Abstract
Objective. Recently, new highly effective direct-acting antivirals (DAAs) against hepatitis C virus (HCV) were introduced. Whether these will alleviate the anticipated increase of liver disease burden in Sweden is unknown, partly because high costs may restrict the use. The objectives were to model the HCV epidemic in Sweden, the burden of disease, and the potential impact of different treatment strategies. Material and methods. HCV disease progression was modeled to 2030. Scenarios were simulated using new DAAs with sustained annual treatment rate (n = 1130), reduced treatment rate (n = 380) to maintain budget, and increased treatment rates (n = 1430 or 2260) to reduce HCV infections. Results. With today’s triple therapies, the estimated number of serious liver complications and death are expected to peak in 2021. Using new DAAs among F0–F4 patients, an unchanged annual treatment rate can reduce the number of HCV infections by 10% by 2030; however, hepatocellular carcinoma (HCC) and mortality will remain unchanged. By reducing to 380 treatments annually and focusing on patients with advanced fibrosis (F3–F4), serious complications will remain constant but the total number of HCV infections will increase. By doubling the number of DAA treatments, HCC-incidence and liver-related deaths would decrease by 65–70% by 2030. Conclusion. Mortality and HCC can be reduced with new DAAs and sustained treatment uptake when restricted to F2–F4 patients, or with increased uptake in F0–F4 patients. Treatment restrictions to limit cost may reduce the positive effects and increase the burden of HCV infection. These results may be important for the future strategies of HCV management.
Introduction
Chronic hepatitis C virus (HCV) infection is a major cause of liver disease in Europe and around the world [Citation1]. In Sweden, the spread of HCV started near the end of the 1960s with culmination in the 1970s, most likely due to increased injection drug use (IDU). With the aging HCV cohort, the number of diagnosed hepatocellular carcinoma (HCC) has gradually increased and HCV infection has become one of the leading causes of liver transplantation in Sweden [Citation2,3]. The burden of HCV-related liver disease is expected to further increase in the coming decade [Citation2,4].
Sweden is a low-endemic country for HCV infection with an estimated prevalence of 0.5% [Citation4]. Mandatory notification of acute and chronic HCV infections was instituted in 1990 and since then laboratories and physicians have reported a total of 54,000 cases of HCV infection to the National Surveillance Register at the Swedish Institute for Infectious Disease Control (SMI; now the Public Health Agency of Sweden) [Citation5]. In addition, clinical notifications include information regarding suspected route of transmission and ∼65% have reported former or ongoing IDU.
The Swedish HCV cohort is well characterized – as validated through several cross-linkage studies using data from national registers – making it suitable for epidemic modeling [Citation2,6,7]. Every person living in Sweden has a specific personal identification number, used in all healthcare contacts and all national registers [Citation8]. These registers have high coverage and validity, enabling nationwide register studies with high completeness [Citation5,8].
Interferon (IFN)-based treatment regimens have been the standard of care for chronic HCV and still are in many parts of the world. These therapies are associated with insufficient response rates and frequent side effects, resulting in a limited number of patients treated [Citation2]. With the recent introduction of direct-acting antivirals (DAAs) and more new DAAs to be introduced in the next few years, substantially higher sustained virological response (SVR) rates, fewer side effects, and more simple regimens can be expected [Citation9–11]. Further, with the adoption of IFN-free regimens, more patients will be eligible for treatment, but higher prices may restrict wide usage.
Recently, studies of the historical HCV epidemiology, the future disease burden using today’s treatment, and strategies for viral eradication were published for 16 countries including Sweden [Citation12–14]. Taking into consideration the initial high costs of new DAAs, the viral eradication strategy is likely out of reach in the near future. To plan and allocate healthcare resources, analyses using different treatment strategies in line with clinical reality are necessary. The aim of this study was to describe the HCV epidemiology in Sweden and to estimate the future disease burden using different treatment uptake strategies, also including scenarios with a restricted treatment policy.
Materials and methods
Published literature and national reports were studied to describe the historical and present HCV epidemiology in Sweden. For the modeling of the future disease burden, a recently presented disease progression model was used [Citation13]. The treatment strategies presented in the previously published analysis [Citation12] differ from the strategies presented here. As described below, these strategies are clinical in approach and consider the probable impact that budget restrictions may have on treatment uptake. For example, it is expected that IFN-free regimens will be limited to patients with advanced fibrosis and cirrhosis.
Population data were obtained by 5-year age and gender cohorts from UN population database with validation from the Swedish Population Registry [Citation15]. The Swedish cohort of individuals with a diagnosed HCV infection has been identified and characterized with data from the National HCV Surveillance Register [Citation5] and through linkage to other national registers (Population, Death, Patient, and Cancer register) [Citation8,15] in previous studies on disease burden, cancer incidence and mortality [Citation2,6,7]. For the model, Swedish HCV cohort data were used to inform the age and gender distribution of the prevalent population, and to validate HCC incidence and mortality. On occasion, data not specific to Sweden were used if it could be well validated and were logical to extrapolate to Sweden. Age- and gender-specific transition probabilities were used to progress patients annually through each disease stage [Citation13].
Baseline characteristics of study population
The anti-HCV prevalence in the general population in Sweden in 2012 (9.5 million) was estimated to be 0.56%. This was based on previous studies and the notifications to the Public Health Agency of Sweden (former SMI) [Citation5]. The anti-HCV prevalence was 0.2–0.5% among blood donors when HCV screening was introduced [Citation16,17] and was 0.4% in a middle-aged urban population [Citation18]. These studies may underestimate the true prevalence due to selection of healthy blood donors and persons with low risk of IDU. From 1990 to 2012, 54,000 individuals (68% men) with positive anti-HCV were reported to SMI [Citation5], with death in 20.5% (before the end of 2010), resulting in a prevalence of 0.43% living with a diagnosed, ongoing, or resolved HCV-infection. Another study demonstrated that in 2006 the diagnosed anti-HCV prevalence was 0.4% in the total population, with the highest prevalence (1.0%) among those born in the 1950s and 1960s [Citation2,7]. Assuming an undiagnosed fraction of about 20% increases the anti-HCV prevalence to ∼0.56% (53,300 individuals) in 2012.
An average viremic rate of 77% [Citation19–21] was used to estimate the prevalence of chronic HCV infection, resulting in 41,100 chronic viremic infections in Sweden (including the undiagnosed fraction). During the past decade, ∼2000 new HCV notifications occurred annually, of which 35% were under 30 years of age [Citation5]. With an estimated viremic rate of 77%, this corresponds to 1540 new viremic cases diagnosed annually. During the past 10 years about 1000 persons in the anti-HCV positive cohort died each year [Citation7].
The genotype (G) distribution in published studies from Sweden was ∼45% G1, 19% G2, 34% G3, and 2% G4 [Citation22–24]. For this analysis, the G distribution was estimated to be 40% (G1a), 10% (G1b), 20% (G2), 30% (G3), and 0% (G4), corresponding to data from the InfCare Hepatitis database (personal communication, Dr Ola Weiland, Karolinska University Hospital, Stockholm, Sweden).
The anti-HCV prevalence among people who inject drugs (PWID) is high (>80%), after a few years with IDU [Citation20,25], and the major reported transmission route in the Swedish HCV-cohort was IDU (65%). The spread of HIV among PWID in Sweden has been low and the prevalence of coinfection with HCV and HIV is estimated to be low [Citation26]. According to the InfCare HIV database, in year 2013, ∼450 patients in the Swedish HIV cohort were coinfected with HCV (personal communication Karolin Falconer, Karolinska University Hospital).
Liver transplantations and mortality
In 2011, Scandiatransplant reported that 156 liver transplants were performed in Sweden, among which 22% were anti-HCV-positive [Citation3]. The number of HCV-related liver transplants from 1990 to 2012 was retrieved from Scandiatransplant and was used for input in the model.
Increased mortality among the HCV-infected population was accounted for using standardized mortality ratios (SMRs) by 5-year age and gender cohorts, as identified through the Swedish HCV cohort from 1990 to 2003 [Citation6]. SMRs were calculated using a 6-month lag time after HCV notification to reduce the risk of selection bias.
For the modeling, treated patients with SVR were considered cured, and the risk of HCC and mortality after SVR was assumed to be at the same level as for the general population.
Treatment models from baseline to the waves of DAAs
For model input, the treatment strategies differed depending on the G-specific efficacy of the drugs, and for each scenario the stages of liver fibrosis were taken into account. The stages of fibrosis in this study refer to the Metavir scoring system [Citation27].
Baseline, first, second, and third waves of DAAs
The baseline time point in our prediction model was set to 2010 during which time PEGylated IFN (PEG-IFN) and ribavirin (RBV) therapy were available. At this time, the Swedish national guidelines recommended treatment of all eligible patients with G2/3 irrespective of fibrosis stage, and of eligible patients with G1 and fibrosis score ≥F2 [Citation28]. The time point for the first wave of DAAs was set to 2012 when the first generation of protease inhibitors (PIs) for HCV G1 was added to PEG-IFN and RBV. This triple therapy was recommended for HCV G1 patients with bridging fibrosis and cirrhosis (F3–F4) only [Citation29]. The baseline scenario considers dual therapy prior to 2012 and triple therapy after this time.
The time point for the second wave, with new highly effective IFN-free DAA combinations, was set to 2015. New DAAs were introduced in 2014 and are anticipated to be widely used by 2015, with a priority to treat patients with advanced fibrosis and cirrhosis. For G1, treatment is expected to include a combination of a potent nucleotide NS5B polymerase inhibitor (NUC) and either a first-generation second wave PI or a HCV NS5A replication complex inhibitor (NS5A-inhibitor) +/− RBV. A few patients may still receive PEG-IFN and RBV in combination with NUC. Expected treatment regimen for G2 is a combination of NUC and RBV, and for G3 a prolonged course of NUC and RBV and/or an NS5A inhibitor, and for some patients possibly NUC combined with PEG-IFN and RBV. The time point for the third wave, using highly effective IFN-free combinations, was set to 2016 [Citation9–11,30–32]. For modeling the second and third waves, treatment strategies differed depending on the expected number of treatments. For the most restrictive scenarios, only persons with F3–F4 fibrosis were treated, but scenarios treating patients with F2–F4 or F0–F4 fibrosis were also modeled.
Number of treatments
In the past 10–15 years, about 1100 individuals were treated annually in Sweden [Citation2], that is, 10,000–15,000 in total, with an estimated 40–50% cure rate across all Gs. The number of treated patients was estimated in a study, demonstrating that 1080 individuals in the Swedish HCV cohort were treated in 2006 (based on cross-linkage to the National Prescription Register) and that RBV and PEG-IFN were sold at an unchanged level during the 2000s [Citation2]. For the modeling of the baseline scenario, an estimated 1130 patients were treated annually, assuming a treatment uptake proportional to the G distribution in Sweden.
Experience with first-generation PIs showed higher costs associated with novel HCV treatment, and the same is anticipated for future DAAs. Thus, a scenario was modeled in which the budget for HCV treatment remained the same as before the introduction of new DAAs. To estimate the number of patients that could be treated with a maintained budget, the first licensed new DAAs were used as an indication of the future cost for DAAs. Compared with the current, IFN-based, standard of care, the drug cost per treated patient was estimated to change by G as follows: G1 – nearly double, G2 – ∼5 times higher, and G3 – ∼8 times higher. Therefore, for the modeling of this “maintained budget” scenario, beginning in 2015, the number of treated patients was reduced from 1130 to 380 (50% of G1, 20% of G2, and 15% of G3) and only patients with F3–F4 fibrosis were treated.
In other scenarios, the number of treatments was kept constant at 1130 annually. Beginning in 2015, the new DAAs with higher SVR were used for the same number of treatments but with different scenarios depending on restriction to stage of fibrosis, treating only ≥F3, ≥F2, or finally ≥F0 (i.e. no restriction).
In a final scenario, beginning in 2016 with the use of only IFN-free treatments, the annual number of treated patients was increased to 1430 and finally doubled to 2260 and modeled by restriction to different fibrosis stages (≥F3, ≥F2, or ≥F0).
SVR rates
The SVR rates used for modeling are presented in . These rates are estimates based on clinical trials with ∼5–10% adjustment for real-life SVR, and reflect both treatment-naïve and -experienced patients. For baseline and first wave scenarios, the real-life data were retrieved from the Swedish InfCare Hepatitis database (personal Communication, Dr Ola Weiland, Karolinska University Hospital, Stockholm, Sweden) and/or publications from Sweden when available, otherwise from other Western countries [Citation33–37].
Table I. Estimated SVR by genotype and stage of fibrosis at baseline dual therapy (PEG-IFN + RBV), first wave (PEG-IFN + RBV + first generation PI for genotype 1), second wave (NUC + second wave PI or NS5A inhibitor for genotype 1, NUC + RBV for genotype 2/3, and NUC + PEG-IFN + RBV for a few patients) and third wave of new DAAs (IFN-free regimens in different combinations). Real-life SVR estimates for treatment-naïve and -experienced patients are combined to a common estimate.
In the model, patients with decompensated cirrhosis were excluded from treatment with PEG-IFN-containing regimens. Since development of HCC has been demonstrated to be one of the first serious complications in patients with compensated liver cirrhosis [Citation38], the SVR for patients with HCC were set to the same rates as for compensated liver cirrhosis, even if the HCC risk increases with worsening of the liver function [Citation39]. During the first wave, transplanted patients with G1 had the same SVR rates as for baseline scenario, since triple therapy was seldom used in these patients.
For the second wave, preliminary and published data from clinical trials were used [Citation10,40–42]. Preliminary data on IFN-free treatment for liver transplanted patients in clinical trials were extrapolated to G-specific SVR rates with adjustment for real-life situations. All treatment combinations (with or without IFN) for G1 patients with decompensated cirrhosis were excluded in this wave, although IFN-free combinations while on transplantation waiting list may be used (off-label or possibly according to labeling). For G2 and G3 patients with decompensated cirrhosis, treatment with IFN-free DAA combinations were assumed for the modeling.
In the third wave, preliminary and published data for different IFN-free combinations were used for prediction of real-life SVR rates [Citation9–11,32,43–45]. High overall SVR rates were assumed for patients with all stages of fibrosis, even in the most difficult-to-treat patients with advanced fibrosis. The predicted rates reflect the estimated real-life data treating different patient populations including IFN-intolerant/IFN-unwilling patients and PWID, probably lowering the SVR rates compared to those reported from trials. Wider treatment uptake in decompensated patients was assumed, with the possibility to treat in all Gs and in all clinics treating hepatitis C, and not just transplant units. Treatment data of patients with decompensated cirrhosis not yet listed on the transplantation list are presently lacking, and preliminary data from liver transplanted patients were used for prediction of SVR rates.
Results
Baseline scenario with PEG-IFN and RBV and first wave triple therapy
The age and gender distribution of viremic HCV infections in 2010 are shown in . Peak viremic prevalence of chronic HCV infection was reached in 2003 with 43,900 infected individuals. With a baseline strategy treating 1130 patients annually with PEG-IFN and RBV, and from 2012 adding first wave triple therapy for G1, the prevalence will decrease to 31,800 viremic cases by 2030 (, ). However, the burden of HCV-related liver disease will increase. The annual number of individuals with incident HCC was forecasted to peak in 2021 with 115 cases. Similarly, decompensated cirrhosis and cirrhosis were expected to increase until 2016 and 2018, respectively. Liver-related mortality was forecasted to peak in 2021 at 190 deaths annually ().
Figure 1. Age and gender distribution of viremic HCV infections in Sweden in 2010 is shown. Abbreviation: HCV = Hepatitis C virus.
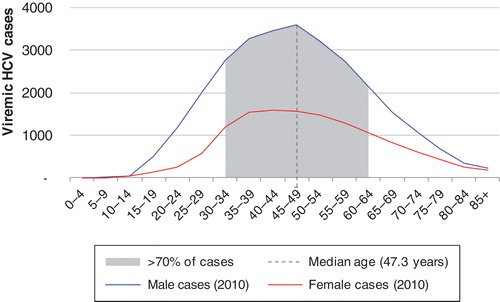
Figure 2. Prediction of future HCV infections and related complications under different treatment strategies is shown: A. prevalence of viremic HCV infections; B. incidence of HCC; C. prevalence of decompensated cirrhosis; D. liver-related mortality. The treatment strategies are: 1. base, dual/triple therapy with PEG-IFN + RBV and first-generation PI for genotype 1, at an annual treatment rate of 1130. Strategies 2–5 consider treatment with future DAAs restricted to F3–F4 fibrosis with different treatment uptake as follows, 2. treat 380 (maintained budget), 3. treat 1130 (maintained number of treated), 4. treat 1430 (a 30% increase), and 5. treat 2260 patients (a doubled increase). Abbreviations: DAA = Direct-acting antiviral; HCC = Hepatocellular carcinoma; HCV = Hepatitis C virus; PEG-IFN = PEGylated interferon; PI = Protease inhibitor; RBV = Ribavirin.
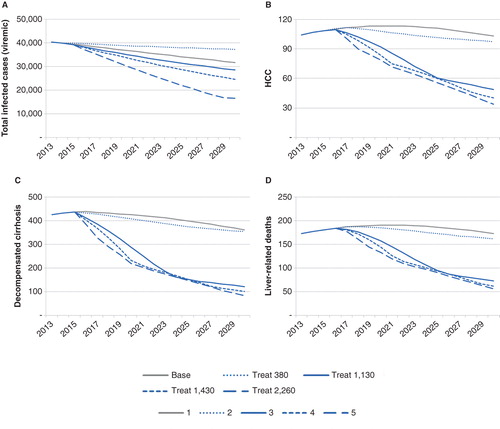
Table II. The impact of different HCV treatment strategies on the liver-related morbidity, mortality, and total viremic infections by 2030, using either no therapy, IFN-based therapies (base, dual/triple) for 1130 patients, or new DAAs in IFN-free regimens for 380 patients (restriction to maintain budget), 760 patients (doubled budget), 1130 (unchanged number), 1430 (30% increase), or 2260 patients (doubled number). The DAA scenarios were treating patients only with fibrosis score ≥F2 or ≥F3.
Increased treatment efficacy in the second and third waves
Reduced number of treatments to maintain the budget
Using second and third wave DAAs, a scenario with a reduction of the annual number of treated patients to 380, to maintain the budget from the first wave triple therapy, was analyzed. When restricting treatment to patients with only F3–F4, the number of serious complications and liver-related deaths were constant compared with the baseline scenario (, ). However, this strategy had very little effect on the total number of infections, resulting in an increase of infected cases compared with the baseline scenario. To decrease the number of infected persons at the same rate as during the baseline scenario would require an annual treatment rate of 940 patients with F3–F4 or 800 with F2–F4, according to the model.
Increased SVR only
In one scenario, the number of treated patients stayed constant at 1130 annually but the SVRs changed with the second (from 2015) and with the third wave (from 2016) of DAAs. Treating persons with F3–F4 only, this strategy reduced the number of serious complications (, ), and by 2025 the number of F3–F4 patients were <1130. To prevent the model from running out of eligible patients to treat, in 2024 treatment access was expanded to F2–F4.
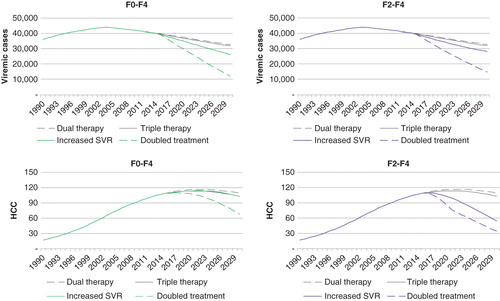
A similar scenario, treating 1130 patients with effective DAAs, but including patients with all stages of fibrosis (F0–F4), would result in treatment of fewer patients with F3–F4 and a less-effective reduction of serious liver complications. In this scenario, the number of serious liver complications would remain the same as for the base case scenario, but the total number of infected patients would be reduced ().
Figure 3. The effects of treating persons with F0–F4 fibrosis compared with restricting treatment to persons with F2–F4 on the future prevalence of viremic HCV infections and incidence of HCC, beginning in 2015 are shown. The therapies in the figure are: “Dual therapy” with PEG-IFN and ribavirin for 1130 patients annually; “Triple therapy”, adding a first-generation protease inhibitor for genotype 1, treating 1130 patients; “Increased SVR” treating 1130 patients with new highly effective DAAs (IFN-free); and “Doubled treatment” treating 2260 patients with DAAs. Abbreviations: DAA = Direct-acting antiviral; HCC = Hepatocellular carcinoma; HCV = Hepatitis C virus; PEG-IFN = PEGylated interferon; SVR = Sustained virological response.
Increased number of patients treated with new DAAs
To achieve substantial impact on the total burden of infected cases, the number of treated patients needs to increase. Treating 1130 patients through first and second waves and then, with the third wave beginning in 2016, either increasing the number of patients with 30% to 1430 or doubling to 2260 were analyzed. Doubling the number of treated to 2260 would effectively reduce the number of patients with liver cirrhosis, incident HCCs, and liver-related deaths, at the most when treating patients with ≥F3 (, ). The same number of treatments to patients with F0–F4 would be more efficient to reduce the number of infected cases but would have less effect on serious liver complications ().
Discussion
Under the scenario of continued use of IFN-based dual and triple therapies, HCV-related liver complications and mortality in Sweden were projected to increase. The model forecasts that IFN-free combinations of highly effective DAAs could significantly reduce the future burden of HCV infection and related liver disease. However, restrictions to maintain the cost of HCV drugs would lead to a substantial reduction of number of treatments with the new DAAs and could result in an increased burden of HCV infection.
According to the model, chronic HCV infection in Sweden peaked in 2003 and slowly declined as a result of a lower new infection rate, high mortality, and treatment. The spread of HCV increased with IDU in the mid-1960s and peaked through the 1970s and early 1980s. The incidence then declined and stabilized at a lower rate. The highest diagnosed anti-HCV prevalence in Sweden is about 1.0% among those born in the 1950s and 1960s, and HCV is more prevalent in men than women [Citation7]. With an aging cohort and lengthy lag time from infection to serious liver complications, an increase in the burden of HCV-related complications is expected in Sweden in the next decade [Citation2]. The addition of first-generation PIs in 2012 had very little impact on HCV disease burden. This was due to insufficient increase in SVR rates, restriction of access to G1, low treatment uptake, and frequent adverse events leading to dose reductions or cessation of treatment [Citation36,39,46]. Thus, the anticipated increase of liver disease indicates a need for new strategies.
Treatment strategies using new DAAs with higher SVR rates and better tolerability could reduce the incidence of serious complications and decrease the number of infected if treatment uptake increases. The results of the modeling indicate that doubling the number of treated patients will be needed to have a major impact on both the number of infections and the number of liver complications, with a 70% reduction in liver-related deaths, as well as a 55% reduction in total viremic cases.
With a limited number of treatments (due to reasons such as budget, access to outpatient wards, limited supply of drugs, etc.) an important issue is priority of whom to treat. To have the highest impact on HCV-related liver disease during the coming decade, the model supports focusing treatment on patients with a high fibrosis score. However, these patients may have a lower chance of SVR. If the aim was only to reduce HCV prevalence in society, it would be more effective to treat patients with no or low fibrosis scores with higher achieved SVR rates, but the burden of liver disease would increase. With an unrestricted possibility to treat, F0–F4 patients could be treated to avoid complications and reduce prevalence.
Using new DAAs, but maintaining the budget as in the past years, will reduce the number of treatments to about one-third of the historical annual number of treatments. With such a reduction and restriction to F3–F4 patients, the model predicts a similar effect on future liver complications as if continuing the baseline IFN-based treatment, but the number of infected persons will increase. To avoid an increase of infected persons, at least 800 ≥F2 or 940 ≥F3 patients need to be treated, which would increase the expenses for HCV treatment.
These comparisons only consider the direct costs of HCV drugs. Studies have demonstrated that new DAAs generally have been well tolerated with few side effects and no impact on working capacity [Citation47]. This is in sharp contrast to the IFN-containing regimens often associated with side effects requiring extra visits, extra blood samples, supportive treatments, and long-term sick leave.
In addition to improved treatment efficacy and increased treatment access, a reduction in HCV infections is dependent on a decrease in the number of new cases. There is a discussion about how to stop the spread of HCV and eradicate the epidemic, which would include treating PWID [Citation48]. This is a group of HCV-infected patients with no explicit contraindications but often excluded from treatments due to concerns about adherence, treatment outcome, side effects, and the risk of re-infection. The major source for new HCV infections in Sweden is IDU, with an extremely high prevalence of anti-HCV (>80%) in PWID [Citation20,25]. The number of PWID in Sweden is uncertain and estimates vary between 8000 and 25,000 [Citation5,49]. In the future, treatment uptake in PWID could most likely be enhanced, as treatment complexity will decrease. However, to significantly reduce the spread of HCV in the PWID population, Sweden will need a higher treatment uptake in this group than in most other countries, as demonstrated in another study [Citation48]. To reduce HCV-incidence moving forward, further studies evaluating treatment strategies for the PWID population are needed.
The possibility to treat and reduce the burden is also dependent on the number of diagnosed patients in the population. A total of 54,000 individuals have been notified for HCV infection from 1990 to 2012 [Citation5]. The proportion of undiagnosed HCV patients was estimated to be low, compared with other European countries [Citation14]. The assumed low rate of undiagnosed infections and the low HCV prevalence in society is supported by several studies [Citation16–18]. The screening programs in Sweden have focused on PWIDs, mostly tested in relation to healthcare or treatment for addiction. Also, a general screening recommendation for those who received blood transfusions before 1992 resulted in an increased frequency of testing, and the anti-HCV prevalence was 0.9% among 65,000 who came for screening [Citation19]. All HCV testing is free of charge but generally offered to risk groups. The rationale for screening of risk groups, certain birth cohorts, pregnant women, or possibly a huge part of the population, has been strengthened when considering that the burden of HCV-related disease can be decreased by strategy of using new DAAs and increased treatment uptake.
The introduction of IFN-free treatment with DAAs is expected to not only provide substantially increased SVR rates with simpler treatment regimens and fewer adverse events but also allow treatment of patients who have previously been unwilling or ineligible to receive PEG-IFN containing treatments. Published and preliminary reports have demonstrated that IFN-free treatments with high SVR rates will be an option for patients for whom IFN was contraindicated [Citation50].
The advantage of our study is the well-characterized cohort of HCV-infected persons in Sweden. Through national registries with high coverage, events of liver complications, liver-related death, and overall deaths are known with minimal loss during follow up. This information makes future predictions more precise. The present prediction model also has advantage compared to previous models, with a total of 36 cohorts composed of 5-year age cohorts for each gender and flexibility in changing inputs of incidence rate, age of infection, background mortality, transplantation and treatment rate over time.
A common problem associated with modeling is the access to good background data. The diagnosed HCV cohort is well characterized but the undiagnosed proportion is of course unknown and had to be estimated. There is an uncertainty of whether the incidence and mortality rates based on the diagnosed population are correct for the undiagnosed, which may be healthier or at a lower risk. Further, some of the cohort characteristics include the first years after the discovery of HCV – when it is possible the sickest were diagnosed first.
Another limitation is that the model does not consider the decreased but remaining risk of HCC and disease progression among cirrhotic patients that achieved SVR [Citation51]. This may result in an underestimation of future burden of disease and overstate the potential impact of treatment. To estimate HCC and decompensation in cirrhotic patients with SVR, incidence rates of 1.02% (HCC) and 0.85% (decompensated cirrhosis) [Citation51] were applied to these cases, after accounting for background mortality. Under the baseline scenario, from 2014 to 2030, there were between 2 and 4 HCC cases annually (average = 3 cases), increasing to between 2 and 8 cases annually under the most effective DAA scenario (average = 7 cases). The risk of decompensation among cirrhotic cases achieving SVR was estimated to be an average of 3 and 6 cases annually (baseline and DAA scenario, respectively). This indicates that liver disease progression after SVR has little impact on the forecasted estimates. However, these estimates are based on incidence rates a few years after treatment and the long-term incidence is unknown.
Also, the predicted SVR rates are rough estimates and may be different in real-life situations once therapies are widely available. Due to simpler regimens, the differences in SVR rates between trial and real life are expected to be lower for the DAAs than for IFN-based therapies. Nevertheless, unexpectedly large differences may derive from the treatment in patient groups for which treatment was previously limited (e.g. PWID and patients with psychiatric comorbidity). The proportion of treatment-naïve and -experienced patients in the HCV cohort and in the population to be treated has been difficult to estimate. Treatment experience is probably higher among people with serious liver disease waiting for treatment with new DAAs and this may reduce the SVR rates.
The impact of comorbidities like coinfections with HBV or HIV (low in Sweden) and excessive alcohol consumption were not considered in this study nor were future interventions such as increased screening for HCV. Alcohol is, however, an important comorbidity in HCV-infected patients in Sweden. In a recent study, 58% of all patients with HCV infection had, at some time in their lives, been notified with an alcohol-related diagnosis or convicted of drunken driving (Stokkeland et al., poster 2224, AASLD 2013) [Citation52]. However, the proportion with continued excessive alcohol consumption after HCV diagnosis is unknown. In a previous study, the risk for decompensation and liver-related death after achieving SVR was low in cirrhotic patients in Sweden [Citation51], potentially indicating limited impact of this comorbidity.
To conclude, this study foresees an increase of HCV-related decompensated cirrhosis, HCC, and liver-related deaths in the coming two decades. This is due to the aging HCV cohort infected for more than 30 years. An increased number of patients need to be treated with new, highly effective DAAs to have a major impact on this growing burden of HCV disease. These results may facilitate disease forecasting and the development of rational strategies for HCV management in Sweden.
Acknowledgment
The model analysis was funded by Gilead, without any interference in the project or the manuscript. The authors also thank Scandiatransplant for providing Swedish data of liver transplantations.
Declaration of interest: Ann-Sofi Duberg has given lectures with honoraria or has been consultant to Roche, MSD, Janssen, Gilead, Abbvie, Medivir, and BMS, and has received research grants from Roche. Sarah Blach is an employee of the Center for Disease Analysis (CDA). Karolin Falconer has given lectures with honoraria and has been consultant to Roche, Gilead, Abbvie and MSD, and has received research grants from Gilead and Roche. Martin Kåberg has given lectures with honoraria from Abbvie, Medivir, Roche, Janssen, MSD, and Reckitt Benckiser. Homie Razavi is an employee of the Center for Disease Analysis (CDA). Soo Aleman has given lectures with honoraria and has been consultant to Roche, MSD, Janssen, Gilead, GlaxoSmithKline, Tillots Pharma, Medivir, BMS, Abbvie, and has received research funding from Gilead.
References
- Lavanchy D. The global burden of hepatitis C. Liver Int 2009;29:74–81.
- Duberg AS, Pettersson H, Aleman S, Blaxhult A, Daviethsdottir L, Hultcrantz R, et al. The burden of hepatitis C in Sweden: a national study of inpatient care. J Viral Hepat 2011;18:106–18.
- Scandiatransplant. The nordic liver transplant registry. 2013. Available from http://www.scandiatransplant.org/. Cited 29 November 2013.
- Duberg A, Janzon R, Bäck E, Ekdahl K, Blaxhult A. The epidemiology of hepatitis C virus infection in Sweden. Euro Surveill 2008;13.
- Folkhälsomyndigheten. Public Health Agency of Sweden, Homepage, Hepatitis C statistics. 2014. Available from http://www.folkhalsomyndigheten.se/amnesomraden/statistik-och-undersokningar/sjukdomsstatistik/hepatit-c/. Cited 27 March 2014.
- Duberg AS, Törner A, Davidsdottir L, Aleman S, Blaxhult A, Svensson Å, et al. Cause of death in individuals with chronic HBV and/or HCV infection, a nationwide community-based register study. J Viral Hepat 2008;15:538–50.
- Duberg A. Hepatitis C virus infection: a nationwide study of associated morbidity and mortality [Dissertation]. Örebro University; Örebro, Sweden: 2009.
- Socialstyrelsen. The National Board of Health and Welfare; Homepage. 2014. Available from http://www.socialstyrelsen.se/english. Cited 27 March 2014.
- Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet 2014;383:515–23.
- Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014;370:211–21.
- Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, et al. Treatment of HCV with ABT-450/r-Ombitasvir and Dasabuvir with Ribavirin. N Engl J Med 2014;370:1594–603.
- Wedemeyer H, Duberg AS, Buti M, Rosenberg WM, Frankova S, Esmat G, et al. Strategies to manage hepatitis C virus (HCV) disease burden. J Viral Hepat 2014;21:60–89.
- Razavi H, Waked I, Sarrazin C, Myers RP, Idilman R, Calinas F, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat 2014;21:34–59.
- Bruggmann P, Berg T, Ovrehus AL, Moreno C, Brandao Mello CE, Roudot-Thoraval F, et al. Historical epidemiology of hepatitis C virus (HCV) in selected countries. J Viral Hepat 2014;21:5–33.
- Statistics Sweden. Startpage. 2013. Available from http://www.scb.se/en_/. Cited 3 December 2013.
- Norda R, Duberg AS, Sönnerborg A, Olcen P. Transmission of hepatitis C virus by transfusion in Orebro County, Sweden, 1990-1992. Scand J Infect Dis 1995;27:449–52.
- Shev S, Hermodsson S, Lindholm A, Malm E, Widell A, Norkrans G. Risk factor exposure among hepatitis C virus RNA positive Swedish blood donors--the role of parenteral and sexual transmission. Scand J Infect Dis 1995;27:99–104.
- Hoffmann G, Berglund G, Elmståhl S, Eriksson S, Verbaan H, Widell A, et al. Prevalence and clinical spectrum of chronic viral hepatitis in a middle- aged Swedish general urban population. Scand J Gastroenterol 2000;35:861–5.
- Duberg AS, Hansdotter F, How AL, Holmström A, Lesko B. [Important with generous sampling for hepatitis C after blood transfusion. The National Board of Health and Welfare’s new recommendation for risk groups]. Lakartidningen 2013;110:1477–9.
- Lidman C, Norden L, Kåberg M, Kall K, Franck J, Aleman S, et al. Hepatitis C infection among injection drug users in Stockholm Sweden: prevalence and gender. Scand J Infect Dis 2009;41:679–84.
- Ydreborg M, Söderström A, Håkanson A, Alsiö Å, Arnholm B, Malmström P, et al. Look-back screening for the identification of transfusion-induced hepatitis C virus infection in Sweden. Scand J Infect Dis 2011;43:522–7.
- Lindh M, Hannoun C. Genotyping of hepatitis C virus by Taqman real-time PCR. J Clin Virol 2005;34:108–14.
- Shev S, Widell A, Foberg U, Fryden A, Hermodsson S, Lindh G, et al. HCV genotypes in Swedish blood donors as correlated to epidemiology, liver disease and hepatitis C virus antibody profile. Infection 1995;23:253–7.
- Westin J, Lindh M, Lagging LM, Norkrans G, Wejstål R. Chronic hepatitis C in Sweden: genotype distribution over time in different epidemiological settings. Scand J Infect Dis 1999;31:355–8.
- Månsson AS, Moestrup T, Nordenfelt E, Widell A. Continued transmission of hepatitis B and C viruses, but no transmission of human immunodeficiency virus among intravenous drug users participating in a syringe/needle exchange program. Scand J Infect Dis 2000;32:253–8.
- Stenkvist J, Weiland O, Sönnerborg A, Blaxhult A, Falconer K. High HCV treatment uptake in the Swedish HIV/HCV co-infected cohort. Scand J Infect Dis 2014;46:624–32.
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289–93.
- Lagging M, Wejstål R, Uhnoo I, Gerden B, Fischler B, Friman S, et al. Treatment of hepatitis C virus infection: updated Swedish Consensus recommendations. Scand J Infect Dis 2009;41:389–402.
- Lagging M, Duberg AS, Wejstål R, Weiland O, Lindh M, Aleman S, et al. Treatment of hepatitis C virus infection in adults and children: updated Swedish consensus recommendations. Scand J Infect Dis 2012;44:502–21.
- Schinazi R, Halfon P, Marcellin P, Asselah T. HCV direct-acting antiviral agents: the best interferon-free combinations. Liver Int 2014;34:69–78.
- Pawlotsky JM, New Hepatitis C. Therapies: The Toolbox, Strategies, and Challenges. Gastroenterology 2014;146:1176–92.
- Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourliere M, et al. Retreatment of HCV with ABT-450/r-Ombitasvir and Dasabuvir with Ribavirin. N Engl J Med 2014;370:1604–14.
- Ackefors M, Nystrom J, Wernerson A, Gjertsen H, Sönnerborg A, Weiland O. Evolution of fibrosis during HCV recurrence after liver transplantation--influence of IL-28B SNP and response to peg-IFN and ribavirin treatment. J Viral Hepat 2013;20:770–8.
- Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol 2008;49:274–87.
- Bruno S, Shiffman ML, Roberts SK, Gane EJ, Messinger D, Hadziyannis SJ, et al. Efficacy and safety of peginterferon alfa-2a (40KD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology 2010;51:388–97.
- Syed E, Rahbin N, Weiland O, Carlsson T, Oksanen A, Birk M, et al. Pegylated interferon and ribavirin combination therapy for chronic hepatitis C virus infection in patients with Child-Pugh Class A liver cirrhosis. Scand J Gastroenterol 2008;43:1378–86.
- Weiland O, Hollander A, Mattsson L, Glaumann H, Lindahl K, Schvarcz R, et al. Lower-than-standard dose peg-IFN alfa-2a for chronic hepatitis C caused by genotype 2 and 3 is sufficient when given in combination with weight-based ribavirin. J Viral Hepat 2008;15:641–5.
- Benvegnu L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut 2004;53:744–9.
- Hezode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol 2013;59:434–41.
- Fried MW, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson I, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naive genotype 1 hepatitis C: The randomized PILLAR study. Hepatology 2013;58:1918–29.
- Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878–87.
- Forns X, Lawitz E, Zeuzem S, Gane E, Bronowicki JP, Andreone P, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in Patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology 2014;146:1669–79.
- Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014;370:1889–98.
- Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014;370:1483–93.
- Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT-450/r-Ombitasvir and Dasabuvir with Ribavirin for Hepatitis C with Cirrhosis. N Engl J Med 2014;370:1973–82.
- Talal AH, LaFleur J, Hoop R, Pandya P, Martin P, Jacobson I, et al. Absolute and relative contraindications to pegylated-interferon or ribavirin in the US general patient population with chronic hepatitis C: results from a US database of over 45 000 HCV-infected, evaluated patients. Aliment Pharmacol Ther 2013;37:473–81.
- Younossi ZM, Stepanova M, Henry L, Gane E, Jacobson IM, Lawitz E, et al. Minimal impact of sofosbuvir and ribavirin on health related quality of life in Chronic Hepatitis C (CH-C). J Hepatol 2014;60:741–7.
- Martin NK, Vickerman P, Grebely J, Hellard M, Hutchinson SJ, Lima VD, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 2013;58:1598–609.
- CAN. CAN Homepage, Drugs in Sweden. 2013. Available from http://www.can.se/sv/In-English/. Cited 4 December 2013.
- Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013;368:1867–77.
- Aleman S, Rahbin N, Weiland O, Davidsdottir L, Hedenstierna M, Rose N, et al. A Risk for hepatocellular carcinoma persists long-term after sustained virologic response in patients with hepatitis C-associated liver cirrhosis. Clin Infect Dis 2013;57:230–6.
- Stokkeland K, Duberg AS, Montgomery SM, Franck J, Hultcrantz R. Alcohol dependence increases the mortality risk in patients with hepatitis C. Hepatology 2013;58(S1):p. 1284A.

