioned media demonstrated a significant increase in proliferation rate when compared to the cells growing in basal media (). Though not significantly different, proliferation of Ishikawa cells growing in conditioned media from EC ASCs vs non-cancer healthy, control patient-ASCs showed that Ishikawa cells proliferated 20 % faster in conditioned media from cancer ASC-conditioned media.
ELISA
Conditioned media from ASCs increased the expression of VEGF by Ishikawa cells nearly 3 times compared with the normal expression (). Though not significantly different, when comparing the expression of VEGF by Ishikawa cells growing on endometrial cancer vs non-cancer healthy, control patients Ishikawa cells secreted 10 % more VEGF in EC ASC-CM.
Table I. VEGF concentration (ELISA) following 48 h Ishikawa culture in basal or conditioned media (N = patients, n = observations per patient).
Due to a limited patient number, the cell proliferation assay using cyquant indicated no significant difference in Ishikawa cell number grown in EC or Ctrl ASC-conditioned media.
Discussion
This is the first study to investigate the role of ASCs in EC development. Adipose tissue is a potentially attractive depot to evaluate biomarkers in relation to cancer detection/diagnosis, and research of other obesity-associated maladies. Clinical applications of ASCs, including systemic and local applications, have begun to show promising possibility of efficacy in patients with a range of diseases, including acute myocardial infarction, peripheral vascular disease, and soft and bony tissue defects including cranial bone loss, Crohn's-related fistula, and skin wounds [Citation15]. This project is innovative in that it explores the applicability of adipose tissue and its associated cellular secretome to better characterize potential mechanisms of EC development. As one of the future steps, western blots will be used to explore the levels of cell mobility-associated proteins. Western blot technique has been extensively used for the quantification of proteins in adipose tissues, but not yet investigated in the context of EC risk.
The main limitation of this pilot study is that associating ASC-secreted factors as biomarkers for cancer status is not necessarily indicative that biomarker concentrations were altered before cancer developed; the possibility remains that malignancy itself caused changes in the biomarkers. To address this challenge, future investigations should utilize a prospective cohort study design, targeting women at high risk and monitoring for obesity-related malignancies. Consistently with our group's previous experience in exploring the reliability of serum-based markers [Citation16–18], one of our future directions will be to evaluate reliability of adipose tissue-based markers. Although early pilot data are presented here, the proposed line of research will ultimately lead to the goal of development screening measures in order to identify women at high risk for EC and other obesity-associated malignancies.
In addition to a low sample size, another limitation worth noting is that this investigation is limited to the exploration of subcutaneous adipose cells. The metabolism of adipose tissue varies in different fat depots [Citation19–21]. In particular, subcutaneous fat is most strongly correlated with obesity-related biological findings in type I EC [Citation22]. Subcutaneous adipose depot provides a practical surrogate source of tissue for screening for biomarkers. In the future steps of this exploratory study we will include omental adipose tissue (visceral fat in the lower abdominal area) as the site that we would most expect to observe changes in biomarkers. Potentially useful markers unique to omental adipose tissue would not provide the screen we are seeking but would provide ‘proof of principle’ for a continued search for correlation in future studies. Thus, in the future, we will be focusing on exploring these two adipose tissue depots to identify biomarkers of EC risk and to better understand EC etiology. We will also be focusing on exploring inherent differences among subgroups in our investigation.
A better understanding of the biology and functioning of the human endometrium in relation to ASCs is vital to improve our knowledge about EC development, female infertility, and for treatment designs to prevent and control these conditions. Additionally, it will also be interesting to explore ASC in ovarian cancer, one of the deadliest global gynecologic malignancies. Correspondingly, searching for biological markers associated with EC risk and novel approaches to identify ‘healthy obese’ from ‘high risk obese’ are important future venues for investigation.
Questions and answers
Q (Diamanti): Did the obese women undergoing bariatric surgery have regular or irregular menses? Are obese women with menstrual irregularities at higher risk of developing endometrial cancer?
A (Linkov): In another study they excluded patients with irregular menstrual periods because they wanted to look at baseline measures such as the incidence of hyperplasia in women at low risk. In our study we included women with both regular and irregular periods. Our sample size was limited but we found five pathologies and some of these were in patients with irregular bleeding.
Q (Beillaux): In relation to the previous question, do you have data on the androgen receptor? If we think of polycystic ovary syndrome and menstrual irregularities we automatically think of hyper androgenism. Is there any such association here?
A (Linkov): As yet, we have not found anything definitive relating to the androgen receptor; however we are still investigating this question.
Q (Fishman): We can virtually cure every woman with type 1 endometrial cancer with surgical intervention. What is your thought about local or systemic progesterone, such as placement of a progesterone IUD rather than bariatric surgery?
A (Linkov): If you have people already diagnosed with endometrial cancer, a progesterone IUD is a good option to preserve the uterus but at the population level, if you want to prevent cancer or preserve the uterus and their health, bariatric surgery is a good option that we are exploring in our research.
Q (Fishman): Hysterectomy is the standard treatment for patients with endometrial cancer. For those that desire fertility, progesterone treatment is an option. Most patients who have endometrial cancer type 1 have a body habitus of excess oestrogen. These women are heavy and if you give oral progesterone you may increase the risk of serious side effects, such as DVT and pulmonary embolism. Women with no cancer but pre-cancer dysplasia, such as hyperplasia would benefit from progesterone IUD rather than bariatric surgery.
Q (Diamanti): Did you perform OGTT in the obese patients with endometrial cancer?
A (Linkov): We did not do this in our study but we believe there are many diabetes cases in the population studied.
Q (Diamanti): But many of these patients have insulin resistance that could have an atypical endometrial pattern and therefore be a different population.
A (Linkov): That is true. We did not exclude them because the sample size was small.
Acknowledgements
Dr Linkov's time has been supported by the American Cancer Society Mentored Research Award.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Mishell Jr MS, Droegemueller W, Herbst AL. Comprehensive gynecology. 3rd ed. St. Louis: Mosby; 1997.
- Crosbie EJ, Zwahlen M, Kitchener HC, et al. Body mass index, hormone replacement therapy, and endometrial cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2010;19:3119–30.
- Ziel HK. Estrogen's role in endometrial cancer. Obstet Gynecol 1982;60:509–15.
- Key TJ, Pike MC. The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer 1988;57:205–12.
- Judd HL, Davidson BJ, Frumar AM, et al. Serum androgens and estrogens in postmenopausal women with and without endometrial cancer. Am J Obstet Gynecol 1980;136:859–71.
- Gambrell RD, Jr., Bagnell CA, Greenblatt RB. Role of estrogens and progesterone in the etiology and prevention of endometrial cancer: review. Am J Obstet Gynecol 1983; 146:696–707.
- Lin CS XZ, Deng CH, Ning H, et al. Defining adipose tissue-derived stem cells in tissue and in culture. 2010; Available from: http://europepmc.org/abstract/MED/20376787.
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005;69(Suppl. 3):4–10.
- Sivridis E. Angiogenesis and endometrial cancer. Anticancer Res 2001;21(6B):4383–8.
- Razmkhah M, Jaberipour M, Erfani N, et al. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-beta1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol. 2011; 266:116–22.
- Prantl L, Muehlberg F, Navone NM, et al. Adipose tissue-derived stem cells promote prostate tumor growth. Prostate 2010;70:1709–15.
- Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev 2006;5:91–116.
- Nishida M. The Ishikawa cells from birth to the present. Human Cell 2002;15:104–17.
- Wadden TA, Butryn ML, Sarwer DB, et al. Comparison of psychosocial status in treatment-seeking women with class III vs. class I-II obesity. Surg Obesity Rel Dis 2006;2:138–45.
- Hong SJ, Traktuev DO, March KL. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Current Opin Organ Transplant 2010;15:86–91.
- Linkov F, Gu Y, Arslan AA, et al. Reliability of tumor markers, chemokines, and metastasis-related molecules in serum. Eur Cytokine Netw 2009;20:21–6.
- Gu Y, Zeleniuch-Jacquotte A, Linkov F, et al. Reproducibility of serum cytokines and growth factors. Cytokine 2009;45:44–9.
- Arslan AA, Gu Y, Zeleniuch-Jacquotte A, et al. Reproducibility of serum pituitary hormones in women. Cancer Epidemiol Biomarkers Prev 2008;17:1880–3.
- Leibel RL, Edens NK, Fried SK. Physiologic basis for the control of body fat distribution in humans. Annu Rev Nutr 1989;9:417–43.
- Björntorp P. Metabolic implications of body fat distribution. Diabetes Care 1991;14:1132–43.
- Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev 1994;74:761–811.
- Nakamura K, Hongo A, Kodama J, et al. Fat accumulation in adipose tissues as a risk factor for the development of endometrial cancer. Oncol Rep 2011;26:65–71.