Abstract
Objective. The molecular adsorbent recirculating system (MARS) is used to purify blood from albumin-bound toxins in patients with liver failure. However, the application of MARS has not demonstrated improved survival in randomized clinical trials and the clinical utility has not been finally established. In our department, the use of MARS is now restricted to the most critically ill patients with acute or acute on chronic liver failure. Material and methods. Since 2005, we have treated 69 patients (30 males/39 females with median age of 49 years ranging from 1 months to 70 years) listed for liver transplantation (LT) with MARS. Median model of end-stage liver disease score in patients older than 12 years of age (n = 56) was 33 (interquartile range 26–39). The flow rate was 35–40 mL/kg/h and treatment kits were changed every 8–12 h. The patients were treated for a median of 27 h (range 1–144 h). Results. Fifty-six patients (81%) were transplanted. Nine died before they could be transplanted, and four patients recovered without transplantation. Forty-six (82%) of the transplanted patients were alive 30 days after transplantation. Ammonium decreased modestly from a median of 148 to 124 µM (p = 0.03) during MARS treatment. We detected worsening of coagulopathy with significant decreases in platelet count and fibrinogen concentrations, and increase in International Normalized Ratio. Phosphate and magnesium decreased significantly during MARS treatment. Conclusion. Continuous MARS therapy may bridge liver failure patients to LT under close observation and treatment of coagulopathy and electrolyte disturbances.
Key Words:
Introduction
Acute liver failure (ALF) and acute on chronic liver failure (AoCLF) have high mortality rates without liver transplantation (LT) [Citation1–3]. Standard therapeutic strategies include treatment of infections and hemorrhages, and supportive treatment of remote organ dysfunctions such as hepatic encephalopathy, renal failure, coagulopathy, circulatory dysfunction and acute respiratory distress syndrome. Patients with hyper acute ALF have a relatively good long-term prognosis if they survive the initial most critical period with hyper-ammonia-related brain edema, whereas patients with less acute ALF and AoCLF most often need LT to survive [Citation4]. Due to organ shortage, several patients die while waiting for a LT. Accordingly, there is a crucial demand for a well-functioning extracorporeal liver system to safely bridge the patients to either spontaneous recovery or to LT.
Randomized controlled trials (RCTs) have not shown improved long-term survival in any of the commercially available extracorporeal liver systems, but final conclusions cannot be drawn at this stage [Citation5–7]. The most frequently used system is the non-biological Molecular Adsorbent Recycling System (MARS, Gambro, Lund, Sweden). In short, using this system the patient’s blood is dialyzed against a high-flux albumin-coated polysulfone filter with a cut-off of 60 kDa and a counter-current albumin-enriched dialysate. The albumin circuit is cleansed by dialyzing it against a conventional dialysate and by letting it pass through charcoal and anion-exchange resin columns. By removing substances such as bilirubin, bile- and fatty acids, mercaptans, phenols, manganese, copper, nitric oxide and cytokines, one think that liver regeneration can be facilitated and that multi-organ dysfunction can be prevented [Citation8,9].
Our hospital has used the MARS system since 2005. Since controlled trials have failed to show improved long-term survival rates when using MARS in patients with AoCLF, and given the lack of other well documented therapeutic options, we restrict the use of this relatively expensive treatment to our most critically ill patients with ALF [Citation5,7,10–12]. Our primary inclusion criterion for treating ALF patients with MARS is that (i) the patient is listed for emergency LT and (ii) that it presents with hepatic encephalopathy grade 3-4 combined with (iii) serum ammonium concentrations higher than 150 µmol/L. We also treat some patients with primary non-function (PNF) after LT. In special cases, we consider other indications such as liver failure after major liver resections and severe intractable pruritus. We herein report that all ALF patients at our institution were treated with MARS while waiting for LT.
Methods
Study population
This is a retrospective report on all patients treated with MARS in the intensive care unit at Oslo University Hospital, Rikshospitalet since the first patient was treated in March 2005 until January 2014. Rikshospitalet is a tertiary center for patients with liver failure primarily admitting potential candidates eligible for LT. The study was approved by the institution’s personal protection ombudsman (overseer) as a quality assurance study.
Molecular adsorbing recirculating system
After correction of coagulopathy, an acute dialysis catheter was inserted either through an internal jugular or subclavian vein. The MARS circuit (Gambro, Lund, Sweden) was used in continuous venovenous hemodiafiltration (CVVHDF) mode and attached a continuous renal replacement therapy (CRRT) device to the circuit. We used CRRT devices from various manufacturers, but all treatments were run in CVVHDF mode. Blood flow rate was set at 120–300 mL/min in adults and 50–90 mL/min in children. Filtration and dialysis flow rates were equal at 30–40 mL/kg/h. MARS was run continuously except for minor breaks when filters were changed every 8–12 h. All treatments were performed without any kind of anticoagulation.
Biochemical parameters
Biochemical parameters are reported from samples drawn 0–1 h prior to starting MARS (baseline), 1–2 h into the treatment, and finally when MARS treatment was ended (1–8 h after treatment). Baseline samples were drawn after insertion of the dialysis catheter, i.e., after correction of coagulopathy. Concentrations of hemoglobin, platelets, white blood cell count (WBC), electrolytes, ammonium, bilirubin, transaminases, lactate dehydrogenase (LDH), alkaline phosphatase (ALP) and gamma-glutamyl transferase (gamma-GT), and level of International Normalized Ratio (INR) were measured in the routine clinical laboratory. Baseline values were used for calculation of the Model of End stage Liver Disease (MELD) score using the formula: (0.957 * ln(serum creatinine) + 0.378 * ln(Serum Bilirubin) + 1.120 * ln(INR) + 0.643) * 10 and pediatric end-stage liver disease (PELD) score using the formula: 10 * ((0.480 * ln(Bilirubin)) + (1.857 * ln(INR)) - (0.687 * ln(Albumin)) + age + growth). Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) score was calculated using baseline values in patients with AoCLF [Citation13]. Acute Physiology and Chronic Health Evaluation (APACHE) II- [Citation14], Sepsis-related Organ Failure Assessment (SOFA)- [Citation15] and Systemic Inflammatory Response Syndrome (SIRS) [Citation16] scores were calculated before and after treatment.
Statistical analyses
Repeated measurements were analyzed with the Wilcoxon signed rank test. Between groups comparisons were done with the Kruskal–Wallis and Mann–Whitney U tests. For parameters that changed significantly during MARS treatment, baseline values and baseline values minus values after last treatment were used as covariates in a univariate logistic regression model to find factors predicting survival without or until transplantation. Baseline values minus values after last treatment were also used in a Cox regression model to explore their ability to predict 30 days post-transplantation survival (survival time was censored at 30 days). The presented p-Values are two-sided, and p < 0.05 was considered significant. The statistical analyses were performed using SPSS 21.0 (IBM®, Chicago, IL).
Results
Study population
Eighty-two patients (37 males and 45 females) aged between were treated with MARS in the study period. Their median age was 43 years, ranging from 1 month to 70 years. Sixty-nine (84%) patients were listed for emergency LT. There were 30 males and 39 females and their median age was 49 years, ranging from 1 month to 70 years). All were mechanically ventilated and their median hepatic encephalopathy score was 4 (range 0–4). Patients with encephalopathy grade 0-2 had PNF of their liver transplant and were listed for urgent re-transplantation.
MELD score in patients 12 years of age or older (n = 56) was 33 (interquartile range (IQR) 26–39). In patients younger than 12 years of age (n = 13), median PELD score was 13 (IQR 11–28). Patients aged 18 years or older with AoCLF (n = 15) had a median CLIF-SOFA score of 15 (IQR 15–17) and corresponding CLIF-SOFA grades of a median of 3 (IQR 3–3).
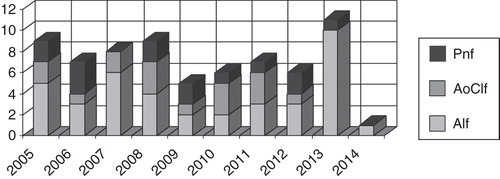
Fifty-six patients (81%) were transplanted, and 46 of these (82%) were alive after 30 days. Median survival time in the 10 patients that died within 30 days post-transplantation was 3 days ranging from 0 to 24 days. Thirteen patients were not transplanted. Of these, nine died while waiting for a liver transplant, and four recovered and were withdrawn from the transplant list (). The patients’ diagnoses are listed in , and the yearly distribution of patients with ALF, AoCLF and PNF are depicted in . There were no difference in MELD/PELD score between survivors and none survivors neither in the transplanted group (p = 0.74) nor in the not transplanted group (p > 0.99). All nine patients that died before they could be transplanted had severe hepatic coma. Six also had evidence of SIRS, one bled from esophageal varices simultaneously with myocardial infarction, and one had intraoperative cardiovascular collapse before the liver could be inserted. One deeply comatose patient with alcoholic cirrhosis and a MELD score of 45 succumbed from intracranial hemorrhage (ICH) after 26.5 h of MARS treatment.
Figure 1. All patients treated with the Molecular Adsorbent Recirculating System at Oslo University Hospital, Rikshospitalet between March 2005 and January 2014.
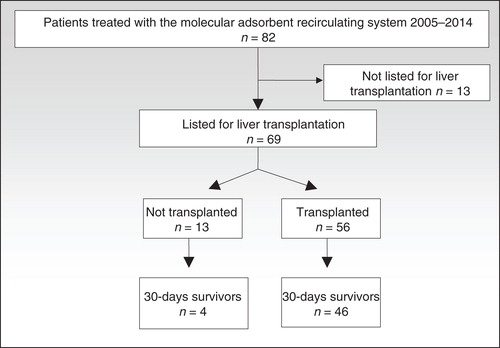
Table I. Indications for molecular adsorbent recirculating system (MARS) treatment in 69 patients listed for liver transplantation in Norway since 2005.
Figure 2. Indications for treating patients listed for liver transplantation with the Molecular Adsorbent Recirculating System.
In patients 18 years of age and older (n = 54), there was a modest reduction in APACHE II-score from a median of 22 (IQR 18–26) prior to start of MARS treatment to 21 (IQR 17–24) (p = 0.03) after the last treatment, whereas the SOFA-score was unchanged (median 15 (IQR 12–17) vs. 14 (13–16) (p = 0.97). Ten patients fulfilled SIRS-criteria prior to and after treatment.
MARS treatment
The patients were treated with MARS for a median of 27 h (range 1–144 h) with a median of three cycles (range 1–15 cycles). The number of cycles with which survivors and non-survivors were treated was similar both in the transplanted group of patients (p = 0.92) and in the not transplanted patients (p = 0.26). Despite running the circuits without anticoagulation, we did not have any episodes of filter clotting. Treatment was not disrupted in any patient due to suspected MARS related complications.
Biochemical parameters
Significant changes in concentrations of several biochemical parameters were seen already after 1–2 h of treatment as compared to baseline (). Hemoglobin and WBC remained stable, but platelet count was reduced with a median of 19 *109/L after 1–2 h and after end of treatment the total drop was 29 *109/L. There was a small but significant increase in sodium of totally 1 mM, whereas potassium and calcium remained stable. Phosphate and magnesium decreased immediately and at end of treatment they were lowered with 0.2 and 0.12 mM, respectively. The liver-failure-related toxicity parameter ammonium decreased modestly during the treatment period. However, no significant decrease could be seen after 2 h of treatment, and in 22 cases ammonium actually increased after MARS treatment was started. There was no difference in ammonium concentrations between survivors and non-survivors in the transplanted group (p = 0.34). However, among the patients who were not transplanted (n = 14) ammonium decreased significantly more in survivors (n = 9) as compared to non-survivors (n = 5) (median decrease (IQR) 68 (62–81) vs. 0 (17–25) (p = 0.01). Bilirubin increased significantly immediately after start of treatment, but at end of treatment the values were modest, but significantly lower than before treatment was started. Transaminases, LDH, ALP and gamma-GT all decreased significantly, as did the renal function parameters creatinine and urea. Neither total plasma protein nor albumin changed during the first 2 h of treatment, but fibrinogen decreased from a median of 1.1–0.8 g/L. The coagulation test INR increased significantly with a value of 0.3 after start of treatment, and after end of treatment, it was still significantly higher than baseline values. In the patient who died of ICH, the course of platelets and INR were 33- 50- 99 *109/L and 4.4- 3.8-3.4 (platelets and plasma were transfused). Fibrinogen decreased from 1.3 to 0.8 and further to values below 0.5 g/L.
Table II. Parameters measured before, during, and after start of treatment with the Molecular Adsorbent Recirculating System (MARS).
In the univariate logistic regression model, none of the baseline parameters (data not shown) or changes in parameters during MARS treatment predicted patient survival without or until transplantation significantly (). Decrease in phosphate during MARS was a negative predictor of 30-day post-transplantation survival and decrease in gamma-GT was a positive predictor in the univariate Cox regression model. However, none of these changes were significant in the multivariate Cox regression analyses ().
Table III. Univariate logistic regression analyses of ability to predict survival without (n = 4) or until (n = 56) liver transplantation in 69 patients listed for liver transplantation. Baseline values minus values after treatment with the Molecular Adsorbent Recirculating System (MARS) are covariates.
Table IV. Cox regression analyses of parameters ability to predict 30-days post liver transplantation survival in Molecular Adsorbent Recirculating System treated patients that were subsequently liver transplanted (n = 56). Baseline values minus values after MARS treatment are covariates. Only parameters that changed significantly during MARS treatment according to Table II were tested.
Discussion
Clinical controlled trials have failed to show that MARS can improve survival rates in patients with liver failure. It is frequently argued that survival studies are confounded by LT, and we fully agree that it would be ethically impossible to exclude patients from transplantation to perform the appropriate RCTs. We therefore consider critically analyzing observational data from MARS-treated patients is in particular important.
Out of 69 high MELD score patients listed for LT as many as 60 patients (87%) either recovered spontaneously or were liver transplanted. Taken the expected high mortality rates without LT, and the relatively long waiting time of about 48 h to receive an emergency liver transplant in Scandinavia into account, we consider the reported survival rate is at an acceptable level [Citation17,18]. One could therefore argue that MARS is a safe therapeutic intervention in patients with ALF listed for LT. Further, taken the theoretical benefits of removing albumin-bound toxins from the blood stream into account, one could argue that it would be good medical practice offering all ALF patients listed for emergency LT MARS treatment. However, our data reveal possible severe side effects of the MARS treatment including coagulopathy and electrolyte disturbances. Indeed, one patient with severe coagulopathy succumbed due to an ICH during MARS treatment. Importantly, our data also question MARS’s efficacy in removing known cerebro-toxic substances like ammonia.
Among numerous substances and mechanisms involved in liver-failure-related brain damage, hyperammonemia-related brain edema is a frequent cause of death for patients with ALF and AoCLF [Citation19]. Therapeutic interventions therefore need to include measures to reduce the levels of circulating ammonium until the patient can be transplanted, or the liver recovers spontaneously. The interventions should be started as soon as possible in particular young patients with hyperacute liver failure since they are at high risk of developing high intracranial pressure (ICP) with consecutive herniation. Overall, we had a relatively small, but statistically significant reduction in ammonia. However, in the smaller group of patients who were not transplanted (n = 13), it is notable that the patients who survived (n = 4) had a substantial and significant reduction in serum ammonia, whereas ammonia did not decrease in non survivors (n = 9), suggesting that MARS may not be able to protect every patient from hyperammonemia. Ideally, an extracorporeal circuit should have an ammonium-reducing capacity exceeding all clinical challenges. Ammonia (NH3) is a small molecule that, in its soluble form (ammonium, NH4+), can be removed from the blood stream by common renal replacement therapy techniques like CRRT. It has recently been shown that ammonia clearance correlates closely with filtration rates during CRRT and that a filtration rate of 90 ml/kg/h is more efficient than 35 ml/kg/h [Citation20]. All our patients were treated with filtration rates between 30 and 40 ml/kg/h on the CRRT-side of the MARS circuit. It is therefore likely that increasing the filtration rates in MARS protocols would increase ammonia clearance, but this needs to be explored in formal trials.
Patients with ALF typically present with coagulopathy, and depending on the severity of the condition high INR values, thrombocytopenia and hypofibrinogenemia are usually detected [Citation21]. Adding an invasive therapeutic intervention potentially worsening the coagulopathy and thereby putting the patients in a state of increased risk of bleeding is highly questionable. Indeed, after starting MARS we detected a statistically significant increase in INR, and decreases in platelet count and fibrinogen. We consider it unlikely that these impairments in coagulopathy solely mirror a spontaneous worsening of liver failure, and our results are in accordance with reports from other authors [Citation22,23]. The patient who succumbed of ICH during MARS presented with severe coagulopathy and in particular hypofibrinogenemia worsened during treatment, emphasizing the necessity of close monitoring and aggressive correction of coagulation disorders. This is in particular important for centers monitoring their comatose liver failure patients with ICP monitors [Citation24,25]. We consider the risk of ICH is too high in these patients and have therefore abandoned inserting ICP monitors and rely upon intermittent transcranial Doppler sonography measurements [Citation26,27].
Like worsening of coagulopathy, electrolyte disturbances regularly occur after start of MARS treatment and close monitoring also of phosphate and magnesium, which are usually not measured at the bedside with blood gas devices, is required [Citation28]. However, it does not seem to be an increased risk of developing hyponatremia possibly contributing to brain edema [Citation29].
There are extracorporeal circuits other than MARS. However, neither the Prometheus system (Fresenius GmBH) [Citation30], single-pass albumin dialysis [Citation31] or plasma exchange therapy [Citation32,33] have been able to show improved survival rates as compared to standard medical therapy, and there is no convincing evidence suggesting that one of the methods is superior compared to others [Citation5]. Although refining existing methods by e.g. increasing filtration rates in the MARS system may be beneficial, we encourage development of other methods like hepatocyte-based bioartificial liver dialysis systems [Citation34,35].
In conclusion, this study indicates that continuous MARS therapy may be of value in bridging liver failure patients to LT under close observation and treatment of coagulopathy and electrolyte disturbances. Increasing flow rates on the CRRT side of the circuit is likely to increase ammonia removal, but this needs to be explored in future studies.
Declaration of interest: The authors declare that they have no conflicts of interest relevant to the manuscript submitted to Scandinavian Journal of Gastroenterology.
Notes
References
- Bernal W, Wendon J. Acute liver failure. N Engl J Med 2013;369:2525–34.
- Kim TY, Kim DJ. Acute-on-chronic liver failure. Clin Mol Hepatol 2013;19:349–59.
- Liou IW. Management of end-stage liver disease. Med Clin North Am 2014;98:119–52.
- O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet 1993;342:273–5.
- Krisper P, Stadlbauer V, Stauber RE. Clearing of toxic substances: are there differences between the available liver support devices? Liver Int 2011;31:5–8.
- Faybik P, Krenn CG. Extracorporeal liver support. Curr Opin Crit Care 2013;19:149–53.
- Banares R, Nevens F, Larsen FS, Jalan R, Albillos A, Dollinger M, et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology 2013;57:1153–62.
- Pares A, Deulofeu R, Cisneros L, Escorsell A, Salmeron JM, Caballeria J, et al. Albumin dialysis improves hepatic encephalopathy and decreases circulating phenolic aromatic amino acids in patients with alcoholic hepatitis and severe liver failure. Crit Care 2009;13:R8.
- Chiu A, Tsoi NS, Fan ST. Use of the molecular adsorbents recirculating system as a treatment for acute decompensated Wilson disease. Liver Transpl 2008;14:1512–16.
- Hassanein TI, Tofteng F, Brown RSJr, McGuire B, Lynch P, Mehta R, et al. Randomized controlled study of extracorporeal albumin dialysis for hepatic encephalopathy in advanced cirrhosis. Hepatology 2007;46:1853–62.
- Vaid A, Chweich H, Balk EM, Jaber BL. Molecular adsorbent recirculating system as artificial support therapy for liver failure: a meta-analysis. ASAIO J 2012;58:51–9.
- Heemann U, Treichel U, Loock J, Philipp T, Gerken G, Malago M, et al. Albumin dialysis in cirrhosis with superimposed acute liver injury: a prospective, controlled study. Hepatology 2002;36:949–58.
- Galbois A, Das V, Carbonell N, Guidet B. Prognostic scores for cirrhotic patients admitted to an intensive care unit: which consequences for liver transplantation? Clin Res Hepatol Gastroenterol 2013;37:455–66.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–29.
- Vincent JL, Moreno R, Takala J, Willatts S, De MA, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med 1996;22:707–10.
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644–55.
- Scandiatransplant. Available from http://www.scandiatransplant.org. 2014.
- Lee WM. Acute liver failure. Semin Respir Crit Care Med 2012;33:36–45.
- Ott P, Vilstrup H. Cerebral effects of ammonia in liver disease: current hypotheses. Metab Brain Dis 2014;29:901–11.
- Slack AJ, Auzinger G, Willars C, Dew T, Musto R, Corsilli D, et al. Ammonia clearance with haemofiltration in adults with liver disease. Liver Int 2014;34:42–8.
- Agarwal B, Wright G, Gatt A, Riddell A, Vemala V, Mallett S, et al. Evaluation of coagulation abnormalities in acute liver failure. J Hepatol 2012;57:780–6.
- Bachli EB, Schuepbach RA, Maggiorini M, Stocker R, Mullhaupt B, Renner EL. Artificial liver support with the molecular adsorbent recirculating system: activation of coagulation and bleeding complications. Liver Int 2007;27:475–84.
- Doria C, Mandala L, Smith JD, Caruana G, Scott VL, Gruttadauria S, et al. Thromboelastography used to assess coagulation during treatment with molecular adsorbent recirculating system. Clin Transplant 2004;18:365–71.
- Blei AT, Olafsson S, Webster S, Levy R. Complications of intracranial pressure monitoring in fulminant hepatic failure. Lancet 1993;341:157–8.
- Larsen FS, Wendon J. Prevention and management of brain edema in patients with acute liver failure. Liver Transpl 2008;14:S90–6.
- Kawakami M, Koda M, Murawaki Y. Cerebral pulsatility index by transcranial Doppler sonography predicts the prognosis of patients with fulminant hepatic failure. Clin Imaging 2010;34:327–31.
- Mohsenin V. Assessment and management of cerebral edema and intracranial hypertension in acute liver failure. J Crit Care 2013;28:783–91.
- Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Kim I, et al. The relationship between hypophosphataemia and outcomes during low-intensity and high-intensity continuous renal replacement therapy. Crit Care Resusc 2014;16:34–41.
- Cordoba J, Garcia-Martinez R, Simon-Talero M. Hyponatremic and hepatic encephalopathies: similarities, differences and coexistence. Metab Brain Dis 2010;25:73–80.
- Santoro A, Faenza S, Mancini E, Ferramosca E, Grammatico F, Zucchelli A, et al. Prometheus system: a technological support in liver failure. Transplant Proc 2006;38:1078–82.
- Ringe H, Varnholt V, Zimmering M, Luck W, Gratopp A, Konig K, et al. Continuous veno-venous single-pass albumin hemodiafiltration in children with acute liver failure. Pediatr Crit Care Med 2011;12:257–64.
- Ye JL, Ye B, Lv JX, Mao WL, Gu B. Changes of ammonia levels in patients with acute on chronic liver failure treated by plasma exchange. Hepatogastroenterology 2014;61:141–5.
- Stenbog P, Busk T, Larsen FS. Efficacy of liver assisting in patients with hepatic encephalopathy with special focus on plasma exchange. Metab Brain Dis 2013;28:333–5.
- Fisher JE, Lillegard JB, McKenzie TJ, Rodysill BR, Wettstein PJ, Nyberg SL. In utero transplanted human hepatocytes allow postnatal engraftment of human hepatocytes in pigs. Liver Transpl 2013;19:328–35.
- Nyberg SL. Bridging the gap: advances in artificial liver support. Liver Transpl 2012;18:S10–14.

