Abstract
Objective. This study aimed to investigate the effect of SagaPro, a product derived from Angelica archangelica leaf, on nocturia. Material and methods. Sixty-nine male patients 45 years or older with at least two nocturnal voids were randomized to receive SagaPro or placebo in a double-blind design for 8 weeks. Voiding diaries were assessed before and after the treatment. Results. The results indicate that SagaPro is safe. The actual number of nocturnal voids (ANV), nocturnal polyuria index (NPi) and nocturnal bladder capacity index (NBC index) decreased in the test population, but there was no significant difference between the treatment groups. Subsequent subgroup analysis showed that SagaPro significantly reduced the NBC index and nocturnal voids per sleeping hour in comparison to the placebo in participants with baseline NBC index above 1.3. When participants with sleep disorders were excluded from this group, ANV was also significantly reduced for the SagaPro group in comparison to the placebo group. Conclusion. SagaPro, made from an extract of the medicinal herb Angelica archangelica, is safe. This study did not show that SagaPro improved nocturia overall compared to placebo. Subgroup analysis suggested a beneficial effect in individuals with decreased nocturnal bladder capacity, which warrants further study.
Introduction
Nocturia is defined as the need to wake from sleep one or more times to void [Citation1]. Having at least two voids per night is associated with impaired health-related quality of life [Citation2]. The prevalence of nocturia increases with increasing age in both men and women. The bothersomeness of nocturia is largely due to the fact that it interferes with the quality of sleep, which may have a significant negative impact on how the individual feels the next day. Nocturia may affect not only nocturics, but also their partners, whose sleep may also be affected [Citation3]. For patients who are aged 60–70 years, the prevalence of nocturia is between 11% and 50%; for those aged 80 years, the prevalence is between 80% and 90%, with about 30% experiencing two or more episodes nightly [Citation4].
Important factors relating to nocturia include benign prostate enlargement and overactive bladder, diabetes mellitus, heart failure, renal failure, peripheral oedema, nephrotic syndrome, hyperproteinaemia and liver diseases [Citation5]. The main causes of nocturia are global polyuria, nocturnal polyuria, decreased bladder capacity and sleep disorders [Citation5]. Among the parameters used to assess the contribution of factors underlying nocturia are nocturnal polyuria index (NPi) and nocturnal bladder capacity (NBC) index [Citation6]. The NPi is usually calculated by dividing the nocturnal urinary volume output by the total urinary output and thus a high NPi indicates increased nocturnal urine production. An NPi of 0.35 normally reflects nocturnal polyuria [Citation6], although the limit may be lower or 0.2 for young people [Citation7]. The NBC index is calculated from the actual number of nightly voids, nocturnal urine volume (nightly voided volume plus the first morning voided volume) and the functional bladder capacity. A high NBC index, which reflects reduced nocturnal bladder capacity, has been found to be more prevalent among Asian men suffering from nocturia than their Caucasian counterparts [Citation8]. An NBC index of 1.3 has recently been suggested as a cut-off point above which reduced nocturnal bladder capacity should be investigated as a cause of nocturia [Citation9].
SagaPro is a phytochemical product from Angelica archangelica leaf that has been used in Iceland for 6 years in the treatment of nocturia. The herb A. archangelica has been used both in folk medicine and as a vegetable. Extensive research has shown that A. archangelica contains several important bioactive compounds, including flavonoids and other polyphenols, terpenes, coumarins and polysaccharides, with various biological effects [Citation10–12]. The roots, fruits and leaves of the herb have been used in folk medicine, and it is one of the most respected medicinal herbs in Nordic countries, where it was cultivated during the Middle Ages [Citation13,14] and exported to other parts of Europe [Citation15]. A water extract from A. archangelica leaf, as is SagaPro, has been found to have antitumour activity in vivo [Citation16]. Among the many bioactive phytochemicals present in SagaPro is isoquercitrin (unpublished results), which could conceivably play a role in the effect of SagaPro on nocturia experienced by the users. Isoquercitrin is a flavonoid that influences the activity of leukotriene LTD4. Leukotrienes (LT) are compounds which are derived from arachidonic acid (20:4n-6) in smooth-muscle cells in the bladder and cause contractions by stimulating receptors. LTD4 also causes contraction in coronary arteries, pulmonary arteries and subcutaneous arterioles. Isoquercitrin inhibits the activity of leukotrienes (LTD4), either by inhibiting the production of LTD4 or by inhibiting binding to receptors in smooth-muscle cells in the bladder [Citation17–19].
The purpose of this study was to examine the safety of SagaPro and its therapeutic effect on nocturia.
Material and methods
This was a parallel, randomized, double-blind, placebo-controlled study to assess the effect of SagaPro on nocturia in men. Included were 69 male volunteers, 45 years of age or older, with an average of at least nocturnal voids per night as determined by a 3-day voiding diary during the screening period.
Exclusion criteria were: residual urine volume greater than 250 ml, total urine output over 24 h exceeding an average of 3000 ml, abnormal urinary findings suggestive of urinary tract infection, significant haematuria or glucosuria requiring further evaluation, patients who had undergone surgical treatment for bladder outlet obstruction/benign prostatic hyperplasia (BPH) within the past 6 months, and patients with a history of urological malignancy (e.g. bladder cancer, prostate cancer), a medical history or active conditions, including known neurological diseases which, in the opinion of the investigators (PI) would prohibit participation in the study. This included, but was not limited to, cancer, renal failure, cirrhosis or chronic liver disease, pancreatic diseases, recent (< 6 months) myocardial infarction or unstable coronary artery disease. Also excluded were patients using medications affecting urination [e.g. loop diuretics (furosemide), antimuscarinic agents, finasteride or dutasteride]. However, individuals who had been using alpha-blockers were eligible for participation in the study. A 7-day washout period was required before starting recordings in the participant diary used for baseline evaluation. Also excluded were those using natural products used for BPH, such as saw palmetto (Sabal serrulata or Serenoa repens), those who had been using SagaPro or other products containing A. archangelica within the 2 months prior to randomization, patients with known allergy to A. archangelica or any other ingredients of SagaPro, patients who had received an investigational product within 30 days before enrolment or expected receipt during this study and men whose work or lifestyle potentially interfered with regular night-time sleep, e.g. shift workers.
Sixty-nine men were randomized to receive either SagaPro or a matching placebo, two tablets per day, and instructed to take the study product in the evening, before going to bed. The tablets were matched in appearance, having the same size and colour coated to ensure blinding. Participants were provided with diary forms to complete a 3-day voiding diary, recording time and volume of voids (over 24 h), time when going to sleep and wake-up time, deviations from planned study product use, if any, and changes in health or medication use, if applicable. The diaries were completed before treatment (and were also used for screening) and after approximately 8 weeks of treatment.
The nocturnal bladder capacity index was calculated as follows:
where ANV is the actual number of nocturnal voids and PNV is the predicted number of nocturnal voids:
where NUV is the nocturnal urinary volume (nightly voided volume plus first morning voided volume) and FBC is the functional bladder capacity, the maximum voided volume in the 3-day diary.
The nocturnal polyuria index was calculated by the formula:
A sample size of 70 participants was calculated to provide a probability of at least 80% that the study would detect a difference between SagaPro and placebo at a two-sided 5% significance level, if the true difference between the groups was 0.7 nocturnal voids and the standard deviation of the response variable was 1.0. The results are expressed as the mean and the numerical changes from baseline values compared statistically with the Student's t test (two sided), with differences between the treatment groups considered significant at p < 0.05.
The patients were fully informed and consented to the study in writing. The study was approved by the National Bioethics Committee of Iceland and the Icelandic Medicines Agency.
Results
Of the total of 69 men, 66 completed the trial and were included in the final outcome. The clinical characteristics of patients completing the trial are shown in .
Table I. Initial clinical characteristics of patients finishing treatment.
Adverse events and safety
Blood pressure and heart rate were stable in both groups during treatment. No severe adverse events were reported, and the number of reports was similar in both treatment groups, 24 in the placebo group and 21 in the SagaPro group. The adverse events were mostly common conditions, the most frequent of which were common cold (four in the placebo group and three in the SagaPro group), diarrhoea (three in each group), vomiting (two in each group) and sore throat (two in the SagaPro group and one in the placebo group). No other adverse events were reported by more than one participant. In no case was it found probable that the adverse event was caused by the treatment, but it was found possible in four cases (one in the placebo group and three in the SagaPro group). One patient in the SagaPro group discontinued treatment because of two of those adverse events, bloating and weight gain, which were later revealed to have been most probably due to gallstones. The remaining adverse events found to be possibly related to the treatment were constipation (SagaPro) and diarrhoea (placebo). Adverse events are listed in .
Table II. Number of adverse events related to the study drug.
Sleeping time
As sleeping time is bound to have a considerable impact on the number of nocturnal voids, the sleeping times were recorded in the diaries. The recorded sleeping times preceding treatment (average of three nights for each patient) ranged from 6 h 13 min to 11 h 32 min, with an average of 520 min. The average sleeping time was reduced for both groups during treatment, and more so in the placebo group, although the treatment groups were not significantly different (p = 0.210) (). This was used to calculate nocturnal voids per sleeping hour by dividing the actual number of nocturnal voids by the hours of recorded sleeping time.
Table III. Average duration of sleep for both treatment groups with standard error of the mean and range.
Effect of SagaPro on nocturia and related parameters on the whole group
No significant difference was found between the treatment groups for ANV, nocturnal voids per hour sleeping time, 24 hand nocturnal voided volume, NPi or NBC index when the whole groups were compared ().
Table IV. Changes in main nocturia-related parameters for the treatment groups.
Subgroup analysis
Subsequent subgroup analysis was carried out to determine whether SagaPro had effect on nocturia dependent on different underlying aetiology. To study participants suffering from nocturnal polyuria, the subgroup of 49 participants (22 from the SagaPro group and 27 from the placebo group) with NPi above 0.35 was studied separately. No significant difference was found between the treatment groups (results not shown).
To determine the effect of SagaPro on participants with reduced nocturnal bladder capacity as a probable cause for nocturia the effect of the herb on individuals with low bladder capacity during the night-time was analysed. As mentioned before, an NBC index of 1.3 has been suggested as a cut-off point above which reduced nocturnal bladder capacity should be investigated as a cause of nocturia. Thus, the 36 participants with a baseline NBC index above 1.3 were studied separately, as shown in . For those 36 participants, voids per sleeping hour and NBC index decreased significantly more in the SagaPro group (p = 0.034 and p = 0.044, respectively). Differences were seen for average and minimal volume per nocturnal voids, both of which increased more in the SagaPro group, albeit not significantly.
Table V. Changes in nocturia-related parameters for the treatment groups for the 36 patients with nocturnal bladder capacity index (NBC index) above 1.3 initially.
As seen in , the baseline values of the treatment groups were very different for many parameters, including ANV, voids per sleeping hour and NBC index. However, as seen in , the effect is not dependent on the baseline values, as the participants with the highest baseline values did not respond better to treatment than those with lower initial values.
Figure 1. (A) Actual number of nocturnal voids (ANV); (B) nocturnal voids (NV) per hour sleeping time; and (C) and nocturnal bladder capacity index (NBC index) before and after treatment, for the 36 participants with baseline NBC index above 1.3. The SagaPro group is shown with triangles and the placebo group with circles. Points lying on the equality line indicate no change after treatment, whereas points lying under the line indicate a reduction and points lying above indicate an increase during the treatment period.
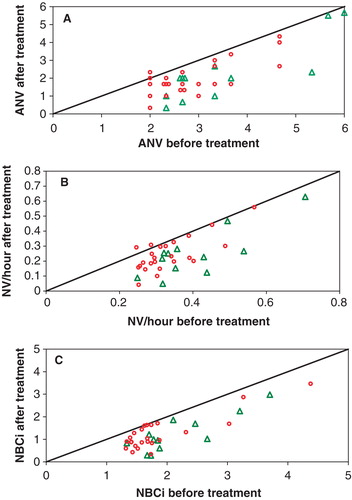
When the seven patients with sleep disorders were excluded from this group, the differences in reduced voids per sleeping hour and NBC index became more pronounced (p = 0.004 and p = 0.026, respectively), with the reduction in ANV also being significantly (p = 0.02) more in the SagaPro group than in the placebo group ().
Table VI. Changes in nocturia-related parameters for the treatment groups for the 29 patients with nocturnal bladder capacity index (NBC index) above 1.3 initially, after exclusion of patients on medication for sleep disorders.
Further studies on nocturnal bladder capacity revealed that average and smallest nocturnal voided volume increased significantly (p = 0.042 and p = 0.022, respectively) in the SagaPro group beyond that of the placebo group for the subgroup of 43 patients with initial average nocturnal voided volume less than 260 ml, whereas no differences were found for the whole group (results not shown).
Discussion
The aim of this study was to investigate the safety of SagaPro and its effect on the number of nocturnal voids and underlying factors in men suffering from nocturia.
The results indicate that SagaPro is safe. The number of nocturnal voids decreased substantially in the group as a whole, but no significant difference was found between the treatment groups, demonstrating a considerable placebo effect.
SagaPro was tested on participants suffering from nocturia of differing aetiology and severity. Apart from global polyuria, which was an exclusion criterion in the study, the main causes of nocturia are nocturnal polyuria, decreased bladder capacity and sleep disorders [Citation5]. Subsequent subgroup analysis aimed to focus on patients with nocturia of different aetiology.
Sleeping times, which contribute greatly to the number of nocturnal voids, were also taken into consideration and were found to be highly diverse in this study, both between individuals and regarding the average sleeping time over the treatment period. Sleeping time was reduced in both groups, slightly more in the placebo group, contributing to the reduction in the number of nocturnal voids. The reduced sleeping time has no obvious explanation. To adjust for this diversity, the number of nocturnal voids per sleeping hour was calculated. This method is reasonable for interpreting the data observed in this study and is useful to overcome difficulties due to large differences in sleeping time at night, especially in small samples. For the group as a whole, the reduction in nocturnal voids per sleeping hour was similar in both treatment groups.
The NPi is used to indicate nocturnal polyuria (increased urine production at night). An NPi of 0.35 normally reflects nocturnal polyuria [Citation5], al though the limit may be lower or 0.2 for young people [Citation8]. In this study, when participants with NPi above 0.35 were studied separately, no significant difference was found between the treatment groups.
An NBC index above 1.3 has been suggested as a cut-off point above which reduced nocturnal bladder capacity should be investigated as a cause of nocturia. Thus, in order to examine the effect on participants with decreased nocturnal bladder capacity, 36 patients with an NBC index above 1.3 were considered separately. In this subgroup, the NBC index was significantly reduced in the SagaPro group, beyond that of the placebo group. Nocturnal voids per hour were also significantly reduced in the SagaPro group. The baseline values are higher in the SagaPro group; however, demonstrates that the reduction is not more pronounced in individuals with high baseline values. It might be of interest to examine further in another study the influence of SagaPro on urodynamic parameters such as urinary flow and residual urine in a larger group of participants with nocturia and reduced bladder capacity. The observed effect may, for example, be related to changes in residual urine volume, which was only measured before treatment in this study.
Sleep disorders can be an important primary cause of nocturia [Citation20]. Nocturnal voids due to sleep disorders increase the number of nocturnal voids and decrease the average void volume, and could thus lead to an elevated NBC index, although the nocturnal bladder capacity is not diminished. When patients on sleep medication were excluded from this group, the above-mentioned differences became more profound and, in addition, the number of nocturnal voids, independent of sleeping time, was significantly reduced in the SagaPro group beyond that of the placebo group. The effect of SagaPro on participants with an NBC index above 1.3 is also reflected in the effect on the average and smallest nocturnal voided volume.
SagaPro is a popular dietary supplement made from an extract of the A. archangelica leaves. The product is primarily used by men over the age of 50 who suffer from lower urinary tract symptoms, to reduce nocturia and thereby improve sleep and general well-being. The exact mechanisms of action of the reported reduction in nocturia are not fully known at present and further studies are needed. This clinical study was carried out to examine the effect of SagaPro on nocturia in men suffering from lower urinary tract symptoms.
In conclusion, SagaPro, made from an extract of the medicinal herb Angelica archangelica, is safe. This study did not show that SagaPro improved nocturia overall compared to placebo. Subgroup analysis suggested a beneficial effect in individuals with decreased nocturnal bladder capacity, which warrants further study.
Acknowledgements
The study was supported by the Technology Development Fund of the Icelandic Research Council. The study was managed and monitored by Encode Clinic, Iceland.
Declaration of interest: Steinthor Sigurdsson, Perla B. Egilsdottir and Sigmundur Gudbjarnason are employed by SagaMedica, the producer of SagaPro.
References
- Staskin D, Kelleher C, Avery K, Bosch R, Cotterill N, Coyne K, Initial assessment of urinary and faecal incontinence in adult male and female patients. In Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence. 4th ed. Plymouth: Health Publications; 2009.
- Tikkinen KA, Johnson TM, Tammela TL, Sintonen H, Haukka J, Huhtala H, Nocturia frequency, bother, and quality of life: how often is too often? A population-based study in Finland. Eur Urol 2010;57:488–96.
- Chartier-Kastler E, Tubaro A. The measurement of nocturia and its impact on quality of sleep and quality of life in LUTS/BPH. Eur Urol Suppl 2006;5:3–11.
- Weiss JP, Blaivas JG. Nocturia. J Urol 2000;163:5–12.
- Udo Y, Nakao M, Honjo H, Ukimura O, Kawauchi A, Kitakoji H, Analysis of nocturia with 24-h urine volume, nocturnal urine volume, nocturnal bladder capacity and length of sleep duration: concept for effective treatment modality. BJU Int 2011;107:791–8.
- Weiss JP, Blaivas JG, Stember DS, Chaikin DC. Evaluation of the etiology of nocturia in men: the nocturia and nocturnal bladder capacity indices. Neurourol Urodyn 1999;18:559–65.
- Walmsley K, Staskin DR. Nocturia: when is it not related to overactive bladder? Curr Urol Rep 2003;4:441–5.
- Mariappan P, Turner KJ, Sothilingam S, Rajan P, Sundram M, Stewart LH. Nocturia, nocturia indices and variables from frequency–volume charts are significantly different in Asian and Caucasian men with lower urinary tract symptoms: a prospective comparison study. BJU Int 2007;100:332–6.
- Burton C, Weiss JP, Parsons M, Blaivas JG, Coats AC. Reference values for the nocturnal bladder capacity index. Neurourol Urodyn 2011;30:52–7.
- Sigurdsson S, Ogmundsdottir HM, Gudbjarnason S. Antiproliferative effect of Angelica archangelica fruits. Z Naturforsch C 2004;59:523–7.
- Sigurdsson S, Ogmundsdottir HM, Gudbjarnason S. The cytotoxic effect of two chemotypes of essential oils from the fruits of Angelica archangelica L. Anticancer Res 2005;25:1877–80.
- Härmälä P, Vuorela H, Törnquist K, Hiltunen R. Choice of solvent in the extraction of Angelica archangelica roots with reference to calcium blocking activity. Planta Med 1992;58:176–83.
- Halldórsson B. Grasnytjar. Copenhagen: August Friedrich Stein; 1783.
- Bjarnason AH. Íslensk flóra með litmyndum. Reykjavík: Iðunn; 1983.
- Newall CA, Anderson LA, Phillipson JD. Herbal medicines – a guide for health-care professionals. London: Pharmaceutical Press; 1996.
- Sigurdsson S, Ogmundsdottir HM, Hallgrimsson J, Gudbjarnason S. Antitumour activity of Angelica archangelica leaf extract. In Vivo 2005;19:191–4.
- Piper PJ. Leukotrienes: possible mediators in bronchial asthma. Eur J Respir Dis Suppl 1983;129:45–64.
- Bjorling DE, Saban MR, Bruskewitz RC, Saban R. Response of the isolated guinea pig bladder to exogenous and endogenous leukotrienes. J Urol 1994;152:1281–6.
- Yoshimura N, Chancellor MB. Interstitial cystitis and bladder research: progress and future directions: highlights of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the Interstitial Cystitis Association (ICA) International Research Symposium October 19–20, 2000, Minneapolis, MN. Rev Urol 2001;3:146–51.
- Scheuermaier K, Meyers M, Surprise M, Loughlin KR, Duffy JF. Reciprocal relationship between age-related sleep disruption and urological symptoms. BJU Int 2011;107:871–3.
