Abstract
We are far from having seen the ideal method of screening for colorectal cancer (CRC) and the downsides of screening have not been fully addressed. Funding of adequately sized screening trials with a 10–15-year perspective for endpoints CRC mortality and incidence is difficult to get. Also, with such time horizons, there will always be an ongoing study to be awaited before feeling obliged to invest in the next. New, promising screening methods may, however, emerge far more often than every 10th year, and the knowledge gap may easily widen unless research is made a key responsibility for any ongoing cancer screening program. Previous lost battles on screening research may be won if accepting that scientific evidence may be obtained within the framework of screening programs – provided that they are designed as platforms for Comparative Effectiveness Research (CER). Accepting that CER-based screening programs should be preferred to non-CER programs and seriously compete for their funding sources, then CER screening programs may not be considered so much as contenders for ordinary clinical research funds. Also, CER within a screening framework may benefit patients in routine clinics as shown by screening research in Nordic countries. The Nordic countries have been early contributors to research on CRC screening, but slow in implementing screening programs.
Background and scope
History has shown that the level of evidence required to justify a screening program is a continuous source for debate. Many programs have been launched long before adequately sized screening trials have shown an effect on cancer mortality or incidence – also for colorectal cancer (CRC) (). Although some CRC screening methods have now been shown effective, we have yet to see the ideal screening method in terms of effectiveness and avoidance of overdiagnosis, overtreatment, and unnecessary anxiety. Thus, implementation of CRC screening is hardly justifiable unless combined with research to fill in knowledge gaps and improve services both for screenees and for patients. This was the case in the 1980s [Citation1, Citation2] and it still holds true [Citation3] – after 30 years of insufficient research.
Figure 1. Time lines from start of recruitment in randomized controlled studies on screening with FOBT, flexible sigmoidoscopy, and CS until year of first publication showing an effect on CRC mortality and/or incidence. From the CS studies, results are not expected for many years yet. Examples of countries where regional or national screening has been implemented before emergence of results from trials are shown in gray boxes at the bottom (not a complete listing). Study periods from start of inclusion until first results showing effect: Minnesota 1976–1993 [Citation21], Nottingham 1981–1996 [Citation22]; Gøteborg 1982–2008 [Citation35]; Funen 1985–1996 [Citation20]; PLCO 1993–2012 [Citation9]; SCORE 1995–2011 [Citation11]; FlexiSig 1996–2010 [Citation8]; NORCCAP 1999–2014 [Citation10]; NordICC 2008 -> [Citation23]; CS, E – the COLONPREV trial in Spain on CS vs/iFOBT 2009 -> [Citation24]; CS, USA – the Veterans Administration CONFIRM trial on colonoscopy vs/iFOBT 2013 -> (http://clinicaltrials.gov/show/NCT01239082); CS, S – the Swedish SCREESCO trial on CS vs/iFOBT vs/controls (three arms) 2014 -> (http://clinicaltrials.gov/show/NCT02078804). Abbreviations: CS = Colonoscopy; iFOBT = Immunochemical fecal occult blood testing.
![Figure 1. Time lines from start of recruitment in randomized controlled studies on screening with FOBT, flexible sigmoidoscopy, and CS until year of first publication showing an effect on CRC mortality and/or incidence. From the CS studies, results are not expected for many years yet. Examples of countries where regional or national screening has been implemented before emergence of results from trials are shown in gray boxes at the bottom (not a complete listing). Study periods from start of inclusion until first results showing effect: Minnesota 1976–1993 [Citation21], Nottingham 1981–1996 [Citation22]; Gøteborg 1982–2008 [Citation35]; Funen 1985–1996 [Citation20]; PLCO 1993–2012 [Citation9]; SCORE 1995–2011 [Citation11]; FlexiSig 1996–2010 [Citation8]; NORCCAP 1999–2014 [Citation10]; NordICC 2008 -> [Citation23]; CS, E – the COLONPREV trial in Spain on CS vs/iFOBT 2009 -> [Citation24]; CS, USA – the Veterans Administration CONFIRM trial on colonoscopy vs/iFOBT 2013 -> (http://clinicaltrials.gov/show/NCT01239082); CS, S – the Swedish SCREESCO trial on CS vs/iFOBT vs/controls (three arms) 2014 -> (http://clinicaltrials.gov/show/NCT02078804). Abbreviations: CS = Colonoscopy; iFOBT = Immunochemical fecal occult blood testing.](/cms/asset/3edf377a-666a-4087-b639-7f5c87144b74/igas_a_1011225_f0001_oc.jpg)
The World Health Organization (WHO) has defined a series of basic criteria to be fulfilled before considering mass public health screening [Citation4]. For cancer screening, this includes high prevalence of the type of cancer in question and its importance as a health problem in the population, detectable precursor or early-stage lesions, a safe and suitable method for detection and an effective treatment shown to give better prognosis when applied to early stage, asymptomatic disease than when detected later as a result of patients seeking advice due to symptoms. So far, there is only CRC screening that has lived up to these standards, demonstrated in a series of randomized controlled studies (RCTs) using fecal occult blood testing (FOBT) [Citation5, Citation6, Citation7] and flexible sigmoidoscopy (FS) [Citation8, Citation9, Citation10, Citation11]. Published results from RCTs on FOBT so far have been limited to guaiac-based FOBT (gFOBT), but there are ongoing studies using immunochemical FOBT (iFOBT), which is specific for human blood. Results from RCTs on colonoscopy (CS) screening are not expected for many years to come ().
Case detection of other gastrointestinal cancers by surveillance of high-risk patients (“case finding,” but sometimes called “screening”) may be beneficial as shown for ultrasound and serum alpha-fetoprotein surveillance of patients with hepatitis B [Citation12, Citation13, Citation14]. The same applies to well-established practice of offering surveillance to members of cancer syndrome families. Population screening and prevention programs for gastric cancer have long been established in high-prevalence countries in Asia, apparently without prior RCTs having cancer mortality or incidence as end points (ref. cervical cancer screening, which was also introduced without prior RCTs). Although the number of new cases of gastric cancer worldwide is estimated to be close to one million per year (1.2 million for CRC), it is declining and population screening programs are not likely to be launched in Europe [Citation15]. There are, however, ongoing trials on eradication of Helicobacter pylori as a primary preventive measure [Citation15]. In this review, I shall discuss mass public health screening for CRC, and how the knowledge gap has been met also for the benefit of patients in routine clinics and challenges for the future.
CRC screening perspectives
Worldwide, CRC is considered a major health problem with ∼1.2 million new cases per year and more than 0.6 million deaths. In Europe, it is the number two incident cancer (447,000 per year) after breast cancer and second to lung cancer in terms of cancer-related cause of death (215,000 per year) [Citation16]. Symptoms are unspecific and often emerging at a late stage beyond prospects of surgical cure. Life-lengthening therapy for advanced CRC has improved, but the gain is largely marginal with exceedingly high costs of new drugs [Citation17]. The more cost-ineffective a treatment is, the more attractive may screening appear – not least to health-care providers and politicians facing galloping costs for treatment of advanced cancer disease.
In Europe, the lifetime risk of CRC is about 5% with a 50% 5-year survival. Life itself being 100% “deadly,” this implies that 97.5% of Europeans will die from something else than CRC. So, why should average risk citizens not responsible for health budgets bother about screening? An analogy to screening may be found in house insurance. Both represent some kind of investment from many (pay an insurance premium or invest time for screening participation with personal inconvenience and exposure to procedure risks) for the benefit of very few (those who will have their house on fire or get CRC at some time in the future). Thus, screening is a question of cost (harm) versus potential benefit as it may be acknowledged by the target population and the health-care provider. These two players may have opposing interests: screenees participate to obtain confirmation of being healthy – probably accepting some inconvenience, but little or no procedural risk to obtain this confirmation. Health-care providers, on the other hand, screen to detect as many concealed early-stage, asymptomatic CRCs as possible. Thus, information material supporting invitees to make a genuinely well-informed decision on participation should be developed jointly by providers and participants. This is often not the case and providers may easily oversell benefits neglecting the downsides of screening [Citation18, Citation19].
Prerequisite principles for screening were laid down by WHO in the 1960s [Citation4]. Many regional and national screening programs have been launched with the best political intentions of doing good, but lacking scientific evidence. This applies also to CRC screening programs. Screening for FOBT was introduced in some countries long before results from large-scale randomized trials emerged in the 1990s [Citation20, Citation21, Citation22] and the same has happened for FS and CS () [Citation23, Citation24].
Nordic work on CRC screening
In a series of important studies to understand physiological gastrointestinal blood loss and develop a new test for FOBT, Jan Dybdahl performed a population-based feasibility study in 1982 obtaining 55% compliance among 754 persons invited to be screened at age 54–64 years using standard Hemoccult-II and a new Tetramethylbenzidine test [Citation25]. Although proven feasible, the benefit of using FOBT as a mass screening method at that time was considered highly questionable in several countries as also pointed out by Dybdahl then [Citation25] and later by others [Citation26].
In 1983, the first ever randomized feasibility study on FS screening was launched – the Telemark Polyp Study (TPS) from which 27-year follow-up results were recently published [Citation27, Citation28]. This was a small-scale RCT with only 400 invited to be screened, but it was population-based with 81% attendance [Citation29] – a much higher compliance than observed in contemporary and succeeding FOBT and FS studies. The key target lesion for CRC prevention through endoscopy screening has been the colorectal adenomatous polyp, but the knowledge about its natural course was very limited in the 1980s. In spite of poor level of evidence, guidelines advised to have all polyps removed followed by what later appeared to be overanxious post-polypectomy surveillance strategies. This restricted in situ studies on growth of polyps. Nevertheless, two in situ studies on polyps were performed in Norway [Citation30, Citation31]. Only in recent years have others pursued this line of research [Citation32]. Statistically, 90–95% of polypectomies may be considered a waste of time representing overtreatment in terms of CRC prevention, but we do not know which 5–10% to go for to prevent overtreatment. Gradually, post-polypectomy surveillance intervals have been extended and groups of patients have been downgraded to not require any surveillance at all [Citation33, Citation34].
Among the early Nordic CRC screening trials, the Danish Funen [Citation6] and the Swedish Gøteborg [Citation35] studies were among the first ever and frequently cited large-scale RCTs on gFOBT screening – both showing a relative CRC mortality reduction of 16% [Citation36]. In more recent years, the Finnish were the first to pioneer randomization of a stepwise introduction of a national gFOBT screening program [Citation37]. Among the large-scale trials on endoscopy screening, the Norwegian CRC Prevention (NORCCAP) trial was one out of four large-scale RCTs on FS launched in the 1990s [Citation8, Citation9, Citation10, Citation11, Citation38]. Of these, the NORCCAP trial was the only study inviting straight from the population registry – thus mimicking invitation routines as expected in a national screening program. After 11-year follow-up, the results were, however, very similar to the other trials – a relative reduction of 27% for CRC mortality and 20% for incidence [Citation10] by intention-to-treat (screen) analysis with 65% attendance.
With a time horizon of at least 10 years and often tens of thousands of participants to obtain sufficient statistical power, screening trials are resource demanding in every sense of the word – often requiring international collaboration. In the 1990s, there was an initiative to launch an RCT comparing gFOBT and FS screening with recruitment of 40,000 participants from 10 European centers. This never materialized, but separate valuable publications on baseline data were published from centers in Sweden [Citation39] and Denmark [Citation40]. After several years of failure to raise money for an RCT on CS screening in Norway (NORCCAP-II), a Nordic collaboration that quickly extended to include several EU countries succeeded in fund-raising for the Nordic Initiative on Colorectal Cancer (the NordICC trial) [Citation23]. The screening phase of this study was successfully completed in 2014 with participating centers in Poland, the Netherlands, Sweden, and Norway. In Sweden, recruitment started in 2014 in the three-armed RCT Screening of Swedish Colons comparing CS with iFOBT versus controls (http://clinicaltrials.gov/show/NCT02078804). This is a good example of large-scale parallel testing of more than one screening modality, similar to a much smaller Dutch study comparing gFOBT, iFOBT, and FS [Citation41]. Imagine the knowledge level we would have been at now if such multiarmed trials had been launched when trials on CS screening were first suggested 20–25 years ago.
In the late 1980s, the reigning Nordic attitude was not to start CRC screening programs unless in combination with research [Citation2]. This attitude prevailed in 2005 [Citation42] in spite of the recommendations on programmatic screening from the EU Commission in 2003 [Citation43] and the World Organization on Digestive Endoscopy (OMED) in 2004 recommending even opportunistic (non-programmatic) CRC screening [Citation44] – which was in opposition to the view taken by the EU Commission only accepting programmatic screening with quality assurance at all levels. This reflects different attitudes to screening organization both within Europe and the USA, but also between the two continents [Citation45]. Both in the feasibility study phase of CRC screening and later in performing large-scale RCTs, the Nordic countries have been early contributors and pioneers, but hesitant implementers of screening programs.
Filling the knowledge gap within screening trials – also for the benefit of patients in routine clinics
The efficacy of a screening method and the effectiveness of a ‘screening-program-to-be’ should be proven before implementing screening programs proper. Also, the level of evidence for current practice should be no less for screening a presumptively healthy population not having asked for this service than for worried patients seeking our health service for advice and treatment – ‘to the best of our ability.’ If anything, level of evidence should probably be higher for the healthy [Citation46] than for the sick accepting some risk if there is a prospect of improving health. The pressure on health-care providers to obtain good-level evidence may therefore be higher in screening than routine clinics. Thus, standards for evidence leading to change of current practice may be improved also for patients in routine clinics through studies performed within a screening framework.
The utmost level of evidence is multiple, well-designed large-scale randomized controlled trials. Randomized trials on mass public screening are resource demanding, time consuming, and expensive with a time frame of 10–15 years for the most valid end points – CRC incidence and mortality. National research funds may not be able to cope with the requirements of screening trials without substantial redistribution of funding otherwise meant to be available for competing applicants in other fields of research. Also, the time horizon of screening trials appears not very attractive to funders, health-care providers, or politicians. A time horizon of 10–15 years further implies that there will likely always be an ongoing study, the results of which ‘we shall have to await before making any decisions on further studies or screening programs.’ This was the case in the late 1980s when initiatives were taken (and failed) to launch large-scale RCTs on FS and/or CS screening – still while we were awaiting the first results from ongoing trials on FOBT screening (). If these initiatives had succeeded, we would long since have known what may be gained by ‘going the extra mile’ with full CS rather than ‘half-way’ FS screening compared to FOBT. Not affording to have several studies running in parallel means that the knowledge gap widens as new, promising screening modalities may emerge far more often than every 10th year. As a result, we will always find ourselves having insufficient evidence of efficacy and effectiveness once a political decision is made of implementing a screening program. Such programs are not run on research funds, but health insurance or taxpayers’ money. Those administering these sources should be more aware of the benefits of funding well-designed RCTs as part of piloting screening rather than launch an ordinary screening program without an explicit research mandate. Although expensive, an RCT pilot is much cheaper than going straight for a program launch – particularly when what may be gained is poorly documented. A screening program built on good intentions with poor evidence may prove to be of no benefit and a misuse of taxpayers’ money. The stepwise randomized introduction of the Finnish CRC screening program using FOBT as screening modality is exemplary in this respect [Citation47, Citation48].
Many fights and frustrations over lost battles on screening research can be surpassed if accepting that proof needed may be obtained within the framework of a piloting screening program – provided that it is designed as a platform for Comparative Effectiveness Research (CER) with a series of randomized and observational studies. One such piloting study was the NORCCAP trial, basically an RCT study on FS screening versus ‘care as usual’ (no screening) [Citation38]. NORCCAP never proceeded to a full program, but a new pilot was launched 10 years after NORCCAP in 2012 – the Bowel Cancer Screening in Norway pilot study (BCSN). Not only is this an RCT comparing once-only FS with biennial iFOBT (immunochemical test for FOBT) in 140,000 invitees, but the idea is to add on further randomization arms if or when the program is to be rolled out on a national basis (http://www.kreftregisteret.no/tarmkreftscreening). The Finnish model of stepwise, randomized introduction of a screening program was adopted by the Swedes when starting gFOBT screening in the Stockholm and Gotland counties in 2008 [Citation49] – to be able to assess effects on CRC mortality. The Danes, having had an early lead in CRC screening studies in the 1980s and 1990s led by Ole Kronborg, implemented countrywide gFOBT screening in 2014 without randomization. Several ongoing gFOBT-based programs are now considering converting to iFOBT.
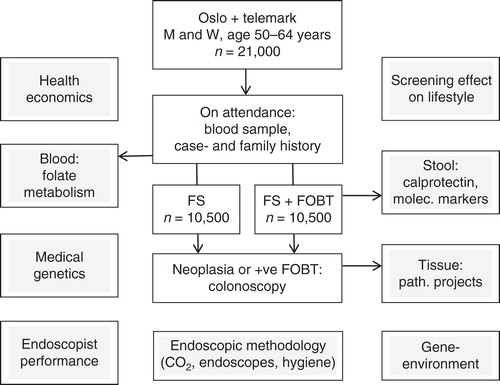
In addition to answering the number one question on impact of CRC screening on incidence and mortality, the NORCCAP trial was designed with a series of additional topics to be addressed as illustrated in . One of these has been the study of possible long-term effects of screening on lifestyle. Recently published results from this showed that possible unfavorable effects of screening on lifestyle are modest, but worth being aware of to consider lifestyle counseling as an integrated part of cancer screening programs [Citation50]. This particular sub-study was strictly screening-related, but other add-on studies may benefit patients in routine clinics. One such example is a series of RCTs on CO2 insufflation carried out within the CER concept of the NORCCAP trial [Citation51, Citation52, Citation53]. Through the Norwegian national quality assurance program Gastronet [Citation54], these studies have strongly contributed to more than 90% of colonoscopies in Norway now being performed with CO2 insufflation and CO2 being recommended in the European Guidelines for CRC screening and diagnosis [Citation55]. Also, the add-on studies on endoscopy performance and patient-reported outcome measures (PROM) in terms of pain and discomfort experienced by screening participants have contributed to further focus on endoscopist training and surveillance in QA programs for the benefit of patients in routine clinics [Citation56, Citation57] – a topic also addressed in other screening studies [Citation58]. Gastronet was the first QA program for CS performance together with the British Global Rating Scale program [Citation59] – both launched independent of each other in 2003. Gastronet was designed within the NORCCAP trial framework in 1999 and subsequently adopted in routine clinics in Norway in 2003 with emphasis on PROM [Citation54]. Both the CO2 and the PROM issues are examples where research within a screening framework has substantially contributed to change of practice and improved services for patients in routine clinics.
Figure 2. The NORCCAP trial on flexible sigmoidoscopy screening (white squares) with add-on study topics (gray squares) having resulted in 48 original scientific publications and 10 PhDs (per January 2015).
There is some concern about the recent emergence of sessile serrated adenomas/polyps (SSA/Ps), once regarded as innocuous hyperplastic polyps, but now considered possibly playing an active part in a polyp–cancer sequence. Endoscopic removal of these may carry a 10 times increased risk of perforation in the proximal colon where these polyps are most often found – without evidence to quantify what may be gained in terms of CRC prevention by having them removed [Citation60]. A recent study from NORCCAP with 11-year follow-up of SSA/Ps left in situ provided evidence that these lesions may be markers of an increased risk of CRC rather than themselves playing an active role in a polyp–cancer sequence [Citation61]. Again, this represents screening trial sub-studies shown to be of benefit for patients in routine clinics where guidelines on how to handle these polyps are about to set an ‘overkill’ standard (just to be ‘on the safe side’) – possibly exposing patients to unnecessary risks of complications [Citation62].
Examples from screening trials contributing to closing the knowledge gap for the benefit of routine patients is listed in .
Table I. Examples of CRC screening trials contributing to reduce the knowledge gap relevant for patients in routine clinics.
Attendance and acceptability
Having found the perfect screening modality (some time in the future) will hardly have any effect if it is not being used or found acceptable by the target population. The ‘perfect screening method’ cannot be expected to be effective if only accepted by, say 10% of the target population. This may vary considerably with time and between countries and cultures. This emphasizes the need to obtain as much screening-related information as possible from the target population, relevant for the target population and presented to the target population when trying to optimize the screening service. Results from your own peer group are expected to be more convincing than results from remote groups in other countries/cultures. In general, attendance for FOBT screening appears to be higher for women (55–60%) than for men (50–55%). For FS screening, the sex difference is not so obvious, but attendance is often 5–10% lower than for FOBT. In spite of these general observations, there are differences poorly accounted for – e.g. how could the population-based TPS on FS screening achieve 81% attendance in 1983 while NORCCAP had 65% in 1999–2001 and BSCN around 50% in 2012–2014 – all in similar Norwegian populations and using the same bowel prep and screening method? Similarly, how did the Danish Funen trial achieve 65% attendance for FOBT in the 1980s while their FOBT screening pilot in 2005–2006 had less than 50% compliance [Citation63]? These observations may reflect declining beliefs in screening in Norway and Denmark – which is in contrast to a stable and high attendance for FOBT screening (70%) in Finland [Citation37]. A Dutch study comparing gFOBT with iFOBT and FS showed attendance rates of 50%, 62%, and 32%, respectively. In spite of only 32% compliance, FS gave the highest yield per 1000 invited (not only per attendee) [Citation41] – emphasizing the need to not only consider compliance, but efficacy of the screening method to address the program effectiveness in a public health perspective. While FS came out as the winner in this Dutch population, it may not be so in, e.g., one of the Nordic populations. Only randomizations within programs or trials may show what is most beneficial for any defined target population.
Acceptability of the screening test itself is one issue, e.g. the advantage of getting away with only one stool sample for iFOBT compared to three for gFOBT, but the unpleasantness of bowel prep for CS and the CS itself for screen-positives and any risks involved are important downsides of screening. This will be an increasing part of the overall acceptability issue of screening as the full implications of participation dawns upon invitees. Again, reliable quality assurance programs conveying these downsides from attendees to the target population are important means of improving services and providing the most reliable, relevant information for consent [Citation19].
The future for CRC screening
The principal discussion on whether to have mass population screening or not will remain – even if all running and planned CRC screening programs convert to a CER concept. It is only a matter of level of discussion – on which level of knowledge do we discuss. The prevalence of CRC may change independent of screening activity and the prospects of cure even if CRC is diagnosed at a symptomatic stage may improve to render screening obsolete (ref. improved prognosis of testis cancer since the 1960s).
New and less invasive screening tests will emerge. Eventually they need to be tested within the framework of existing screening programs.
CS will remain the cornerstone for workup of screen-positives. Apart from surgery, there is no alternative to endoscopic treatment of lesions detected.
The quality of CS and training of endoscopists will attract increasing attention [Citation55, Citation64, Citation65].
Overdiagnosis and overtreatment of colorectal lesions will be addressed. We have seen an increasing understanding of progression and regression of polyps [Citation32, Citation66] and acknowledgment that some adenomas may carry no increased risk of CRC [Citation34] and some lesions may be less active players in a polyp–cancer sequence than anticipated [Citation61]. Virtual microscopy will develop to aid in this process [Citation67].
PROM like pain, discomfort, and satisfaction with information and services will attract more attention as part of addressing the acceptability of screening.
Conclusion
The Nordic countries have been slow in implementing CRC screening for its population, but reasonably active in contributing to research on CRC screening – also contributing to raise standards in health services for patients in routine clinics. Granted that competition for funding is inevitable, research on screening should not be considered a contender for usually very limited clinical research funds, but may be a serious competitor for funding of screening programs that are not based on CER principles.
Declaration of interest: The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
References
- Screening for colorectal cancer. Am Fam Physician 1989;40:40, 2, 5 passim.
- GHoff. [Screening for cancer – a current health service? An updating of screening for breast cancer, cervix cancer and colorectal cancer]. Tidsskr Nor laegeforen 1987;107:1864–8; Screening for cancer – et aktuelt helsetilbud? En oppdatering av screening for kreft i bryst, livmorhals og tykktarm/endetarm.
- MBretthauer, GHoff. Comparative effectiveness research in cancer screening programmes. BMJ 2012;344:e2864.
- JMGWilson, GJungner. Principles of practice of screening for disease. WHO, Geneva, Switzerland; 1968.
- JSMandel, TRChurch, JHBond, FEderer, MSGeisser, SJMongin, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med 2000;343:1603–7.
- OKronborg, ODJorgensen, CFenger, MRasmussen. Randomized study of biennial screening with a faecal occult blood test: results after nine screening rounds. Scand J Gastroenterol 2004;39:846–51.
- JHScholefield, SMoss, FSufi, CMMangham, JDHardcastle. Effect of faecal occult blood screening on mortality from colorectal cancer: results from a randomised controlled trial. Gut 2002;50:840–4.
- WSAtkin, REdwards, IKralj-Hans, KWooldrage, ARHart, JMNorthover, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010;375:1624–33.
- RESchoen, PFPinsky, JLWeissfeld, LAYokochi, TChurch, AOLaiyemo, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012;366:2345–57.
- OHolme, MLoberg, MKalager, MBretthauer, MAHernan, EAas, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. Jama 2014;312:606–15.
- NSegnan, PArmaroli, LBonelli, MRisio, SSciallero, MZappa, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial – SCORE. J Natl Cancer Inst 2011;103:1310–22.
- MSherman. Hepatocellular carcinoma: screening and staging. Clin Liver Dis 2011;15:323–34; vii-x.
- BHZhang, BHYang, ZYTang. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417–22.
- JGChen, DMParkin, QGChen, JHLu, QJShen, BCZhang, et al. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen 2003;10:204–9.
- VPasechnikov, SChukov, EFedorov, IKikuste, MLeja. Gastric cancer: Prevention, screening and early diagnosis. World J Gastroenterol 2014;20:13842–62.
- JFerlay, ESteliarova-Foucher, JLortet-Tieulent, SRosso, JWCoebergh, HComber, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in. 2012;Eur J Cancer 2013;49:1374–403.
- DSchrag. The price tag on progress--chemotherapy for colorectal cancer. N Engl J Med 2004;351:317–19.
- SWoloshin, LMSchwartz, WCBlack, BSKramer. Cancer screening campaigns--getting past uninformative persuasion. N Engl J Med 2012;367:1677–9.
- PHSchwartz, EEdenberg, PRBarrett, SMPerkins, EMMeslin, TFImperiale. Patient understanding of benefits, risks, and alternatives to screening colonoscopy. Fam Med 2013;45:83–9.
- OKronborg, CFenger, JOlsen, ODJorgensen, OSondergaard. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348:1467–71.
- JSMandel, JHBond, TRChurch, DCSnover, GMBradley, LMSchuman, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993;328:1365–71.
- JDHardcastle, JOChamberlain, MHRobinson, SMMoss, SSAmar, TWBalfour, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996;348:1472–7.
- MFKaminski, MBretthauer, AGZauber, EJKuipers, HOAdami, Mvan Ballegooijen, et al. The NordICC Study: rationale and design of a randomized trial on colonoscopy screening for colorectal cancer. Endoscopy 2012;44:695–702.
- EQuintero, ACastells, LBujanda, JCubiella, DSalas, ALanas, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012;366:697–706.
- JHDybdahl, KHaug, KBakkevold, KOOlsen, KVetvik. Screening for occult faecal blood loss in a community by means of Hemoccult II slides and a tetramethylbenzidine test. Scand J Gastroenterol 1984;19:343–9.
- PMoayyedi, EAchkar. Does fecal occult blood testing really reduce mortality? A reanalysis of systematic review data. Am J Gastroenterol 2006;101:380–4.
- EThiis-Evensen, GSHoff, JSauar, FLangmark, BMMajak, MHVatn. Population-based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Telemark Polyp Study I. Scand J Gastroenterol 1999;34:414–20.
- EThiis-Evensen, MKalager, MBretthauer, GHoff. Long-term effectiveness of endoscopic screening on incidence and mortality of colorectal cancer: a randomized trial. United European Gastroenterol J 2013;1:162–8.
- GHoff, MVatn, EGjone, SLarsen, JSauar. Epidemiology of polyps in the rectum and sigmoid colon. Design of a population screening study. Scand J Gastroenterol 1985;20:351–5.
- GHoff, AFoerster, MHVatn, JSauar, SLarsen. Epidemiology of polyps in the rectum and colon. Recovery and evaluation of unresected polyps 2 years after detection. Scand J Gastroenterol 1986;21:853–62.
- BHofstad, MHVatn, SNAndersen, HSHuitfeldt, TRognum, SLarsen, et al. Growth of colorectal polyps: redetection and evaluation of unresected polyps for a period of three years. Gut 1996;39:449–56.
- PJPickhardt, DHKim, BDPooler, JLHinshaw, DBarlow, DJensen, et al. Assessment of volumetric growth rates of small colorectal polyps with CT colonography: a longitudinal study of natural history. Lancet Oncol 2013;14:711–20.
- WSAtkin, RValori, EJKuipers, GHoff, CSenore, NSegnan, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition – Colonoscopic surveillance following adenoma removal. Endoscopy 2012;44:SE151–63.
- MLøberg, MKalager, ØHolme, GHoff, H-OAdami, MBretthauer. Long-term colorectal cancer mortality after adenoma removal. New Engl J Med 2014;371:799–807.
- ELindholm, HBrevinge, EHaglind. Survival benefit in a randomized clinical trial of faecal occult blood screening for colorectal cancer. British J Surg 2008;95:1029–36.
- PHewitson, PGlasziou, EWatson, BTowler, LIrwig. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol 2008;103:1541–9.
- NMalila, TPalva, OMalminiemi, HPaimela, AAnttila, THakulinen, et al. Coverage and performance of colorectal cancer screening with the faecal occult blood test in Finland. J Med Screen 2011;18:18–23.
- MBretthauer, GGondal, KLarsen, ECarlsen, TJEide, TGrotmol, et al. Design, organization and management of a controlled population screening study for detection of colorectal neoplasia: attendance rates in the NORCCAP study (Norwegian Colorectal Cancer Prevention). Scand J Gastroenterol 2002;37:568–73.
- HBrevinge, ELindholm, SBuntzen, JKewenter. Screening for colorectal neoplasia with faecal occult blood testing compared with flexible sigmoidoscopy directly in a 55-56 years’ old population. Int J Colorectal Dis 1997;12:291–5.
- MRasmussen, CFenger, OKronborg. Diagnostic yield in a biennial Hemoccult-II screening program compared to a once-only screening with flexible sigmoidoscopy and Hemoccult-II. Scand J Gastroenterol 2003;38:114–18.
- LHol, MEvan Leerdam, Mvan Ballegooijen, AJvan Vuuren, Hvan Dekken, JCReijerink, et al. Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut 2010;59:62–8.
- MHakama, GHoff, OKronborg, LPahlman. Screening for colorectal cancer. Acta Oncol (Madr) 2005;44:425–39.
- PBoyle, PAutier, HBartelink, JBaselga, PBoffetta, JBurn, et al. European code against cancer and scientific justification: third version (2003). Ann Oncol 2003;14:973–1005.
- PRozen, SJWinawer. Report of the OMED Colorectal Cancer Screening Committee Meeting, New Orleans, 2004 – in collaboration with the IDCA. Eur J Cancer Prev 2004;13:461–4.
- GHoff, JADominitz. Contrasting US and European approaches to colorectal cancer screening: which is best? Gut 2010;59:407–14.
- GHoff. Different standards for healthy screenees than patients in routine clinics? World J Gastroenterol 2013;19:8527–30.
- NMalila, AAnttila, MHakama. Colorectal cancer screening in Finland: details of the national screening programme implemented in Autumn 2004. J Med Screen 2005;12:28–32.
- NMalila, TOivanen, OMalminiemi, MHakama. Test, episode, and programme sensitivities of screening for colorectal cancer as a public health policy in Finland: experimental design. BMJ 2008;337:a2261.
- JBlom, SKilpelainen, RHultcrantz, STornberg. Five-year experience of organized colorectal cancer screening in a Swedish population - increased compliance with age, female gender, and subsequent screening round. J Med Screen 2014;21:144–50.
- PBerstad, MLoberg, IKLarsen, MKalager, OHolme, EBotteri, et al. Long-term lifestyle changes after colorectal cancer screening: randomised controlled trial. Gut 2014; [Epub ahead of print].
- MBretthauer, GHoff, EThiis-Evensen, TGrotmol, STHolmsen, VMoritz, et al. Carbon dioxide insufflation reduces discomfort due to flexible sigmoidoscopy in colorectal cancer screening. Scand J Gastroenterol 2002;37:1103–7.
- MBretthauer, EThiis-Evensen, GHuppertz-Hauss, LGisselsson, TGrotmol, ESkovlund, et al. NORCCAP (Norwegian colorectal cancer prevention): a randomised trial to assess the safety and efficacy of carbon dioxide versus air insufflation in colonoscopy. Gut 2002;50:604–7.
- MBretthauer, ABLynge, EThiis-Evensen, GHoff, OFausa, LAabakken. Carbon dioxide insufflation in colonoscopy: safe and effective in sedated patients. Endoscopy 2005;37:706–9.
- GHoff, MBretthauer, GHuppertz-Hauss, EKittang, AStallemo, OHoie, et al. The Norwegian Gastronet project: Continuous quality improvement of colonoscopy in 14 Norwegian centres. Scand J Gastroenterol 2006;41:481–7.
- RValori, JFRey, WSAtkin, MBretthauer, CSenore, GHoff, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition – Quality assurance in endoscopy in colorectal cancer screening and diagnosis. Endoscopy 2012;44:SE88–105.
- MBretthauer, ESkovlund, TGrotmol, EThiis-Evensen, GGondal, GHuppertz-Hauss, et al. Inter-endoscopist variation in polyp and neoplasia pick-up rates in flexible sigmoidoscopy screening for colorectal cancer. Scand J Gastroenterol 2003;38:1268–74.
- IKLarsen, TGrotmol, MBretthauer, GGondal, GHuppertz-Hauss, BHofstad, et al. Continuous evaluation of patient satisfaction in endoscopy centres. Scand J Gastroenterol 2002;37:850–5.
- WAtkin, PRogers, CCardwell, CCook, JCuzick, JWardle, et al. Wide variation in adenoma detection rates at screening flexible sigmoidoscopy. Gastroenterology 2004;126:1247–56.
- JSint Nicolaas, Vde Jonge, RAde Man, Fter Borg, DLCahen, WMoolenaar, et al. The Global Rating Scale in clinical practice: a comprehensive quality assurance programme for endoscopy departments. Dig Liver Dis 2012;44:919–24.
- GHoff, MBretthauer, KGarborg, TJEide. New polyps, old tricks: controversy about removing benign bowel lesions. Bmj 2013;347:f5843.
- OHolme, MBretthauer, TJEide, EMLoberg, KGrzyb, MLoberg, et al. Long-term risk of colorectal cancer in individuals with serrated polyps. Gut 2014. [Epub ahead of print].
- DKRex, DJAhnen, JABaron, KPBatts, CABurke, RWBurt, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107:1315–29. quiz 4, 30.
- JLindebjerg, MOsler, CBisgaard. Colorectal cancers detected through screening are associated with lower stages and improved survival. Dan Med J 2014;61:A4758.
- LKWanders, SCvan Doorn, PFockens, EDekker. Quality of colonoscopy and advances in detection of colorectal lesions: a current overview. Expert Rev Gastroenterol Hepatol 2014;1–14. [Epub ahead of print].
- PDunckley, GElta. Quality assurance of training. Best Pract Res Clin Gastroenterol 2011;25:397–407.
- FLoeve, RBoer, AGZauber, MVan Ballegooijen, GJVan Oortmarssen, SJWinawer, et al. National Polyp Study data: evidence for regression of adenomas. Int J Cancer 2004;111:633–9.
- MGoetz. Real-time histology in colonoscopy. Gastroenterol Clin North Am 2013;42:567–75.
- GHoff, MHVatn, SLarsen. Relationship between tobacco smoking and colorectal polyps. Scand J Gastroenterol 1987;22:13–16.
- WSAtkin, AHart, REdwards, CFCook, JWardle, PMcIntyre, et al. Single blind, randomised trial of efficacy and acceptability of oral picolax versus self administered phosphate enema in bowel preparation for flexible sigmoidoscopy screening. Bmj 2000;320:1504–8; discussion 9.
- SThomas-Gibson, PRogers, SCooper, RMan, MDRutter, NSuzuki, et al. Judgement of the quality of bowel preparation at screening flexible sigmoidoscopy is associated with variability in adenoma detection rates. Endoscopy 2006;38:456–60.
- KKGarborg, MLoberg, JMatre, OHolme, MKalager, GHoff, et al. Reduced pain during screening colonoscopy with an ultrathin colonoscope: a randomized controlled trial. Endoscopy 2012;44:740–6.

