Abstract
Endometriosis is a common gynecologic disorder histologically characterized by the displacement of endometrial tissue to extra-uterine locations. A significant cause of infertility and pelvic pain, the global socioeconomic burden of endometriosis is staggering. Laparoscopy remains the gold standard for the diagnosis of the condition. However, the invasive nature of surgery, coupled with the lack of a laboratory biomarker for the disease, results in a mean latency of 6–7 years from onset of symptoms to definitive diagnosis. Unfortunately, the delay in diagnosis may have significant consequences in terms of disease progression. The discovery of a sufficiently sensitive and specific biomarker for the non-surgical detection of endometriosis promises earlier diagnosis and prevention of deleterious sequelae, and remains a top research priority. The enigmatic pathophysiology of endometriosis presents unique challenges to biomarker development that are now well outlined. Within the past decade, significant advancements in understanding the molecular hallmarks of endometriosis have occurred, and promising biomarker candidates are emerging.
Key Words::
Introduction
Endometriosis is a debilitating gynecologic disease characterized by the implantation of endometrial tissue in ectopic locations, including the pelvic peritoneum, ovaries and bowel. The prevalence of endometriosis in reproductive age women is 6–10 % and as high as 35–50 % in women with pain and/or unexplained infertility [Citation1]. A heritable component to endometriosis is well supported, though the specific genes involved remain an area of active investigation. The risk for first degree relatives of women with severe endometriosis is six times higher than for relatives of unaffected women [Citation2], and monozygotic twin studies demonstrate high concordance rates not only for histologically confirmed endometriosis but also for disease stage [Citation3]. Though incomplete in accounting for the entirety of reported clinical manifestations of the disease, Sampson's theory of retrograde menstruation is the most widely accepted description of endometriosis pathogenesis [Citation4]. This theory holds that endometriosis originates from the implantation of sloughed endometrial tissue refluxed into the pelvis via the fallopian tube(s) during menstruation.
The survival and implantation of endometrial tissue in ectopic locations involves inhibition of apoptosis and escape from immune clearance, apposition and cell-substratum interactions, invasion via matrix degradation, local cellular proliferation and neuroangiogenesis [Citation5]. Nascent peritoneal implants typified by the red vesicular phenotype are comprised of functional endometrial glands and stroma that are responsive to sex steroids, as evidenced by the observation of lesional menstruation [Citation6]. Repetitive episodes of menstrual shedding and attendant local inflammatory reaction may predispose toward fibrosis and adhesion formation. Collectively, these processes set conditions for the pain and infertility associated with endometriosis. Considerable evidence exists to support the conceptualization of endometriosis as an estrogen-dependent disorder. More recently, endometriosis has been associated with inflammation, attenuated progesterone action at the level of the endometrium and neuroangiogenesis. The elucidation of the molecular mechanisms associated with these processes has provided new perspectives in disease pathophysiology toward novel diagnostic and treatment approaches.
The gold standard for the diagnosis of endometriosis remains direct visualization of lesions at surgery preferably coupled with histologic confirmation of endometrial glands and stroma in biopsies of suspected lesions. The requirement for invasive surgery for the diagnosis of peritoneal implants likely contributes to an average latency of 6.7 years from onset of symptoms to definitive diagnosis [Citation7]. Delayed diagnosis and treatment may have significant consequences, as endometriosis is more advanced in women whose diagnostic laparoscopy is delayed, supporting progression of disease over time [Citation8]. Indeed, longitudinal placebo-controlled trials with second look laparoscopy have demonstrated that 71–83 % of untreated lesions will progress or remain stable over a 12-month period [Citation9]. Consequently, the discovery of a non-surgical biomarker for the diagnosis of endometriosis is considered a main priority and an area of active research.
Important considerations and potential confounders in endometriosis biomarker development
Disease heterogeneity
Endometriosis is a phenotypically heterogeneous condition, appearing as superficial peritoneal lesions, endometriotic cysts within the ovary, and/or deeply infiltrative lesions with extension into adjacent pelvic organs to include bowel, bladder and ureter. Though each of these lesional types shares the histologic features of endometrial glands and stroma, increasing evidence supports their consideration as three separate subtypes: peritoneal, ovarian, and rectovaginal [Citation10]. These disease subtypes may arise by distinct pathogenic mechanisms. Peritoneal endometriosis, by virtue of superficial and relatively transient localization, is best supported by retrograde menstruation with subsequent implantation, whereas ovarian endometriosis may originate from coelomic metaplasia of superficial epithelium in an estrogen rich microenvironment. Finally, deep infiltrating endometriosis (DIE) affecting the rectovaginal space may represent direct extension from subserosal adenoymotic lesions of the lower uterine segment. Preclinical and molecular lines of evidence support the classification of endometriosis as three separate anatomic subtypes. The anatomic distinctions are important in the context of biomarker development. Currently, ultrasound and magnetic resonance imaging provide adequate diagnostic accuracy for ovarian endometriosis and to a lesser extent DIE, but no reliable detection method exists for the more prevalent peritoneal form of the disease. Therefore, the population for whom a biomarker is most relevant is women with pain and/or infertility and without radiologic evidence of ovarian or deep infiltrating endometriosis. This population is thought to represent the majority of women with the disease.
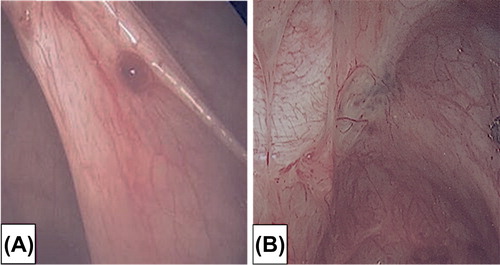
Like anatomic distribution, a temporal component to disease heterogeneity complicates efforts at biomarker development, particularly for the peritoneal endometriosis subtype. Over time, a stagewise phenotypic progression is observed with peritoneal endometriotic lesions progressing from red vesicular to black powder-burn and culminating in a fibrotic appearance (). The sequence is likely a consequence of cyclic inflammatory reaction to the peritoneal endometriotic lesion. The earliest lesion is the red vesicular subtype cytoarchitecturally defined as a cluster of communicating glands that are more biochemically active than black powder-burn lesions [Citation11]. Of the peritoneal lesional subtypes, the red vesicular lesion most closely resembles the endometrial tissue of origin, as reflected by their responsiveness to cyclic ovarian steroid hormones. Laparoscopy timed to menstruation observed these lesions to be focally hemorrhagic in response to progesterone withdrawal [Citation6]. Lesional bleeding could be the precursor to the development of fibrin mediated adhesions. As a consequence of cyclic inflammatory reactions, lesions eventuate to cicatrization. A temporal progression of peritoneal lesions from red vesicular to fibrotic stages is supported by a large prospective surgical study finding red vesicular lesions predominantly in younger (20–25 year old) women and white plaques predominantly in older (41–45 year old) women [Citation12].
Inaccuracy of surgical diagnosis
Partially owing to the aforementioned lesional heterogeneity, the potential exists for inconsistency and inaccuracy in the laparoscopic diagnosis of endometriosis. Endometriotic lesions evidence variable characteristics at laparoscopy, rendering their visual diagnosis less certain. A study involving women with chronic pelvic pain undergoing laparoscopy determined laparoscopic visualization of lesions to be an inaccurate approach [Citation13]. Of 122 excised peritoneal lesions from 54 patients, only 54 % were confirmed to be endometriotic on histologic review. Consequently, 20 patients who were surgically staged as having minimal to mild endometriosis at laparoscopy were misdiagnosed. This study highlights the value of histology over macroscopic inspection as the gold standard in defining specimens in clinical and molecular research studies. Biopsy from each affected area with detailed annotation of pathologic specimens is important toward prevention of false negative histology for the patient and in the accuracy of reported results for the progress of endometriosis research.
Variable symptomatology
A major limitation to the progress of biomarker development in endometriosis is the lack of correlation between the extent of disease and symptoms of pain or infertility. The incongruence of the widely used rAFS staging system with pain is well documented. Endometriosis can be asymptomatic, and the presence of endometrial tissue in the pelvis transiently may not be necessarily pathologic.
Clustering of endometriosis with other estrogen-dependent pathology
The estrogen (specifically, 17-β estradiol) dependence of endometriosis is well established. Prolonged intervals of unopposed estrogen predispose women, female primates, and even men to the disorder, whereas natural or surgical menopause is generally palliative if not curative [Citation14]. Estradiol is the proliferative signal for both eutopic and ectopic endometrial growth, and alterations of estrogen signaling have been associated with the disease. In a hypogonadal athymic nude mouse model, endometrial explant cultures treated with estradiol secrete matrix metalloproteinases (MMPs) and establish ectopic peritoneal lesions when introduced into recipient animals [Citation15]. Once established, endometriotic lesions evidence a molecular program designed to maintain local estrogen bioavailability. Specifically, levels of the ERβ isoform of the estrogen receptor and aromatase are significantly elevated in endometriotic lesions relative to eutopic endometrium [Citation16].
Like endometriosis, endometrial polyps, uterine leiomyomata, and adenomyosis are estrogen-dependent conditions, and each has been observed to cluster with endometriosis in affected women. A retrospective study of 431 women with infertility revealed endometrial polyps in 68 % of women with laparoscopically confirmed endometriosis as compared to a 20 % prevalence of polyps in infertile women without endometriosis [Citation17]. Similarly, adenomyosis has been shown to be significantly associated with peritoneal endometriosis in infertile patients [Citation18].
These associations have important implications for biomarker development, particularly endometrial biopsy-derived candidates. Clustered pathologies may confound the interpretation of molecular profiles in the delineation of a biomarker that is unique to endometriosis. Consequently, screening for endometrial polyps, leiomyomata and adenomyosis is an important consideration in the cataloging of biobank specimens used for biomarker discovery.
Influence of menstrual cycle phase in biomarker interpretation
An ideal biomarker for endometriosis would maintain sensitivity and specificity regardless of when the sample was taken relative to the menstrual cycle. However, for biomarkers whose expression is estrogen or progesterone regulated, cyclic variation may exist and influence interpretation. Cycle-based laboratory assessment is not unprecedented in the gynecologic setting as evidenced by the requirement for serum follicle stimulating hormone (FSH) and estradiol sampling on day 3 of the menstrual cycle for accurate ovarian reserve assessment.
The cyclic variation in the endometrial molecular signature presents a significant challenge with regard to endometrial-based biomarker development. Whole genome molecular profiling of normal endometrium demonstrated tremendous variance between samples taken from the proliferative, early-secretory, mid-secretory and late-secretory phases of the cycle [Citation19]. Subsequent comparison of global gene expression profiles in the endometrium from women with versus without endometriosis showed specimens to cluster more by cycle phase than by disease status [Citation20], highlighting the importance of phase specific delineation of pipelle samples intended for use in endometrial biomarker development.
Candidates for an endometriosis biomarker
Properties of an ideal biomarker
In view of the above considerations and potential confounders, critical properties of a biomarker for endometriosis include high sensitivity, high specificity, simplicity, reproducibility and minimal invasiveness (). The acceptable sensitivity and specificity of radiologic approaches to detect ovarian endometriosis (i.e. endometrioma or ‘chocolate cysts’) and deep infiltrating endometriosis (i.e. rectovaginal nodules) focus the need for a test to detect the peritoneal form of the disease. Women with only peritoneal endometriosis are typically staged at rAFS stage I–II, but may evidence more advanced stages depending on the extent of peritoneal involvement and associated adhesions. Given the tendency for endometriosis to cluster with other estrogen-dependent conditions, a biomarker must be sufficiently specific to delineate among them. The chronic inflammatory nature of endometriosis further challenges the specificity of tests based on mediators of inflammation. Optimally, a biomarker for endometriosis will offer the convenience of sampling at any point during the menstrual cycle. However, a cycle phase specific test frame is acceptable toward maximizing the sensitivity and specificity of the assay. A non-invasive test in the form of a peripheral blood or urine sample is preferred, though a semi-invasive approach such as an office-based endometrial pipelle biopsy is a reasonable alternative to surgery. Finally, a biomarker for endometriosis must produce consistent results across a spectrum of geographic, ethnic and biometric demographics. To date, an ideal biomarker meeting these criteria has not been validated.
Table I. Properties of an ideal biomarker for endometriosis.
Approaches to biomarker discovery
Myriad approaches to biomarker discovery in endometriosis have been applied to include both unbiased and targeted. Unbiased discovery methods leverage the power of high throughput genomic or proteomic platforms and systems biology principles toward the identification of candidate molecules. In targeted approaches, biologically plausible molecules are screened for biomarker candidacy either as a single molecule or as a panel of molecules. Importantly, the two approaches are not mutually exclusive, as unbiased methods may unveil biological processes or even candidates for orthogonal and targeted testing.
A thorough understanding of disease pathogenesis provides a solid foundation for targeted biomarker discovery. Though often regarded as an enigmatic disease, the molecular features that underpin the pathophysiology of endometriosis are clarifying. Biological categories investigated for association with endometriosis include glycoproteins, cytokines and chemokines, growth factors, apoptosis markers, cell adhesion markers, hormones, autoantibodies and cell populations. A brief list of selected endometriosis biomarker candidates is provided in . For a thorough description of over 100 peripheral (serum, plasma and urinary) biomarker candidates reported in the 25 years prior to mid-2009, the reader is referred to the excellent systematic review provided by May et al. [Citation21]. The same group consolidated endometrial candidates from studies published over a 25-year interval prior to mid-2010 [Citation22]. In both systematic reviews, studies were scored (0–11 in the former and 0–10 in the latter) using a modification of the QUADAS (Quality Assessment of Diagnostic Accuracy Studies) criteria. Unfortunately, a fair number of the studies included in these reviews are challenged by the aforementioned confounders, are underpowered and/or lack suitable controls. For purposes of this review, biomarker candidates will be grouped by the route of sampling.
Table II. Summary of selected endometriosis biomarker candidates.
Peripheral blood
A serum or plasma biomarker, though attractive, remains elusive. Perhaps the most extensively studied molecule for endometriosis detection is the glycoprotein, Cancer Antigen 125 (CA-125). Though CA-125 may have value in the detection of advanced stage disease, particularly endometriomas [Citation23], the absolute level of the analyte appears to lack sufficient sensitivity to be useful in the diagnosis of peritoneal endometriosis. Relative changes in the CA-125 level over the menstrual cycle evidenced promise. In a cohort presenting for infertility, CA-125 elevation during menses was more pronounced in women with endometriosis, with an 83 % increase in the CA-125 level showing 93 % sensitivity and 92 % specificity for the diagnosis of the disorder [Citation30]. This study was relatively small and requires independent validation. More recently, a panel of plasma analytes which included CA-125, VEGF, Annexin V and Glycodelin sampled during the menstrual phase of the cycle demonstrated 81 % sensitivity and 81 % specificity for the detection of ultrasound negative endometriosis based on a multivariate logistic regression analysis [Citation28].
Technological advances in mass spectrometry and biocomputational analysis have accelerated the proteomic search for a non-surgical biomarker. Differential protein expression in the serum, plasma, urine or endometrium of women with endometriosis relative to women without the disease may provide candidates for biomarker development.
Urinary
Relative to serum, urine evidences a significantly narrower dynamic range of proteins, allowing more rapid preparation of specimens for proteomic interrogation. Using MALDI-TOF MS, several groups have reported differential peptide profiles in the urine of women with endometriosis relative to that of women without endometriosis at surgery [Citation31,Citation32]. MALDI-TOF does not allow direct identification of peptides or proteins that are differentially expressed. The development of a urinary diagnostic is in the early stages. Like serum, urine reflects an amalgam of systemic processes, and it will be important to assess the ability of a urinary assay to differentiate endometriosis from other inflammatory conditions.
Endometrial
Though more invasive than serology, endometrial tissue is accessible via biopsy in the office setting and offers the potential advantage of improved specificity. Devices such as the Pipelle suction-based sampler are commonly used in the office without the need for anesthesia. An endometrial diagnostic assay is preferably obtained in the proliferative phase, as this avoids interruption of an unanticipated pregnancy that would be undetectable by current pregnancy tests in the mid-to-late secretory phase. This diagnostic assay is predicated on reliable differential expression of a biomarker between women with and without endometriosis during the proliferative phase of the menstrual cycle.
At the transcriptomic level, significant differences in gene expression exist in eutopic endometrium from women with versus without endometriosis. Such differences suggest a heritable or acquired abnormality of the endometrium may provide a selective survival and implantation advantage toward the formation of endometriotic lesions. Both array-based global [Citation20] and targeted gene expression studies [Citation22] of the endometrial transcriptome have identified genes and pathways that may be involved in disease pathogenesis and reveal potential candidates for the development of an endometrial-based biomarker.
Several groups have reported unique proteomic profiles using the SELDI-TOF MS platform in the endometrium of women with endometriosis compared to women without the disease [Citation33,Citation34]. Importantly, the SELDI-TOF MS methodology provides differential proteomic profiles in the form of mass/charge (m/z) peaks without attendant characterization of the peptides or proteins. Nonetheless, the more recent of these studies described a panel of differentially expressed (m/z) peaks in the endometrial proteome of women with versus without endometriosis as diagnostic of endometriosis with 89–91 % sensitivity and 80–90 % specificity [Citation33].
Clarification of the role of neuroangiogenesis in endometriosis has translated to potential as a biomarker. Initial independent studies involving immunohistochemical detection of the PGP9.5 marker for nerve fibers in the endometrium proved highly sensitive (95 % and 98 %) and specific (100 % and 83 %) for the identification of endometriosis [Citation29,Citation35]. The latter study determined the diagnostic potential of the test to be independent of menstrual cycle phase at the time of endometrial sampling. The specificity of nerve fiber assays is challenged by the finding of similar endometrial innervation and neuronal growth fiber expression in women with adenomyosis, with expression reported to be more correlated with pelvic pain than diagnosis [Citation36]. Studies involving larger populations are needed to validate the utility of endometrial nerve fiber density as a biomarker for endometriosis.
Conclusion
Endometriosis is a chronic inflammatory disorder for which novel diagnostic and therapeutic approaches are urgently needed. The past decade has witnessed an explosive increase in molecular and clinical studies aimed at addressing these needs. The development of a reliable biomarker for the early detection of endometriosis is challenged by clinical heterogeneity, inaccuracy of visual inspection in the diagnosis of early stage endometriosis [Citation13], clustering of endometriosis with other estrogen-dependent disorders, and the potential confounding effect of menstrual cycle phase in biomarker interpretation. These confounders highlight the importance of proper collection and meticulous curation of tissue bank specimens used in biomarker development and validation. Well-developed protocols for the standardization of biobank procedures in endometriosis research have been published [Citation37]. Unbiased and targeted biomarker discovery approaches using large, well curated biorepositories offer promise in the identification of a biomarker for endometriosis.
Questions and answers
Q (Diamanti): Is the infertility in patients with endometriosis secondary to the pathology or is there some genetic basis?
A (Burney): A meta-analysis showed that the presence of endometriosis decreased IVF pregnancy rate by 50 % and depended on the stage of the disease. Endometriosis caused impairment of several aspects; egg and embryo development, transit across fallopian tubes and implantation. The reason for implantation problems may be a delay in progesterone action on the endometrium, creating dyssynchrony between embryo availability and endometrial receptivity.
Q (Anderson): Does adenomyosis have a completely different pathophysiology?
A (Burney): We consider them related conditions to an extent. Endometriosis may represent three different entities, with rectovaginal disease an extension of adenomyotic disease.
Q (Villa): My question is an extension of the previous discussion. In some cases we have diagnosed intestinal endometriosis. Is this an independent condition?
A (Burney): Deeply infiltrative endometriosis affecting the rectosigmoid colon is a particularly aggressive form of the disease, possibly the adenomyotic form.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
The views expressed are those of the author(s) and do not reflect the official policy of the Department of the Army, the Department of Defense or the U.S. Government.
References
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am 1997;24:235–58.
- Simpson JL, Elias S, Malinak LR, et al. Heritable aspects of endometriosis. I. Genetic studies. Am J Obstet Gynecol 1980;137:327–31.
- Hadfield RM, Mardon HJ, Barlow DH, et al. Endometriosis in monozygotic twins. Fertil Steril 1997;68:941–2.
- Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927;14:442–69.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril 2012;98:511–9.
- Burney RO, Lathi RB. Menstrual bleeding from an endometriotic lesion. Fertil Steril 2009;91:1926–7.
- Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011; 96:366–73, e8.
- D’Hooghe TM, Debrock S. Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Hum Reprod Update 2002;8:84–8.
- Sutton CJ, Ewen SP, Whitelaw N, et al. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril 1994;62: 696–700.
- Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil Steril 1997;68: 585–96.
- Vernon MW, Beard JS, Graves K, et al. Classification of endometriotic implants by morphologic appearance and capacity to synthesize prostaglandin F. Fertil Steril 1986; 46:801–6.
- Koninckx PR, Meuleman C, Demeyere S, et al. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril 1991;55:759–65.
- Marchino GL, Gennarelli G, Enria R, et al. Diagnosis of pelvic endometriosis with use of macroscopic versus histologic findings. Fertil Steril 2005;84:12–5.
- Punnonen R, Klemi PJ, Nikkanen V. Postmenopausal endometriosis. Eur J Obstet Gynecol Reproduct Biol 1980;11: 195–200.
- Bruner KL, Matrisian LM, Rodgers WH, et al. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest 1997;99:2851–7.
- Bukulmez O, Hardy DB, Carr BR, et al. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology 2008;149:1190–204.
- Shen L, Wang Q, Huang W, et al. High prevalence of endometrial polyps in endometriosis-associated infertility. Fertil Steril 2011;95:2722–4, e1.
- Kunz G, Beil D, Huppert P, et al. Adenomyosis in endometriosis – prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum Reprod 2005;20:2309–16.
- Talbi S, Hamilton AE, Vo KC, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006;147:1097–121.
- Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 2007;148:3814–26.
- May KE, Conduit-Hulbert SA, Villar J, et al. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update 2010;16:651–74.
- May KE, Villar J, Kirtley S, et al. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update 2011;17:637–53.
- Mol BW, Bayram N, Lijmer JG, et al. The performance of CA-125 measurement in the detection of endometriosis: a meta-analysis. Fertil Steril 1998;70:1101–8.
- Agic A, Xu H, Rehbein M, Wolfler MM, et al. Cognate chemokine receptor 1 messenger ribonucleic acid expression in peripheral blood as a diagnostic test for endometriosis. Fertil Steril 2007;87:982–4.
- Othman Eel D, Hornung D, Salem HT, et al. Serum cytokines as biomarkers for nonsurgical prediction of endometriosis. Eur J Obstet Gynecol Reproduct Biol 2008;137:240–6.
- Cho S, Ahn YS, Choi YS, et al. Endometrial osteopontin mRNA expression and plasma osteopontin levels are increased in patients with endometriosis. Am J Reproduct Immunol 2009;61:286–93.
- Agic A, Djalali S, Wolfler MM, et al. Combination of CCR1 mRNA, MCP1, and CA125 measurements in peripheral blood as a diagnostic test for endometriosis. Reprod Sci 2008;15:906–11.
- Vodolazkaia A, El-Aalamat Y, Popovic D, et al. Evaluation of a panel of 28 biomarkers for the non-invasive diagnosis of endometriosis. Hum Reprod 2012;27:2698–711.
- Al-Jefout M, Dezarnaulds G, Cooper M, Tokushige N, Luscombe GM, Markham R, et al. Diagnosis of endometriosis by detection of nerve fibres in an endometrial biopsy: a double blind study. Hum Reprod 2009;24:3019–24.
- Kafali H, Artuc H, Demir N. Use of CA125 fluctuation during the menstrual cycle as a tool in the clinical diagnosis of endometriosis; a preliminary report. Eur J Obstet Gynecol Reproduct Biol 2004;116:85–8.
- El-Kasti MM, Wright C, Fye HK, et al. Urinary peptide profiling identifies a panel of putative biomarkers for diagnosing and staging endometriosis. Fertil Steril 2011;95: 1261–6, e1–6.
- Tokushige N, Markham R, Crossett B, et al. Discovery of a novel biomarker in the urine in women with endometriosis. Fertil Steril 2011;95:46–9.
- Fassbender A, Verbeeck N, Bornigen D, et al. Combined mRNA microarray and proteomic analysis of eutopic endometrium of women with and without endometriosis. Hum Reprod 2012;27:2020–9.
- Wang L, Zheng W, Ding XY, et al. Identification biomarkers of eutopic endometrium in endometriosis using artificial neural networks and protein fingerprinting. Fertil Steril 2010;93:2460–2.
- Bokor A, Kyama CM, Vercruysse L, et al. Density of small diameter sensory nerve fibres in endometrium: a semi-invasive diagnostic test for minimal to mild endometriosis. Hum Reprod 2009;24:3025–32.
- Zhang X, Lu B, Huang X, et al. Endometrial nerve fibers in women with endometriosis, adenomyosis, and uterine fibroids. Fertil Steril 2009;92:1799–801.
- Sheldon E, Vo KC, McIntire RA, et al. Biobanking human endometrial tissue and blood specimens: standard operating procedure and importance to reproductive biology research and diagnostic development. Fertil Steril 2011;95:2120–2, 2, e1–12.