Abstract
Objective. Meta-analyses have indicated effect of probiotics on irritable bowel syndrome (IBS). However, few long-term trials have been conducted and uncertainty remains as to effectiveness and long-term effect in a primary care setting. We aimed to investigate the effect of probiotics compared with placebo in the management of IBS in primary care during a 6-month treatment period and with a 6-month follow-up. Material and methods. We randomized IBS patients fulfilling Rome III criteria to receive two capsules twice daily either containing placebo or a probiotic mixture of Lactobacillus paracasei ssp paracasei F19, Lactobacillus acidophilus La5 and Bifidobacterium Bb12 in an amount of 1.3 × 1010 CFU per capsule. Primary endpoint was proportion of responders defined as patients reporting adequate relief (AR) at least 50% of the time in the 6-month treatment period. Secondary outcomes were proportions of patients reporting AR at different time points, and change in gastrointestinal symptoms and health-related quality of life (HrQOL) from baseline to 6 and 12 months. Results. A total of 131 patients were included in this study. The proportion of responders in the treatment period was 52% (35/67) in the probiotic group versus 41% (26/64) in the placebo group, p = 0.18. Overall we found no difference between the groups in change in gastrointestinal symptoms after treatment. Patients improved in HrQOL, but with no statistically significant difference between the groups. Conclusion. During a 6-month treatment period, we were not able to detect a positive effect of probiotic when compared with placebo.
Introduction
Irritable bowel syndrome (IBS) is characterized by chronic or recurrent symptoms attributed to the gastrointestinal (GI) tract in the absence of an organic explanation. IBS is very common affecting about 12% in Western populations [Citation1]. The exact pathophysiological mechanisms are unknown, and many physiological explanations have been raised, such as abnormal GI motility, visceral hypersensitivity, altered brain gut function, low-grade chronic inflammation and alteration in intestinal flora.
In recent years, an increasing focus has been on a possible influence on the gut microbiota. The current working hypothesis is that abnormal microbiota activate mucosal innate immune responses, thereby increasing epithelial permeability and activating nociceptive sensory pathways and dysregulating the enteric nervous system [Citation2]. Clinical evidence for this is supported by the fact that: 1) IBS symptoms can develop in predisposed individuals following an enteric infection [Citation3], 2) treatments targeting the microbiota such as antibiotics [Citation4], probiotics [Citation5] and prebiotics [Citation6] have been suggested to be effective and 3) the fecal microbiota has proved to be significantly altered in IBS patients compared to healthy controls [Citation7,8].
Trials with probiotics have been conducted and meta-analyses indicate effect on global symptoms with number needed to treat (NNT) ranging from 4 to 21 [Citation5,9,10]. Unfortunately, these trials have been very heterogeneous using different probiotic strains, doses and outcomes. Furthermore, many trials had methodological limitations. Proofs of efficacy have often been based on reported change in composite or specific symptoms scores not integrating clinical relevance for the patient. Since IBS is a chronic and relapsing condition and severity as well as symptoms may vary in the same patient over time, long-term efficacy trials are needed. Unfortunately, only few long-term trials have been conducted and none of them with long follow-up [Citation11–13]. Probiotics do not colonize the GI tract, but may in theory create an aberrant flora giving the patient symptoms after cessation of treatment. A long follow-up provides opportunity to determine treatment durability and potential worsening of symptoms after cessation of treatment.
The aim of this study was to investigate the effect of probiotics compared with placebo in the management of IBS in a primary care population during a 6-month treatment period and with a 6-month follow-up.
Methods
We conducted a randomized double-blind, placebo-controlled trial with two parallel groups. Patients were included from January 2009 to June 2010 and followed for 1 year.
Participants and setting
The target group was patients aged 18–50 years, consulting with GI complaints and suspected of IBS by their general practitioner (GP). GPs referred patients to the study, where they were assessed for eligibility. Enrollment took place at the Research Unit of General Practice in Odense, Denmark. Inclusion criteria were informed written consent and fulfillment of Rome III criteria. Exclusion criteria were a) presence of alarm signals (unexplained weight loss >3 kg within the past 3 months, rectal bleeding, unexplained fever, unexplained anaemia, family history of inflammatory bowel disease (IBD) or colorectal cancer (CRC), abnormal physical examination), b) medicine or alcohol abuse, c) pregnancy, d) severe comorbidity interfering with evaluation of outcomes and e) duration of symptoms less than 1 year in patients aged above 40. The last criterion was a consequence of the Danish guidelines on CRC. All eligible patients were first allocated to a study concerning the diagnostic process of IBS [Citation14]. Patients were excluded if receiving an organic diagnosis during the diagnostic program. All patients with a final diagnosis of IBS were included in this study and randomly allocated to capsules with probiotics or placebo. Two capsules were to be taken twice daily for 6 months. After 3 and 6 months patients attended study visits with a study nurse, where residual capsules were counted and questions about symptoms and adverse effects assessed. After completing the 6-month intervention patients were followed for another 6 months and attended a final 12-month study visit. Every month patients completed a questionnaire concerning symptoms (adequate relief (AR) and GI symptoms). Further questionnaires concerning HrQOL were answered at baseline, 6 months and 12 months. Information on demographics, previous symptoms and previous use of resources was collected in a structured interview at first encounter.
The probiotic
The probiotic capsules contained Lactobacillus paracasei ssp paracasei F19, Lactobacillus acidophilus La5 and Bifidobacterium Bb12 in doses of 3 × 109 to 7 × 109 yielding a total content of all strains of 1.3 × 1010 CFU per capsule. To achieve the same dose as in former trials conducted with the same probiotic mixture [Citation15,16], 4 capsules were to be consumed. The probiotic capsules were provided by the Danish-Swedish cooperative dairy company Arla Foods. The placebo capsules contained, like the active capsules, maltodextrin. Patients were advised against taking any other kind of probiotic during the study period but consumption of lactic acid bacteria in ordinary sour milk products was permitted.
Endpoints/questionnaires
Primary endpoint was proportions of patients reporting AR of their IBS symptoms at least 50% of the time during the 6-month treatment period. Once a month patients had to answer the question: “in the past seven days have you had adequate relief of your irritable bowel syndrome pain or discomfort?(yes/no) The question was translated into Danish by an independent bilingual secretary. Secondary endpoints were change in GI symptoms from baseline to 3, 6 and 12 months, measured by the Gastrointestinal Symptom Rating Scale modified for use in patients with IBS (GSRS-IBS) [Citation17], and change in health-related quality of life from baseline till after 6 and 12 months measured by Irritable Bowel Syndrome Quality of Life measurement (IBS-QOL) [Citation18,19]. We further assessed proportion of patients reporting AR at each time point (every month) during 12 months.
Compliance
Patients were considered compliant, if they ingested at least 80% of the planned capsules. We counted the number of capsules remaining in each returned box in order to determine the degree of compliance.
Compliance was verified by an subanalysis of a random sample of fecal samples (after 3 months of treatment), investigating the presence of Lactobacillus paracasei ssp paracasei F19 (F19), Lactobacillus acidophilus La5 (LA-5) and Bifidobacterium Bb12 (BB-12). A total of 52 samples were analyzed, of which 29 samples were from patients who had received probiotics. Colonies were isolated on specific substrates. The presence of the probiotic strain was then confirmed by counting all the characteristic colonies on the different substrates, by microscopy and by a qualitative analysis using RAPD-PCR [Citation20].
Randomisation and masking
To ensure balance between the groups during the trial the patients were randomized into blocks of four. The size of the block was unknown to the investigator. Concealed allocation was ensured by the distribution of investigational products through an organization within Arla Foods, otherwise not involved in this study. The capsules appeared completely identical and were packed in identical boxes labeled with participant number based on a randomization list. The randomization list was kept at Arla Foods during the entire study phase. The investigator handed out boxes consecutively and registered the participant number for each patient. The blinding was maintained until the data analysis was completed.
The study was approved by the local ethics committee in the Region of Southern Denmark (Project no. S-20080078, 07.17.2008). Informed consent was obtained from all patients. The trial was registered in Clinical trials: NCT01151657
Statistics
We wanted, in line with Simrén [Citation15], to detect a 30% therapeutic benefit in proportions of responders after 6 months of treatment and with 80% power at α = 0.05, using a two-sided test and assuming a 15% placebo response. At least 70 persons should be included, but as we suspected dropouts, given the long treatment period and follow-up, we included all patients eligible, thus resulting in 131 patients.
The primary endpoint was analyzed based on the intention to treat (ITT) principle with drop outs counting as non-responders and missing values regarded as not having AR. A subgroup analysis excluding patients not being bothered by symptoms (= reporting AR) at baseline was performed. We further analyzed the primary endpoint based on the per-protocol population and excluding patients not being bothered by symptoms at baseline.
Change in GI symptoms was analyzed for the population of patients having answered the baseline monthly letter and the 3-, 6- and 12-monthly letter, respectively. Change in HrQOL was analyzed for the population having answered the baseline questionnaire and the 6- and 12-month questionnaire, respectively. Proportions of patients having AR at every time point (month) were analyzed as ITT with missing values regarded as not having AR.
Normally distributed continuous measures are reported as mean and SD, and group comparisons were performed using Student's t-tests. Categorical data are reported as absolute number and percentage and compared using Chi-squared tests. All tests were two-sided and for the primary endpoint statistical significance was accepted at the 5% level.
Regarding the secondary endpoints no formal adjustment for multiplicity was performed.
Due to a high drop-out rate, we performed sensitivity analyses, regarding change in GI symptoms and change in HrQOL, taking the baseline values into account.
Results
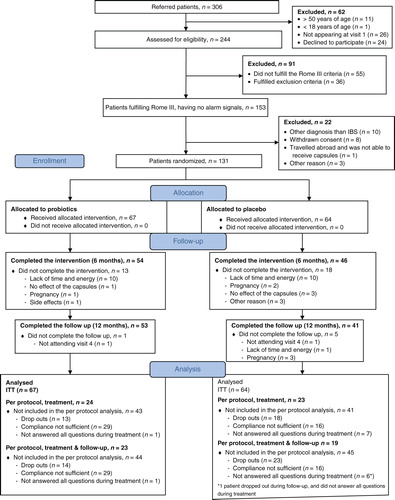
A total of 306 patients were referred for the study, of these 244 patients were assessed for eligibility, leaving153 patients investigated and 131 patients included and randomly allocated to probiotics or placebo. A total of 67 patients were assigned to probiotic capsules and 64 patients to placebo: of these 54 patients (81%) and 46 patients (72%) completed the intervention, respectively. Dropouts were younger and had a shorter history of symptoms. Reasons for dropout are shown in . Of the 100 patients completing the intervention, 55 patients were fully compliant during all 6 months. Some 97 (74%) of the included patients were women. Mean age was 31 years and mean duration of symptoms 6.8 years. It was equally distributed in the two groups ().
Table I. Baseline characteristics of the randomised patients.
Among the patients completing the intervention 2% of the monthly letters were missing in the 6-month treatment period.
Primary outcome
After 6 months of treatment the number of responders were 35 (52%) in the probiotic group versus 26 (41%) in the placebo group, yielding an absolute risk difference of 11%, 95% CI-interval (–5, 29), p = 0.18. In the subgroup analysis excluding patients not being bothered by symptoms (= reporting AR) at baseline (n = 79) the number of responders was 15 (39%) in the probiotic group versus 12 (29%) in the placebo group, p = 0.34. In the per-protocol analysis excluding patients not being bothered by symptoms (= reporting AR) at baseline (n = 23) the numbers were 7 (47%) versus 2 (25%), p = 0.31.
Secondary outcomes
After 3 months of treatment, we observed a difference between the groups regarding total score as well as diarrhea, satiety and bloating. However, after 6 months of treatment no differences were observed. After the additional 6 months of follow-up a difference in total score, pain and satiety was observed. All the observed differences were in favour of the placebo group ().
Table II. Change in gastrointestinal symptoms from baseline, GSRS-IBSa.
Patients improved in HrQOL during the trial, but with no difference between the groups (). The sensitivity analyses taking into account the baseline values did not change the estimates.
Table III. Change in health-related quality of life from baseline, IBS-QOLa.
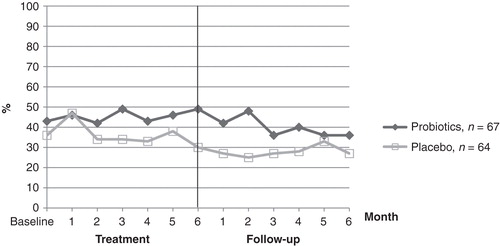
Reported AR at each time point (months) in the two groups is shown in . The proportion of patients having AR was fluctuating but declined during follow-up in both groups. In the probiotic group the proportion of patients having AR was most pronounced at 3 and 6 months, while in the placebo group the greatest effect was seen after 1 month of treatment. We observed no worsening in symptoms in the probiotic group after cessation of treatment when compared with placebo.
Compliance
Of the patients completing the treatment 55 patients were compliant as defined in the study protocol, 25 patients (46%) in the probiotic group and 30 patients (65%) in the placebo group respectively. There was no difference between compliant and non-compliant patients concerning age, sex and IBS subtypes. In the probiotic group 18 (62%) of the non-compliant patients were, however, compliant in 3 of the 6 treatment months.
We found probiotic strains in 23 (79%) samples from the probiotic group (LA-5 in 3(10%), BB-12 in 20(74%), and F19 in 11(39%)). None of the three probiotic strains were found in the feces of the subjects in the placebo group.
Adverse effects
No serious adverse affects were reported. In the probiotic group one patient reported an itching rash on forearms and thighs and dropped out and another patient reported a transient rash at elbows and thumbs but continued treatment.
Discussion
In this long-term treatment trial in a primary care population, we were not able to show that the probiotic mixture containing Lactobacillus paracasei ssp paracasei F19, Lactobacillus acidophilus La5 and Bifidobacterium Bb12 had superior efficacy compared to placebo. Although the group treated with probiotics had a greater proportion of responders, this was not statistically significant. The effect of treatment was fluctuating in both groups. After treatment it declined.
The study was a 6-month, randomized, double-blind, placebo-controlled trial which is the optimal design when investigating efficacy of treatments. The length of the study provided the opportunity to investigate whether long-term treatment is feasible in IBS, a disorder characterized by chronic and intermittent symptoms. Further this is the first study with a long follow-up providing the possibility of examining durability of treatment.
A major strength of the study is the recruitment of patients from primary care, since most IBS patients are managed here. All included patients fulfilled the Rome III criteria. The criteria have demonstrated a good sensitivity relative to a clinical diagnosis of IBS made by GPs in primary care [Citation21], and we believe that our population represents IBS patients in primary care well.
We used the subjective global assessment AR, as we believe it is a clinically relevant outcome including symptom burden and using the patients´ own reference system. Furthermore, AR is validated [Citation22] and recommended for use in IBS trials [Citation23,24].
Certain limitations of the study should be acknowledged. Due to the length of the study it was reasonable to include patients with intermittent symptoms and we chose to include all patients in the analysis irrespective of baseline AR status. Based on a large patient-level meta-analysis a Rome Foundation report has concluded that AR is not impacted by baseline severity [Citation22]. However, effect of baseline severity on ARs validity is much debated [Citation25,26]. The subgroup analysis of the primary outcome including only patients not having AR at baseline revealed a lower overall number of responders, but almost the same difference in proportions of responders between the groups.
The study was a long-term trial with a duration exceeding that of most studies conducted [Citation5]. To make it feasible we decided, that the patients should answer the AR question once monthly. Whether this is acceptable could be questioned. However, we believe that the responder definition of having AR 50% of the time during treatment covers a long time span, ensuring a true picture of the patient´s continuous symptoms. The monthly interval between measurements is comparable with the design in the two former trials lasting 6 months [Citation11,13].
A proportion of 46% of the patients completing the intervention in the probiotic group were compliant during the whole treatment period (6 months). This is lower than in former studies. One reason could be that the patients were to consume four capsules daily, which is more than in other studies. Furthermore, the patients were younger than in other studies [Citation12,13,15,16], most of the patients had no comorbidity and were not used to taking medication daily. The missing effect could partly be explained by the low compliance, on the other hand the non compliance could also be explained by lacking effect. Of the non-compliant patients 18 (62%) actually consumed probiotic in sufficient quantities during 50% of the treatment months. The level of compliance was confirmed by the fecal analyses, where the probiotic strains could be detected in 79% of the samples in the active group.
Simrén et al. [Citation15] and Sondergaard et al. [Citation16] used the same probiotic mixture as in this study, but in fermented milk. Neither was able to detect an effect of the probiotic. Simrén used the same responder definition and found after 8 weeks of treatment a numerically higher proportion of responders in the probiotic group. He observed the greatest improvement in the first 4 weeks in contrast to this study. The effect was sustained in the study of Simrén, but with no difference between the probiotic group and the placebo group at the end of the study. It is debatable whether effect after 1 month is clinically relevant in a group of patients with a chronic disorder. During the 6-month treatment, we found the same proportion of responders and difference between the groups as observed by Simrén after 8 weeks. We included more patients in our trial, but the observed difference (11%) was not within the range of statistical difference (impute significant). The power calculation was based on finding a difference of 30% between the groups. It could be argued that this was rather optimistic in this population, given the observed effect sizes in meta-analyses [Citation5,9,10]. We find it questionable, however, whether the difference of 11% is clinically relevant. In a population suffering from a benign disorder, one could, however, argue that even a slight reduction of symptoms in a minor group of patients is worthwhile as long as the therapy is safe. No serious adverse effects have been reported in IBS-trials on probiotics [Citation5,9,10], which was also the case in our study. On the other hand there is a risk of keeping a large part of the patients in the belief that they need medicine even though there might be no clinical efficacy [Citation27].
Until now only three long-term studies have been conducted [Citation11–13]. The Finnish trials used a probiotic mixture (Lactobacillus rhamnosusGG, L.rhamnosus LC705, Bifidobacteriuum breve Bb99/B.animalis spp.lactis Bb12 and propionibacterium freudenreichii spp. Shermanii JS) and concluded that the probiotic mixture was effective in alleviating IBS symptoms after 5 and 6 months of treatment, respectively. In an Israeli trial, using capsules containing L. reuteri, no effect was proven. Proofs of efficacy were in all studies based on change in composite symptom scores, but whether the change was clinically relevant to the patients was only briefly discussed, and only in the 5-month trial was concurrent improvement in quality of life observed [Citation12]. In the present study we found a greater improvement in some of the GI symptoms after three months in the placebo group, but after 6 months no difference between the groups was observed. This supports the finding of no significant difference in the primary outcome. The observations should, however, be interpreted with caution because of the possibility of multiplicity.
IBS patients are known to have impaired health-related quality of life [Citation28]. In this study patients improved in HrQOL during the intervention and follow-up, probably due to a Hawthorn effect since no difference between the groups was observed.
To conclude, in this primary care population we were not able to detect a positive effect of a 6-month treatment with probiotics when compared with placebo.
Acknowledgement
The authors wish to thank all patients who participated in the study. Further, we wish to thank Professor Jakob Kragstrup, DMedSci, PhD, for contributions during the planning of the study and the ARLA Food employees Ulla Svensson, PhD and Eva Ohman, who handled the supply of capsules and the fecal analyses. We also thank Lise Stark for the English proofreading. The study was funded by The Danish Dairy Research Foundation, The Mads Clausen Foundation, The Foundation of the Danish Medical Association and Trygfonden to LMB; from the Council for Quality Assurance in Primary care in the Region of Southern Denmark to DEJ, and from The Research Fund of Odense University Hospital to ODM. Arla Foods supplied the capsules for the study and analysed the fecal samples. The entire funding source had no involvement in the study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:712–21.
- Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 2013;62:159–76.
- Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: the incidence and prognosis of post- infectious irritable bowel syndrome. Aliment Pharmacol Ther 2007;26:535–44.
- Sachdev AH, Pimentel M. Antibiotics for irritable bowel syndrome: rationale and current evidence. Curr Gastroenterol Rep 2012;14:439–45.
- Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx- Orenstein AE, Brandt LJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut 2010;59:325–32.
- Silk DB, Davis A, Vulevic J, Tzortzis G, Gibson GR. Clinical trial: the effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther 2009;29:508–18.
- Kassinen A, Krogius-Kurikka L, Makivuokko H, Rinttila T, Paulin L, Corander J, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 2007;133:24–33.
- Malinen E, Rinttila T, Kajander K, Matto J, Kassinen A, Krogius L, et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol 2005;100:373–82.
- McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol 2008;14:2650–61.
- Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol 2009;9:15.
- Niv E, Naftali T, Hallak R, Vaisman N. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome–a double blind, placebo-controlled, randomized study. Clin Nutr 2005;24:925–31.
- Kajander K, Myllyluoma E, Rajilic-Stojanovic M, Kyronpalo S, Rasmussen M, Jarvenpaa S, et al. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther 2008;27:48–57.
- Kajander K, Hatakka K, Poussa T, Farkkila M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther 2005;22:387–94.
- Begtrup LM, Engsbro AL, Kjeldsen J, Larsen PV, de Muckadell OS, Bytzer P, et al. A positive diagnostic strategy is noninferior to a strategy of exclusion for patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2013;11(8):956–962.
- Simren M, Ohman L, Olsson J, Svensson U, Ohlson K, Posserud I, et al. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome - a randomized, double-blind, controlled study. Aliment Pharmacol Ther 2010;31:218–27.
- Sondergaard B, Olsson J, Ohlson K, Svensson U, Bytzer P, Ekesbo R. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: a randomized, placebo-controlled trial. Scand J Gastroenterol 2011;46:663–72.
- Wiklund IK, Fullerton S, Hawkey CJ, Jones RH, Longstreth GF, Mayer EA, et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol 2003;38:947–54.
- Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci 1998;43:400–11.
- Drossman DA, Patrick DL, Whitehead WE, Toner BB, Diamant NE, Hu Y, et al. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol 2000;95:999–1007.
- Janet Håkansson. Report IBS III. Arla Foods GFDP 2011.
- Engsbro AL, Begtrup LM, Kjeldsen J, Larsen PV, de Muckadell OS, Jarbøl DE, et al. Patients suspected of irritable bowel syndrome-cross-sectional study exploring the sensitivity of Rome III criteria in primary care. Am J Gastroenterol 2013;108:972–80.
- Spiegel B, Camilleri M, Bolus R, Andresen V, Chey WD, Fehnel S, et al. Psychometric evaluation of patient-reported outcomes in irritable bowel syndrome randomized controlled trials: a Rome Foundation report. Gastroenterology 2009;137:1944–53.
- Bijkerk CJ, de Wit NJ, Muris JW, Jones RH, Knottnerus JA, Hoes AW. Outcome measures in irritable bowel syndrome: comparison of psychometric and methodological characteristics. Am J Gastroenterol 2003;98:122–7.
- Camilleri M, Mangel AW, Fehnel SE, Drossman DA, Mayer EA, Talley NJ. Primary endpoints for irritable bowel syndrome trials: a review of performance of endpoints. Clin Gastroenterol Hepatol 2007;5:534–40.
- Whitehead WE, Palsson OS, Levy RL, Feld AD, VonKorff M, Turner M. Reports of “satisfactory relief” by IBS patients receiving usual medical care are confounded by baseline symptom severity and do not accurately reflect symptom improvement. Am J Gastroenterol 2006;101:1057–65.
- Passos MC, Lembo AJ, Conboy LA, Kaptchuk TJ, Kelly JM, Quilty MT, et al. Adequate relief in a treatment trial with IBS patients: a prospective assessment. Am J Gastroenterol 2009;104:912–19.
- Salmon P. The potentially somatizing effect of clinical consultation. CNS Spectr 2006;11:190–200.
- Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 2000;119:654–60.