Abstract
Presently the majority of women diagnosed with epithelial ovarian cancer (EOC) have advanced stage disease (III–IV) with a poor 5-year survival rate (12–30 %). This significantly contrasts when early stage disease is detected, which has a 5-year survival rate approximating 90 %. Therefore, detection of early stage disease is critical to making an impact on outcome. By using genetic algorithms, modifications of transvaginal ultrasonography and use of novel biomarkers, we propose a risk assessment profile to identify at-risk women and enable ovarian cancer screening to become a reality. Such a novel algorithm starts by applying classic genetic pedigree assessment and uses a panel of multiple biomarkers that identify both phenotypic and genotypic expression of high-risk markers followed with conventional ultrasound and advanced ultrasound techniques such as microvascular contrast-enhancement as a secondary test. We presently employ a multidisciplinary program incorporating genetics, molecular biology, tumor immunology, gynecologic oncology and diagnostic imaging to identify asymptomatic high risk women.
Introduction
EOC is the most lethal gynecologic malignancy, with an estimated 140,000 annual deaths globally. The majority of women continue to be diagnosed with advanced stage disease (III–IV), and despite advances in diagnostic technologies, this figure has remained unchanged over five decades. The prognosis of advanced stage EOC remains quite poor, with a 5-year survival rate of 12–15 %, and these women often require morbid operations and multiple adjuvant chemotherapeutics. In contrast, the 5-year survival rate of early stage (I) EOC approximates 90 %, requires much less morbid operations and may not require adjuvant chemotherapy. Thus, detection of early stage EOC and subsequent treatment would improve not only quality of life as well but overall survival. Unfortunately, to date, efforts to detect early stage EOC and screening for ovarian cancer have not been effective [Citation1]. Indeed, the current detection modalities of bimanual exam, CA125, and transvaginal sonogram together allow us to detect only 30–45 % of women with early stage disease. Our proposal for identifying those women who do not yet have phenotypic expression of EOC, but are in fact at risk, may allow for early detection and possibly prevention of EOC. We believe detection and treatment of at risk individuals will shift the paradigm of the treatment of EOC analogous to the role of cervical dysplasia in cervical cancer. We propose that the clinical applications of the molecular biology and genetics unique to EOC can be combined with novel diagnostic imaging to create detailed risk assessment profiles that will identify those women at risk to ultimately provide timely counseling and treatment to prevent EOC.
Genetics
One of the most important risk factors of EOC is genetic predisposition. Identifying those with genetic predisposition for EOC will be the first step toward risk assessment and identifying and treating pre- invasive EOC.
Diagnosis of genetic predisposition involves risk assessment based on a patient's personal and family history of cancer and the formal genetic evaluation of the family pedigree. Management includes a formal pedigree assessment by board certified experts such as a genetic counselor followed by directed testing for known genetic mutations associated with ovarian cancer or other related malignancies of the patient as well as other at-risk individuals identified by the pedigree assessment. Additional testing involves extensive pre- and post-test counseling best performed by a multidisciplinary team involving genetic counselors and their physicians to aid the patient in understanding the implications of their condition and available treatment options. Because the number and type of gene mutations associated with hereditary ovarian cancer continues to increase, a negative work-up for a genetic mutation does not necessarily preclude management in the presence of a positive family pedigree. In fact, a family history of ovarian cancer in a first-degree relative triples a woman's lifetime risk of developing ovarian cancer [Citation2].
Inheritance of high-penetrance cancer susceptibility genes places women at greatly increased risk of developing EOC, such as BRCA1, BRCA2 and those associated with Lynch syndrome. Women who have inherited a BRCA1 or BRCA2 gene mutation have a 35–70 % and 10–30 % respective lifetime risk of developing EOC. Those with Lynch syndrome associated mutations (MLH1MSH2, MSH6, PMS2, EPCAM) have an estimated 9–12 % lifetime risk of developing EOC. Women of Eastern European, or Ashkenazi Jewish descent, are at higher risk of being positive carriers of BRCA1 or BRCA2 mutations. A number of familial ovarian cancer syndromes have also been identified such as Cowden syndrome and Li-Fraumeni syndrome. Cowden syndrome is associated with mutation of the PTEN gene and increases risk of breast, thyroid and endometrial cancer in addition to ovarian cancer. Li Fraumeni syndrome is associated with mutation of TP53 and involves an increased risk of breast, sarcoma, brain and adrenocortical cancers.
Inheritance of a genetic mutation accounts for 10–20 % of epithelial ovarian cancers with the majority being sporadic [Citation3]. However, an increasing number of genes (over 21 gene mutations) have recently been identified such as BRIP1, RAD51D, RAD51C, PALB2 and BARD1 which are associated with a higher risk in developing EOC. Newer estimates suggest up to 20–25 % of women may carry a germ-line mutation associated with inherited ovarian carcinoma. This percentage is likely an underestimate with the anticipated identification of more genes associated with higher risk of developing EOC. Although a positive patient personal or family history with risk factors is currently recommended prior to initiating a work-up for identifying known inherited gene mutations, almost one-third of women with hereditary ovarian carcinoma have no close relatives and 35 % are greater than 60 years of age at diagnosis. There is consideration of expanding recommendations to include any women diagnosed with EOC regardless of age or family history [Citation4].
Biomarkers
Biomarkers have long played a role in the management of EOC. CA125 is a glycoprotein antigen that is the most commonly known and measured ovarian cancer tumor marker. While CA 125 concentration is elevated in the majority (85–90 %) of women with advanced stage EOC it is elevated in 47 % of women with early stage disease. Therefore, according to the United States Preventive Services Task Force Statement in 2013 [Citation5], the use of CA125 is not effective for screening and is not suggested for diagnosing ovarian cancer (2012).
Multiple publications have identified hundreds of potential biomarkers for detecting ovarian cancer in addition to CA125. One of the most clinically interesting panels include Human epididymis protein 4 (HE4), transthyretin (TTR), apolipoprotein A1 (ApoA1) and transferrin. These protein biomarkers, in combination with CA125, have been used in panels with encouraging results in early stage EOC detection. Anderson et al. proposed a panel of biomarkers including CA 125, HE4, and mesothelin. Serum specimens of 34 ovarian cancers and 70 matched control subjects provided evidence of increased three tumor markers 3 years before clinical diagnosis, however lead time was short [Citation6]. Recently, Yurkovetsky et al. identified a panel consisting of CA-125, HE4, CEA, and VCAM-1 (vascular cell adhesion molecule 1). This panel was applied to sera from 139 patients with early-stage ovarian cancer, 149 patients with late-stage ovarian cancer, and 1102 healthy women. This four-biomarker panel provided the highest diagnostic power of 86 % sensitivity for early-stage and 93 % sensitivity for late-stage ovarian cancer at 98 % specificity [Citation7]. The multivariate index assay OVA1 has been approved by the United States Food and Drug Administration (FDA) for triage of pelvic masses since 2009. The test consists of CA125, beta2-microglobulin, transferrin, ApoA1 and TTR. Based on serum concentrations of these biomarkers, OVA1 assigns a single numerical score ranging from zero to ten with cutoffs set as 5.0 for premenopausal women and 4.4 for postmenopausal women. Pelvic masses with scores higher than these thresholds are considered likely cancerous. A recent study on 516 women yielded an improvement of sensitivity – as high as 93 % – and negative predictive value while decreasing specificity and positive predictive value when replacing the CA 125 with the OVA1 multivariate index assay [Citation8]. These results, while requiring validation, suggest that combinations of biomarkers may provide improved detection as the first step in a multimodal screening protocol.
In parallel with the efforts to identify potential protein and lipid biomarkers, attention has been recently focused on the evolving role of micro RNAs (miRNAs) in the regulation of ovarian metastasis. miRNAs are approximately 22-nucleotide noncoding RNAs that post-transcriptionally regulate messenger RNA (mRNA) translation into protein of a large number of target genes [Citation9]. miRNAs globally influence gene expression, which ultimately determines cellular behavior by targeting complementary gene transcripts for translational repression or degradation of the mRNA transcript [Citation10]. Similar to other cancers, the initiation and development of ovarian cancer is characterized by disruption of oncogenes and tumor suppressor genes by both genetic and epigenetic mechanisms [Citation11]. Previous miRNA expression profiling studies of ovarian cancer have defined differentially expressed miRNAs in ovarian cancer relative to the corresponding normal control [Citation12]. Resnick et al. reported differences in serum miRNAs between normal women and patients with ovarian cancer; among 21 miRNAs that the study identified, five miRNAs were found to be overexpressed (miR-21, miR-29a, miR-92, miR-93, and miR-126), and three miRNAs (miR-127, miR155, and miR-99) were underexpressed in the sera of patients with ovarian cancer, suggesting a panel of miRNAs can be set as a screening tool for ovarian cancer [Citation13]. Recent efforts have focused on establishing miRNAs as novel molecular biomarkers for ovarian cancer and defining peripheral blood-derived miRNAs as novel circulating biomarkers [Citation14].
In the future, the most sensitive and specific biomarker panel would include a combination of both serum/urine protein and miRNA biomarkers to detect phenotypic and genotypic expression of pre-invasive EOC.
Screening trials
Current guidelines recommend against ovarian cancer screening for the general population. This is in part due to the lack of efficacy in current trials. The Prostate, Lung, Colorectal and Ovarian (PLCO) screening trial included a randomized controlled trial of screening post-menopausal women in the general population with CA125 and conventional trans- vaginal ultrasound. The study concluded that this screening resulted in no change in mortality [Citation1]. Further, results indicated a 15 % major complication rate in those who underwent surgical intervention from positive screening results. Another study, the Japanese Shizuoka Cohort Study of Ovarian Cancer Screening, a randomized controlled trial, performed screening consisting of physical exam, annual ultrasound and CA125 on 82,000 women and reported a sensitivity of 77 % and a stage shift with an increased diagnosis of Stage I EOC (63 % vs 38 %). While these are promising results, the mortality rates are pending [Citation15]. The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) involved screening 202,638 low-risk postmenopausal women. Their screening trial differs from the PLCO in the use of algorithms to interpret CA125 fluctuations as seen using the risk of ovarian cancer algorithm (ROCA). Although initial results in 2009 yielded an encouraging sensitivity and specificity [Citation16]; the outcome of UKCTOCS will not be available by 2015. In our own experience at the National Ovarian Cancer Risk Assessment Program, based on screening per current guidelines of high-risk women, we detected a low rate (less than 2 %) of EOC in high risk women receiving prophylactic surgery who had a normal physical and transvaginal ultrasound examination. High-risk eligibility for our program was defined as those with a personal breast/ovarian cancer history, a family history suggestive of genetic ovarian cancer syndrome, a recognized mutation carrier or history of prolonged use of infertility medications. Patients were screened every 6 months with transvaginal ultrasound. A total of 1281 high-risk women from 1990–2004 – 624 BRCA1 + women, 495 BRCA2 + women and 162 BRCA- women with a pedigree consistent with inherited cancer syndrome had prophylactic removal of bilateral salpingo- oophorectomy with 1.3 % (17) cases confirming serous malignancies. Five out of 17 malignancies were identified in Stage I. Our data suggest that current screening guidelines are ineffective in detection of pre-invasive ovarian conditions.
Imaging studies
Transvaginal sonogram (TVS) is the initial diagnostic modality of choice for the evaluation of the adnexa but likewise has proven ineffective for screening and detection of early stage EOC. Van Nagell et al. reported detection of four asymptomatic women with surgically confirmed Stage I EOC out of 57,000 conventional TVS performed. We previously reported on our experience with 4526 women at high risk for ovarian cancer who were screened with TVS which demonstrated the limited value of this modality. Despite a normal TVS as close as 6 months prior, all ovarian, primary peritoneal, and fallopian tube cancers detected in asymptomatic women were diagnosed at an advanced stage (III) [Citation17]. Similar findings were encountered in the PLCO trial. Conventional TVS used in this screening process again did not result in a stage shift, with 77 % of serous cancer diagnoses in the intervention group diagnosed at an advanced stage (III or IV) and just 15 % detected at an early stage [Citation1]. These numbers confirm that conventional TVS is an ineffective primary screening method.
In order to improve the efficacy of sonography, several techniques have been combined with gray scale morphologic assessment. Three-dimensional ultrasound with power Doppler and microvascular contrast-enhancement are examples of techniques that have improved sonographic characterization ovarian lesions. Results in several studies have shown that these advanced techniques can be used to differentiate benign and malignant adnexal masses [Citation18]. We previously reported on our experience evaluating 1600 women who underwent two-dimensional and three-dimensional sonography with 71 operations performed and 14 malignancies confirmed. While both two-dimensional and three-dimensional ultrasounds had a 100 % sensitivity rate, three-dimensional sonography resulted in 75 % specificity vs 54 % for the two-dimensional sonography, confirming the superiority of three-dimensional sonography in adnexal mass characterization.
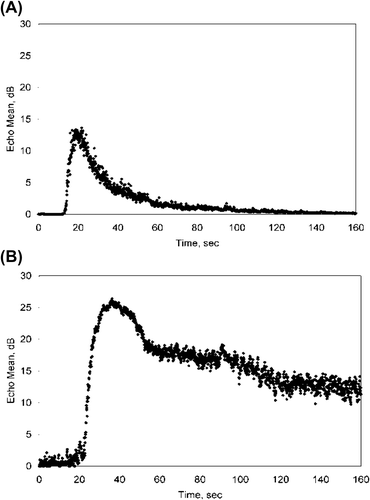
Fleischer et al. [Citation19] have demonstrated that using pulse inversion harmonic imaging with microvascular contrast-enhanced ultrasonography may be even more accurate in aiding preoperative differentiation of benign and malignant ovarian tumors. Microbubble contrast agents can help delineate and allow detection of the abnormal microvasculature unique to malignant tissue. Solid tumors induce a new vascular supply from pre-existing host venules (tumor neovascularization), stimulating the capillary endothelium to form new leaky vascular channels with incompetent basement membranes that allow extravasation of erythrocytes into the parenchyma. The contrast agents are five to ten microns in diameter, similar in size to erythrocytes, and are able to traverse capillaries and venules such as to elucidate the neovasculature in tumors and thus differentiate benign from malignant adnexal masses. Pulse-inversion techniques optimize the sonographic detection of microbubble contrast agents, providing a medium to quantify this difference. demonstrates a comparison of contrast enhancement kinetics param-eters in a benign (A) versus malignant (B) ovarian tumor. We reported on specific quantifiable flow parameters useful in separating benign from malignant adnexal masses such as time to peak, peak enhancement, half wash out time and area under the curve (AUC). AUC corresponds to vascular volume, which is typically increased in tumors. Our findings showed significant differences in three of the four parameters with benign masses having longer 1/2 wash out time (140 seconds vs 46 seconds), greater enhancement (23 dB vs 12 dB) and larger AUC (2013 vs 524) than malignant masses [Citation19]. In our preliminary data, microvascular ultrasound detected 20/20 malignancies including eight stage I cancers, while conventional ultrasound led to detection of only 12/20 cases. This data demonstrates the value of microvascular contrast-enhanced sonography and indicates this modality ultimately may be used to detect pre-invasive ovarian disease for risk assessment.
Conclusion
EOC continues to be a lethal disease primarily due to late-stage detection and ultimate resistance to therapy. Key to changing outcomes in EOC is a paradigm shift targeting detection of early rather than advanced stage ovarian cancer. We believe this can occur by increasing detection of the at-risk individual by the establishment of a detailed risk assessment profile involving genetics, disease specific biomarkers and advanced microvascular ultrasound techniques.
Questions and answers
Q (Lane): Could early diagnosis in ovarian cancer improve the prognosis? Should we be doing marker arrays in asymptomatic women?
A (Fishman): Usually the diagnosis is made late and there is a poor outcome. As of now, there are no serum or urine biomarkers which can detect early disease. If we consider whether primary physicians should do better examinations we must realize that there is a problem relating to training, since it is well documented that 75 % of bimanual examinations give incorrect results. Ultrasound is very important and both transvaginal US and transabdominal US should be performed as part of an annual examination. It is often not done because of the expense.
Q (Beillaux): Is there still a place for CA125 analysis?
A (Fishman): Yes, for follow-up of women with ovarian cancer, not for the diagnosis of the condition.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011;305:2295–303.
- Schorge JO, Modesitt SC, Coleman RL, et al. SGO White Paper on ovarian cancer: etiology, screening and surveillance. Gynecol Oncol 2010;119:7–17.
- Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009;361:170–7.
- Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. PNAS 2011;108:18032–7.
- Screening for Ovarian Cancer: Clinical Summary of U.S. Preventive Services Task Force Reaffirmation Recommendation. AHRQ Publication 2012; 12–05165-EF-5. Accessed 31 March 2014 from: http://www.uspreventiveservicestaskforce.org/uspstf12/ovarian/ovarcancersum.htm
- Anderson GL, McIntosh M, Wu L, et al. Assessing lead time of selected ovarian cancer biomarkers: a nested case-control study. J Natl Cancer Inst 2010;102:26–38.
- Yurkovetsky Z, Skates S, Lomakin A, et al. Development of a multimarker assay for early detection of ovarian cancer. J Clin Oncol 2010;28:2159–66.
- Ware Miller R, Smith A, DeSimone CP, et al. Performance of the American College of Obstetricians and Gynecologists’ ovarian tumor referral guidelines with a multivariate index assay. Obstet Gynecol 2011;117:1298–306.
- Lopez J, Percharde M, Coley HM, et al. The context and potential of epigenetics in oncology. Br J Cancer 2009; 100:571–77.
- Guo SW, Zilberberg MD, Hummelshoj L. Endometriosis and ovarian cancer. Lancet Oncol 2012;13:e189–90; author reply e190.
- Chen H, Hardy TM, Tollefsbol TO. Epigenomics of ovarian cancer and its chemoprevention. Front Genet 2011;2:1–8. doi: 10.3389/fgene.2011.00067
- Creighton CJ, Fountain MD, Yu Z, et al. Molecular profiling uncovers a p53-associated role for microRNA-31 in inhibiting the proliferation of serous ovarian carcinomas and other cancers. Cancer Res 2010;70:1906–15.
- Resnick KE, Alder H, Hagan JP, et al. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol 2009;112:55–9.
- Kuhlmann JD, Rasch J, Wimberger P, et al. MicroRNA and the pathogenesis of ovarian cancer – a new horizon for molecular diagnostics and treatment? Clin Chem Lab Med 2012;50:601–15.
- Kobayashi H, Yamada Y, Sado T, et al. A randomized study of screening for ovarian cancer: a multicenter study in Japan.2008;18:414–20.
- Menon U, Gentry-Maharaj A, Hallett R, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol 2009;10:327–40.
- Fishman DA, Cohen L, Blank SV, et al. The role of ultrasound evaluation in the detection of early-stage epithelial ovarian cancer. Am J Obstet Gynecol 2005;192:1214–21; discussion 1221–22.
- Marret H, Sauget S, Giraudeau B, et al. Contrast-enhanced sonography helps in discrimination of benign from malignant adnexal masses. J Ultrasound Med 2004;23:1629–39; quiz 1641–42.
- Fleischer AC, Lyshchik A, Andreotti RF, et al. Advances in sonographic detection of ovarian cancer: depiction of tumor neovascularity with microbubbles. AJR 2010;194:343–8.