Abstract
Objective. Galactose elimination capacity (GEC) is used as a quantitative measure of liver metabolic function with prognostic value in adults with acute and chronic liver failure. Almost no data are available regarding GEC in children, however. This study thus aims to meet the previously unmet clinical need for age-related data on GEC in children. Material and methods. We studied galactose elimination in 10 healthy children (median age 10.7 years; range 7 months to 16 years) and 30 children with chronic liver disease (median age 8.6 years; range 3 months to 16 years). GEC was estimated from the linear decrease in concentration of galactose in arterialized capillary blood from the ear following intravenous infusion of galactose. Results. In both groups of children, GEC (μmol/min/kg body weight) was highest in the youngest children and decreased with age, although at a significantly lower level in the children with liver disease (p = 0.05). GEC was significantly higher in healthy children than in healthy adults, diminishing to the adult level by the age of 16 years. Conclusions. GEC was found to be higher in children than in adults until the age of 16 years. Moreover, GEC was significantly lower in children with chronic liver disease than in healthy children, underlining that GEC testing also has potential clinical usefulness as a quantitative measure of liver metabolic function in children.
Introduction
The capacity of the body to eliminate the sugar galactose is used as a quantitative measure of liver metabolic function [Citation1]. In adults, galactose elimination capacity (GEC) is known to be related to body weight and decrease slowly with age [Citation2,Citation3]. GEC is of prognostic value in adults with acute liver failure and chronic liver disease, irrespectively of etiology [Citation4–9], and it is a good predictor of outcome in patients undergoing liver resection for cancer [Citation10]. In combination with the Child-Pugh score, GEC predicts survival in patients with acute or chronic liver failure and has been used to identify candidates for urgent liver transplantation [Citation11,Citation12].
In children, GEC has been measured following heart surgery using adult reference values [Citation13], but has otherwise only been measured sporadically [Citation14]. As a quantitative measure of liver metabolic function is as relevant for children as for adults, there is an unmet need for age-related GEC values in children.
The purpose of the present study was thus to describe GEC in healthy children aged from a few months to adolescence for use in interpreting GEC in children with chronic liver disease.
Material and methods
Subjects
The study was performed between 2002 and 2008 and followed a cross-sectional design encompassing measurement of GEC in 10 healthy children and 30 children with chronic liver disease. The median age of the healthy children was 10.7 years (range 7 months to 16 years), while that of the children with liver disease was 8.6 years (range 3 months to 16 years).
The liver disease diagnoses were as follows (): alpha-1-antitrypsin deficiency (7 patients), autoimmune hepatitis (6 patients), biliary atresia (3 patients), Wilson's disease (3 patients), familiar Indian childhood cirrhosis (2 patients), primary sclerosing cholangitis (2 patients), cystic fibrosis (2 patients), chronic hepatitis B infection (1 patient), progressive familiar intrahepatic cholestasis (1 patient), and sustained severe but unexplained liver enzyme elevation (3 patients). None of the patients had a Child-Pugh score exceeding grade A.
Table I. Biochemical characteristics of 30 children with liver disease.
GEC measurement
The children were fasted overnight with free access to drinking water. The next morning, galactose solution (Kabi, Sweden) (0.5 g/ml) was administered intravenously over a 5-min period in a dose of 0.75 g/kg body weight. In cases where the total amount of galactose would exceed 40 g, the dose was reduced to 0.5 g/kg body weight (equivalent to that used in adults). 100-μl samples of arterialized capillary blood from the ear were collected every 5 min from 25 to 60 min following galactose administration [Citation15]. Urine was collected by voiding for 4 h following galactose administration for determination of urinary excretion of galactose. The galactose concentration in the blood and urine samples was determined enzymatically [Citation16].
GEC was calculated as described by Tygstrup [Citation1]:
where D is the infused dose of galactose (μmol), U is the amount of galactose excreted into the urine (μmol), and the time of intercept is the intercept with time in minutes of a linear regression of the time course of the blood concentration of galactose. The constant, 7 min, was added to correct for the delay in galactose distribution between the blood and the hepatocytes.
Ethics
The study was performed in accordance with the Helsinki Declaration and was approved by the Ethics Committee of Aarhus County (VEK no. 20020293). All the patients were recruited from the Department of Paediatrics, Aarhus University Hospital. The healthy children were recruited among parents who responded to a poster displayed in the hospital restaurant. Written informed (verbal and written) consent was obtained from the parents of each of the healthy subjects.
Statistics
The relationship between GEC and age was determined by non-linear regression for the healthy children and for the children with liver disease using STATA statistical software 11 (StataCorp, USA).
Results
In both the healthy children and the children with liver disease, GEC was highest in the youngest children and decreased with age (). The curvilinear relationship between GEC (μmol/min/kg) and age (years) was significantly lower in the children with liver disease than in the healthy children (children with liver disease: GEC = 46.20 − 10.27 ln(age/5) + 1.259 [ln(age/5)]; healthy children: GEC = 53.71 − 10.27 ln(age/5) + 1.259 [ln(age/5)]). The mean difference was 7.51 μmol/min/kg (95% CI: 0.02; 15.00, p = 0.05). There was no significant gender difference in GEC (data not shown).
Figure 1. Age-dependency of GEC in children. Galactose elimination capacity (GEC) in relation to age in 10 healthy children (•; solid line) and 30 children with liver disease (Δ; broken line). The symbols indicate measured values and the lines indicate the fitted regression curves (see text).
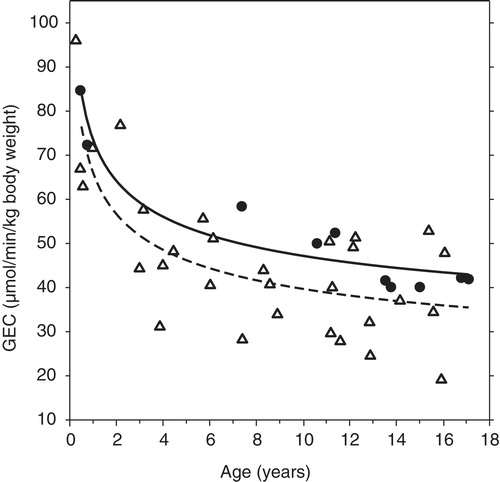
In healthy children, GEC was significantly greater than in healthy adults. At age of 16 years, GEC had decreased to the average adult value (41.5 μmol/min/kg body weight at age 25 years [Citation3]).
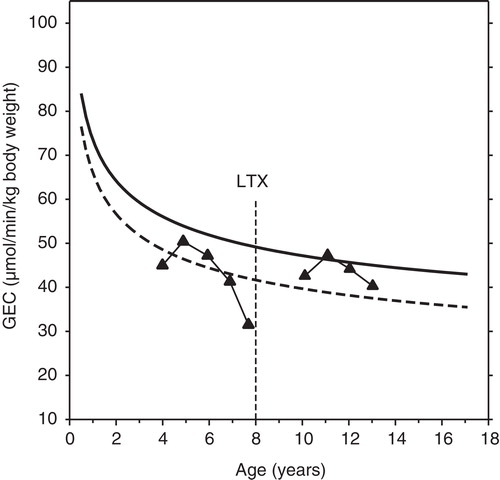
The decline in GEC from age 5 to 8 years in children with alpha-1-antitrypsin deficiency is shown in . Following liver transplantation at age 8 years, GEC recovered to the level seen in healthy children of the same age.
Discussion
The main findings of the present study are that GEC is higher in children than in adults, GEC decreases with age, and that GEC is lower in children with liver disease than in healthy children of the same age. Our data comprise the first measurements of GEC in children from infancy to adolescence. The age-dependent decrease in GEC underscores the need for such a description, and its clinical usefulness is evident from the fact that GEC was lower in children with liver disease than in healthy children of the same age.
As the recruitment of healthy children for studies involving venous infusion of galactose and repeated sampling of blood from the ear is difficult, our study only encompasses 10 healthy children. Nevertheless, the data cover a wide spectrum of age, and the age-dependency of the decrease in GEC was very clear. In order to determine the limits of variation of GEC in healthy children it will be necessary to study a larger group of healthy children of different ages.
At all ages, GEC was lower in children with chronic liver disease than in healthy children. As there was some overlap, however, the discriminative or diagnostic value of the test was low, as expected in view of the variety of the liver diagnoses. The strength of the test lies in the quantitative and hence prognostic information it provides about the metabolic liver function independent of the diagnosis. It is interesting that even if the children were not clinically severely ill, the GEC was sensitive enough to on average detect a significant reduction in the liver metabolic function in children with liver disease compared with that of the healthy children. The relationships between GEC and age in may be used clinically to judge whether or not a given GEC value is indicative of severely compromised liver function. As illustrated in , repeated measurements may be of particular value.
Figure 2. Example of GEC before and after liver transplantation in a child with severe liver disease. In this boy with alpha-1-antitrypsin deficiency the age-dependent decline in GEC was markedly more pronounced than in healthy children. Following liver transplantation at the age of eight his GEC normalized and exhibited a normal age-dependent decline. The symbols connected by straight lines indicate measured values. The regression curves shown for comparison are those illustrated in .
The age-dependence of GEC was very strong in both healthy children and children with liver disease: GEC was fourfold greater in the youngest children than in the adolescents and adults. As GEC is a measure of the in vivo liver-specific maximum capacity for galactose removal (Vmax) [Citation17], the decrease with age is probably due to changes in the activity of hepatic galactokinase and changes in the relative weight of the liver. Galactose is one of the hexoses in lactose, and hence a major source of energy in milk. Hepatic galactokinase activity is high in the human fetus [Citation18,Citation19]. Liver weight is about 5% of body weight in infants aged less than 1 year and gradually decreases to 2–3% of body weight in adults [Citation20,Citation21].
The present study indicates that GEC can be used to test liver metabolic function in children. A lower GEC or a faster than expected age-dependent decrease in GEC may indicate deterioration in liver metabolic function. GEC may be particularly valuable in children on oral anti-coagulation therapy since the international normalized ratio (INR) cannot be used to calculate the PMELD and Child-Pugh scores in such children.
In summary, GEC was highest in the youngest children and declined with age in both healthy children and children with chronic liver disease. Moreover, GEC was significantly lower in children with chronic liver disease than in healthy children in spite of a relatively low number of healthy children included in the study. These findings indicate clinical usefulness of GEC as a quantitative measure of liver metabolic function in children. The results of the present study may help to design future studies on liver function tests in children.
Acknowledgements
The study was supported by the National Institutes of Health (R01-DK074419), the Danish Medical Research Council (09-061564), the NovoNordic Foundation, the A.P. Møller Foundation for the Advancement of Medical Science and the Aase og Ejnar Danielsens Fond.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Tygstrup N. Determination of the hepatic elimination capacity (Lm) of galactose by single injection. Scand J Clin Lab Invest Suppl 1966;18:118–25.
- Marchesini G, Bua V, Brunori A, Bianchi G, Pisi P, Fabbri A, Galactose elimination capacity and liver volume in aging man. Hepatology 1988;8:1079–83.
- Schnegg M, Lauterburg BH. Quantitative liver function in the elderly assessed by galactose elimination capacity, aminopyrine demethylation and caffeine clearance. J Hepatol 1986;3:164–71.
- Herold C, Regn S, Ganslmayer M, Ocker M, Hahn EG, Schuppan D. Can quantitative tests of liver function discriminate between different etiologies of liver cirrhosis? Dig Dis Sci 2002;47:2669–73.
- Herold C, Heinz R, Niedobitek G, Schneider T, Hahn EG, Schuppan D. Quantitative testing of liver function in relation to fibrosis in patients with chronic hepatitis B and C. Liver 2001;21:260–65.
- Herold C, Heinz R, Radespiel-Tröger M, Schneider HT, Schuppan D, Hahn EG. Quantitative testing of liver function in patients with cirrhosis due to chronic hepatitis C to assess disease severity. Liver 2001;21:26–30.
- Merkel C, Gatta A, Zoli M, Bolognesi M, Angeli P, Iervese T, Prognostic value of galactose elimination capacity, aminopyrine breath test, and ICG clearance in patients with cirrhosis. Comparison with the Pugh score. Dig Dis Sci 1991;36:1197–203.
- Merkel C, Marchesini G, Fabbri A, Bianco S, Bianchi G, Enzo E, The course of galactose elimination capacity in patients with alcoholic cirrhosis: possible use as a surrogate marker for death. Hepatology 1996;24:820–3.
- Jepsen P, Vilstrup H, Ott P, Keiding S, Andersen PK, Tygstrup N. The galactose elimination capacity and mortality in 781 Danish patients with newly-diagnosed liver cirrhosis: a cohort study. BMC Gastroenterol 2009;9:50–6.
- Redaelli CA, Dufour JF, Wagner M, Schilling M, Hüsler J, Krähenbühl L, Preoperative galactose elimination capacity predicts complications and survival after hepatic resection. Ann Surg 2002;235:77–85.
- Salerno F, Borroni G, Moser P, Sangiovanni A, Almasio P, Budillon G, Prognostic value of the galactose test in predicting survival of patients with cirrhosis evaluated for liver transplantation. A prospective multicenter Italian study. AISF Group for the Study of Liver Transplantation. Associazione Italiana per lo Studio del Fegato. J Hepatol 1996;25:474–80.
- Schmidt LE, Ott P, Tygstrup N. Galactose elimination capacity as a prognostic marker in patients with severe acetaminophen-induced hepatotoxicity: 10 years' experience. Clin Gastroenterol Hepatol 2004;2:418–24.
- Narkewicz MR, Sondheimer HM, Ziegler JW, Otanni Y, Lorts A, Shaffer EM, Hepatic dysfunction following the Fontan procedure. J Pediatr Gastroenterol Nutr 2003;36:352–7.
- Narkewicz MR, Smith D, Gregory C, Lear JL, Osberg I, Sokol RJ. Effect of ursodeoxycholic acid therapy on hepatic function in children with intrahepatic cholestatic liver disease. J Pediatr Gastroenterol Nutr 1998;26:49–55.
- Tygstrup N. Effect of sites of blood sampling in determination of the galactose elimination capacity. Scand J Clin Lab Invest 1977;37:333–8.
- Kurz G, Wallenfels K D-Galactose, UV test mit Galactose-dehydrogenase. In: Bergmeyer HU, editor. Methoden der enzymathischen analyse. Weinheim, Germany: Verlag Chemie; 1970. pp 1241–4.
- Keiding S, Johansen S, Winkler K, Tønnesen K, Tygstrup N. Michaelis-Menten kinetics of galactose elimination by the isolated perfused pig liver. Am J Physiol 1976;230:1302–13.
- Donnel GN, Bergren WR, Cleland RS. Galactosemia. Pediatr Clin North Am 1960;7:315–32.
- Shin-Buehring YS, Beier T, Tan A, Osang M, Schaub J. The activity of galactose-1-phosphate uridyltransferase and galactokinase in human fetal organs. Pediatr Res 1977;11:1045–51.
- Vink CL. Liver function and age. Clin Chim Acta 1959;4:674–682.
- Noda T, Todani T, Watanabe Y, Yamamoto S. Liver volume in children measured by computed tomography. Pediatr Radiol 1997;27:250–2.
