Abstract
Osteoarthritis (OA) is one of the most frequent musculoskeletal disorders in the elderly population. OA is characterised by a gradual loss of extracellular matrix in the articular cartilage of joints. OA can only be managed by artificial joint replacement when joint destruction becomes severe. Therefore, it is preferable to administer conservative therapy that is easy, simple and effective in inhibiting OA progression at the early stage. Heat shock protein 70 (Hsp70) has a protective effect on the cartilage and inhibits the apoptosis of chondrocytes. Heat stimulation by microwave to the joints can increase Hsp70 expression in chondrocytes, and at the same time, Hsp70 expression partially enhances matrix metabolism of the cartilage. These findings suggest that hyperthermia can be positively applied to the treatment of OA. Hyperthermia is therefore expected to be an inexpensive and less-invasive conservative therapy for OA.
Introduction
Osteoarthritis (OA) is one of the most frequent musculoskeletal disorders in the elderly population. OA is characterised by a gradual loss of extracellular matrix in the articular cartilage of joints. Since the joint function is severely impaired, personal and social activities of patients with OA are limited. Because the articular cartilage, which is the matured hyaline cartilage, does not have blood vessels, the cells required to repair damaged cartilage are not provided. Chondrocytes themselves have limitations in their proliferative potential and repair capacity, therefore, when OA progresses and the articular cartilage degenerates, it becomes difficult to treat. Intra-articular injection of hyaluronan has been applied as a conservative therapy for OA Citation[1], but its effect on advanced OA is limited. Orally administered supplements such as glucosamine and chondroitin sulphate reduce the pain due to OA, and inhibit the narrowing of the joint-space width Citation[2], Citation[3]. However, they cannot change the natural course of OA or greatly interrupt its progression. When OA has progressed, the wide range of cartilage tissue should be repaired. Therefore, at present, surgical procedures for injuries of localised articular cartilage (e.g. autologous transplantations of the articular cartilage Citation[4], Citation[5] and chondrocytes Citation[6]) cannot be applied for OA. OA can only be managed by artificial joint replacement when joint destruction becomes severe. However, this is associated with some problems including the invasiveness, and high cost of the procedure and also the long-term prognosis. Therefore, it would be preferable to develop a conservative therapy that is easy, simple and can also effectively inhibit the progression of OA at the early stage.
Thermotherapy is widely used for musculoskeletal disorders as a physical therapy in clinical settings Citation[7]. Hyperthermia with hot packs, paraffin baths, ultra-short waves and microwaves are generally performed as well as therapeutic exercise for joint diseases such as OA and rheumatoid arthritis. There are two types of effects of hyperthermia on soft tissues: local and distant effects. The distant effects are mainly the heat transfer by blood flow and biological reaction via the autonomic nervous system. On the other hand, the local effects include an increase of elasticity of collagen fibres by heat Citation[8], pain relief due to increasing the pain threshold Citation[9], decreasing muscle tension by reducing electrical pulses in the muscular spindle Citation[10], increase of local blood flow Citation[11], and acceleration of tissue metabolism. Hyperthermia, which is applied to the rehabilitation for musculoskeletal disorders, is generally thought to be an adjunctive therapy. The local effects of hyperthermia such as pain control and reduction of muscle tension are utilised in a range of joint motion exercise. However, the intensity and duration of heat stimulation used for hyperthermia are empirically determined, and its effect has not been scientifically proven. In addition, the direct effect of heat on metabolism and repair of the articular cartilage, which is the mainly affected tissue of OA, is unknown.
This article investigated the role of a stress protein, heat shock protein (Hsp) 70, in chondrocytes, and the effect of heat on the articular cartilage. Furthermore, the application of hyperthermia for articular cartilage with OA is also herein considered.
Anatomy of articular cartilage
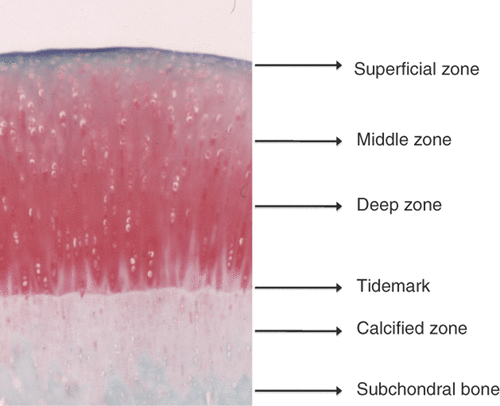
Articular cartilage possesses a zonal structure that includes the superficial, middle, and deep zones, each with a distinct cellular phenotype and matrix composition (). Chondrocytes decrease in number from the superficial zone to the deep zone and increase in size. Articular cartilage contains 2% chondrocytes and the extracellular matrix. The extracellular matrix is composed of approximately 20% collagen (primarily Type II collagen), approximately 10% proteoglycan, and 70% water. Collagen forms a meshwork structure in the cartilage matrix to maintain its shape. Collagen orientation changes in the different layers of articular cartilage. Proteoglycan is negatively charged and has large capacity to retain and maintain water in the cartilage matrix, which contributes to the viscoelasticity necessary for the cartilage to function as a shock absorber. Chondrocytes are the only cell type of the articular cartilage. They produce collagen and proteoglycan, and metabolise the extracellular matrix. Chondrocytes themselves release growth factors and cytokines, and these factors regulate metabolism of the cartilage matrix by autocrine and paracrine mechanisms. Adult human cartilage is avascular, thus, there is no external cell supply to compensate for cell loss caused by necrosis, apoptosis, or other cellular mechanisms.
Figure 1. Structure of cartilage. The main difference observed between the chondrocytes in hyaline cartilage is their morphological variation between the zones. Chondrocytes in the superficial zone are flattened and elongated, whereas the cells in the middle zone appear rounded and those in the deep zone have an ellipsoid morphology.
Etiology of OA
OA is a progressive disease that induces degeneration of the articular cartilage, subsequent reparative proliferation of the bone, and secondary synovitis. The radiological changes are characterised by a combination of bony proliferation, such as osteophyte formation and sclerosis of subchondral bone, and joint space narrowing which corresponds to degeneration of cartilage (). Histologically, the surface of articular cartilage becomes irregular and reveals fibrillation and clefts. The cartilage thickness gradually decreases, and finally cartilage disappears and eburnated subchondral bone is exposed. The laminar structure of articular chondrocytes is completely destroyed, and cluster formation is often observed in chondrocytes during the early phase (). The pathological basis of OA has not yet been clarified, but it is thought that its onset and progression are related to biological or chemical stress as well as non-physiological mechanical stress () Citation[12]. If the mechanical stress increases to a level excessively higher than the physiological levels, the cartilage matrix is impaired and OA could occur. This process of disease progression is proven by the fact that (1) cartilage degeneration in OA starts from the weight-bearing area Citation[13], and (2) many OA-related animal models are prepared by making mechanical changes to the joints Citation[14], Citation[15]. Locally produced humoral factors, e.g. inflammatory cytokines (IL-1, IL-6, IL-17 and TNF-a) and metalloproteinases (MMP-1, MMP-3, MMP-8, MMP-13 and aggrecanases), are thought to be important, in addition to mechanical stress Citation[16–19]. Chondrocytes undergo apoptosis in response to stress, and apoptotic chondrocytes are frequently found in OA cartilage. Therefore, apoptosis has attracted the attention of researchers as a potential etiological factor in the progression of OA Citation[20–24]. D’Kima et al. demonstrated caspase inhibitors to reduce the severity of cartilage lesions in experimental OA. Caspases are activated during apoptosis, thus suggesting that apoptosis of chondrocytes may be a potential target for therapeutic intervention in OA Citation[25].
Figure 2. Osteoarthritis of the knee. The knee joint is the most common site of osteoarthritis. The anteroposterior radiograph reveals the valgus deformity of the joint, joint space narrowing, osteophyte formation and sclerosis of subchondral bone. In knee osteoarthritis, medial femorotibial alterations often predominate.
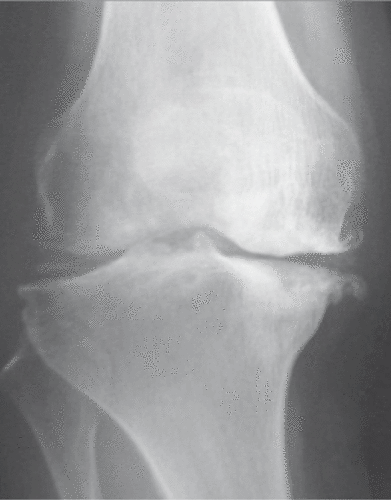
Figure 3. Histological features of cartilage in osteoarthritis. Osteoarthritic cartilage demonstrates severe fibrillation leading to cracking that extends into the tidemark. There is an obvious decrease in the numbers of chondrocytes in the late phase of the disease (A), while cluster formations, corresponding to chondrocyte proliferation, are sometimes observed to be adjacent to the area of fibrillation in the early phase (B).
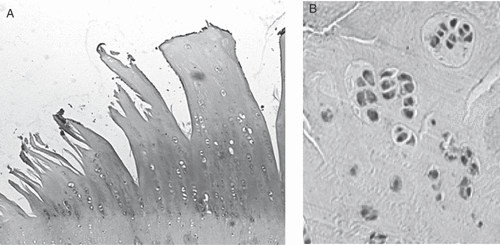
Figure 4. A schematic drawing of the events involved in the initiation and progression of osteoarthritis. Potential causative factors are listed above and the cellular and morphological changes are listed below Citation[12]. Articular cartilage is an important component of the joint, and it is always exposed to pressure produced by weight bearing and muscle contraction. If the mechanical stress increased to a level excessively higher than the physiological levels, the cartilage matrix will be impaired and osteoarthritis could occur. In addition to mechanical stress, its onset and progression could be induced by inflammatory cytokines produced by chondrocytes in cartilage or the synovial tissue in the joint. Inflammatory cytokines contribute to the dysregulation of the chondrocyte function. The association of increased production of metalloproteinases induced by inflammatory cytokines with cartilage damage has been established. The apoptosis of chondrocytes caused by various types of stress has attracted the attention of researchers as an important etiologic factor in OA progression.
![Figure 4. A schematic drawing of the events involved in the initiation and progression of osteoarthritis. Potential causative factors are listed above and the cellular and morphological changes are listed below Citation[12]. Articular cartilage is an important component of the joint, and it is always exposed to pressure produced by weight bearing and muscle contraction. If the mechanical stress increased to a level excessively higher than the physiological levels, the cartilage matrix will be impaired and osteoarthritis could occur. In addition to mechanical stress, its onset and progression could be induced by inflammatory cytokines produced by chondrocytes in cartilage or the synovial tissue in the joint. Inflammatory cytokines contribute to the dysregulation of the chondrocyte function. The association of increased production of metalloproteinases induced by inflammatory cytokines with cartilage damage has been established. The apoptosis of chondrocytes caused by various types of stress has attracted the attention of researchers as an important etiologic factor in OA progression.](/cms/asset/5813f899-8116-4760-8726-9ce0757b2bc7/ihyt_a_410924_f0004_b.gif)
Current clinical application of hyperthermia to OA
In a systemic review of the literature on the thermal modalites in OA, evidence exists that an increase in the temperature of the joint may provide the short-term relief of joint pain, although treatment with hot packs did not demonstrate any significant beneficial effect when used to treat OA Citation[26]. This may be due to several reasons. Firstly, superficially applied treatments such as hot packs heat only skin and subcutaneous tissues, while deep heating apparatus such as ultrasound may increase temperature by 4–5 °C at depths of 8 cm Citation[26]. Secondly, the few published studies in this area tend to have serious methodological limitations to accurately distinguish the treatment effects from placebo effect. Thirdly, thermal therapy is usually practiced for only a short time. Heat-generating sheet used on knee OA 6 hrs per day at the longest is reported to be effective for alleviating pain, and improving both stiffness and gait impairment Citation[27].
Expression of Hsp70 in OA chondrocytes
Heat shock protein 70 (Hsp70) is a member of a family of highly conserved proteins which are synthesised in cells after stress loading, and protects cells from various types of stress. A study using spontaneous OA mice showed that expression of several HSPs is increased in the articular cartilage from the early stage of OA Citation[28]. In addition, studies using clinical specimens report that Hsp70 expression levels in chondrocytes are correlated with the histological severity of OA Citation[29], Citation[30]. It is not clear what induces the expression of Hsp70 in OA chondrocytes, but the non-physiological mechanical stress involved in OA induces Hsp70 in chondrocytes in vitro Citation[31], Citation[32].
Roles of Hsp70 in chondrocytes
The roles of Hsp70 in chondrocytes were investigated by Hsp70 gene transduction in several studies. Cartilage metabolism is accelerated in chondrocyte-like cells in vitro by adenovirus vector-mediated gene transduction of Hsp70 Citation[33], and chondrocytes are protected from cytotoxic stress Citation[34]. In addition, transduced Hsp70 gene in articular chondrocytes dramatically inhibits apoptosis of chondrocytes induced by nitric oxide (NO) Citation[35]. The inhibition of apoptosis by Hsp70 may not be based on its influence on the level of cytosolic cytochrome C released from NO-stimulated mitochondria, but on the inhibition of caspase 3 activation Citation[35]. Glutamine and MG132, which induce Hsp70, protect chondrocytes from cytotoxic stress when they are added to cultured chondrocytes Citation[36], Citation[37], and reduce degeneration of the articular cartilage when they are administered intra-articularly to experimental OA rats Citation[38]. Glutamine and MG132 might produce effects other than that via Hsp70 on chondrocytes, but the direct effect of Hsp70 on the articular cartilage in vivo was investigated by Grossin et al. Citation[39]. They reported that cartilage degeneration induced by mono-iodoacetate was inhibited by Hsp70 gene transduction into the articular cartilage of the patellae Citation[39]. Future studies should investigate the therapeutic effects of Hsp70 in spontaneous and injury-induced OA models, in addition to drug-induced cartilage degeneration.
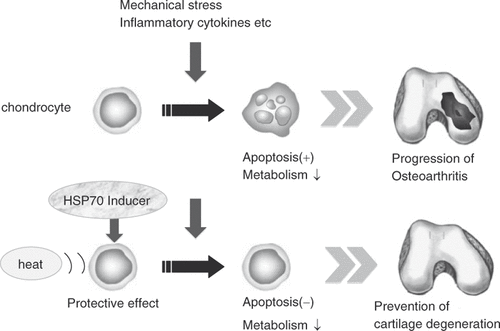
These studies indicate that Hsp70 has a protective effect on the cartilage and inhibits apoptosis of chondrocytes. Induction of Hsp70 may be useful for slowing the progression of OA (). OA progresses even though Hsp70 expression increases in human OA possibly because (1) the expression level of Hsp70 is insufficient in comparison to the stress added to chondrocytes, and (2) the cytoprotective action of Hsp70 did not function properly.
Figure 5. Strategy for applying hyperthermia to the treatment of OA. Hsp70-induction in chondrocytes by heat stimulation and/or HSP inducer, such as glutamine, MG132, GGA and curcumin can have a therapeutic effect on OA by inhibiting chondrocyte apoptosis as well as increasing the cartilage metabolism.
Effect of heat stimulation on cultured chondrocytes
Hojo et al. applied heat stimulation to cultured chondrocytes, and evaluated the proteoglycan metabolism Citation[40]. Proteoglycan metabolism is increased at 39 °C and 41 °C, and decreased at 43 °C. The metabolism at 43 °C decreases further when the duration of heat stimulation is prolonged Citation[40]. This suggests that consideration of duration as well as temperature of heat stimulation is required in the clinical setting. A narrow therapeutic range of temperature seems to pose problems in clinical settings since the effect of hyperthermia shows a significant variation in response to small differences in the applied temperature. There is joint fluid in the joints in vivo and there is also free water in the extracellular matrices of the articular cartilage. Therefore, it is not possible for the cartilage in the deep areas to have a higher temperature than the skin due to microwaves that are clinically used with outputs that do not burn the skin. Furthermore, the abovementioned free water should be dispersed when differences in the temperature occur, so the possibility of a localised high temperature is low. The activity of chondrocytes decreased by heat stimulation at 43 °C can be controlled by adding glutamine that induces Hsp70 Citation[34].
In vivo effect of hyperthermia on the articular cartilage in an animal model
Conversive heat, such as ultrasound and microwaves which are converted into heat energy and reach the deep region, should be used to apply heat stimulation to the deep-lying articular cartilage in joints. Tonomura et al. applied heat stimulation to the knee joints of rabbits for 20 minutes using a clinically available 2.45-GHz microwave applicator, and observed the temperature in joints increase as the intensity of heat stimulation by microwave gets higher Citation[41]. They also reported the joint temperature of the rabbits to reach approximately 40 °C at an intensity of 40W, and that the expression of proteoglycan and type II collagen in the articular cartilage was observed to remarkably increase at this intensity. At the same time, Hsp70 has been confirmed to accumulate in the chondrocytes Citation[41]. In that study, the pre-treatment of joints with quercetin that inhibits expression of Hsp70 reduced the effect of heat stimulation by microwaves on increasing the expression of proteoglycan, but its effect on type II collagen was not observed to change Citation[41]. Consequently, heat stimulation by microwave to the joints can increase matrix metabolism of the cartilage partially via Hsp70 expression. However, since there are large species differences in regard to the distance from the stimulation site to joints and the amount of subcutaneous fat and muscle between human and rabbits, it is therefore necessary to investigate optimal conditions of heat stimulation in humans. In addition, further studies on the effects of heat stimulation on the catabolic factors or cell viability of chondrocyte should be performed.
Conclusions
The number of studies is small, but they demonstrate the effect of Hsp70 and hyperthermia that induces Hsp70 on protecting chondrocytes and increasing metabolism of the articular cartilage. This suggests that hyperthermia can be positively applied to OA treatments. However, the effect of hyperthermia on OA has not been sufficiently investigated in clinical studies Citation[42], and the associated mechanism is still unknown. To effectively perform thermotherapy to the articular cartilage, it is necessary to determine the optimal intensity and duration of heat stimulation in humans. Since thermo-tolerance and intracellular accumulation of Hsp70 are observed after heat stimulation Citation[43], the interval of heat stimulation should also be studied. Hsp70 inducers, such as geranylgeranylaceton (GGA) Citation[44] and curcumin Citation[45], as well as glutamine Citation[37] and MG132 Citation[36] could be applied to treat OA. Hsp70 inducers or hyperthermia may provide an inexpensive and less invasive conservative therapy for OA.
Acknowledgements
This study was supported by a Grant in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 20390404).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jubb RW, Piva S, Beinat L, Dacre J, Gishen P. A one-year, randomised, placebo (saline) controlled clinical trial of 500–730 kDa sodium hyaluronate (Hyalgan) on the radiological change in osteoarthritis of the knee. Int J Clin Pract 2003; 57: 467–474
- Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE, Gossett C. Long-term effects of glucosamine sulphate on osteoarthritis progression: A randomized, placebo-controlled clinical trial. Lancet 2001; 357: 251–256
- Pavelka K, Gatterova J, Olejarova M, Machacek S, Giacovelli G, Rovati LC. Glucosamine sulfate use and delay of progression of knee osteoarthritis: A 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med 2002; 162: 2113–2123
- Yamashita F, Sakakida K, Suzu F, Takai S. The transplantation of an autogeneic osteochondral fragment for osteochondritis dissecans of the knee. Clin Orthop Relat Res 1985; 201: 43–50
- Hangody L, Kish G, Karpati Z, Udvarhelyi I, Szigeti I, Bely M. Mosaicplasty for the treatment of articular cartilage defects: Application in clinical practice. Orthopedics 1998; 21: 751–756
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994; 331: 889–895
- Sarzi-Puttini P, Cimmino MA, Scarpa R, Caporali R, Parazzini F, Zaninelli A, Atzeni F, Canesi B. Osteoarthritis: An overview of the disease and its treatment strategies. Semin Arthritis Rheum 2005; 35: 1–10
- Lehmann JF, Masock AJ, Warren CG, Koblanski JN. Effect of therapeutic temperatures on tendon extensibility. Arch Phys Med Rehabil 1970; 51: 481–487
- Lehmann JF, Brunner GD, Stow RW. Pain threshold measurements after therapeutic application of ultrasound, microwave, and infrared. Arch Phys Med Rehabil 1958; 39: 560–565
- Mense S. Effects of temperature on the discharges of muscle spindles and tendon organs. Pflugers Arch 1978; 374: 159–166
- Abramson DI, Belly Y, Tuck S, Jr, Mitchell R, Chandrasekharappa G. Changes in blood flow, oxygen uptake and tissue temperatures produced by therapeutic physical agents. III. Effect of indirect or reflex vasodilsatation. Am J Phys Med 1961; 40: 5–13
- Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007; 213: 626–634
- Dieppe P, Kirwan J. The localization of osteoarthritis. Br J Rheumatol 1994; 33: 201–203
- Muir H, Carney SL. Pathological and biochemical changes in cartilage and other tissues of canine knee resulting from induced joint instability. In: Helminen HJ, Kirivanta I, Tammi M, et al., editors. Joint Loading: Biology and Health of Articular Structures. Bristol: John Wright; 1987. pp 47–63
- Hoch DH, Grodzinsky AJ, Koob TJ, Albert ML, Eyre DR. Early changes in material properties of rabbit articular cartilage after menisectomy. J Orthop Res 1983; 1: 4–12
- Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: Associations with degenerative changes. Arthritis Rheum 2001; 44: 585–594
- He W, Pelletier JP, Martel-Pelletier J, Laufer S, Di Battista JA. Synthesis of interleukin 1beta, tumor necrosis factor-alpha, and interstitial collagenase (MMP-1) is eicosanoid dependent in human osteoarthritis synovial membrane explants: Interactions with antiinflammatory cytokines. J Rheumatol 2002; 29: 546–553
- Lubberts E, Koenders MI, van den Berg WB. The role of T cell interleukin-17 in conducting destructive arthritis: Lessons from animal models. Arthritis Res Ther 2005; 7: 29–37
- Cawston TE, Wilson AJ. Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract Res Clin Rheumatol 2006; 20: 983–1002
- Blanco FJ, Guitian R, Vazquez-Martul E, deToro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum 1998; 41: 284–289
- Hashimoto S, Takahashi K, Amiel D, Coutts RD, Lotz M. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis Rheum 1998; 41: 1266–1274
- Healy ZR, Lee NH, Gao X, Goldring MB, Talalay P, Kensler TW, Konstantopoulos K. Divergent responses of chondrocytes and endothelial cells to shear stress: Cross-talk among COX-2, the phase 2 response, and apoptosis. Proc Natl Acad Sci USA 2005; 102: 14010–14015
- Thomas CM, Fuller CJ, Whittles CE, Sharif M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage 2007; 15: 27–34
- Pennock AT, Robertson CM, Emmerson BC, Harwood FL, Amiel D. Role of apoptotic and matrix-degrading genes in articular cartilage and meniscus of mature and aged rabbits during development of osteoarthritis. Arthritis Rheum 2007; 56: 1529–1536
- D’Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum 2006; 54: 1814–1821
- Brosseau L, Yonge KA, Robinson V, Marchand S, Judd M, Wells G, Tugwell P. Thermotherapy for treatment of osteoarthritis. Cochrane Database Syst Rev 2003; CD004522
- Seto H, Ikeda H, Hisaoka H, Kurosawa H. Effect of heat and steam-generating sheet on daily activities of living in patients with osteoarthritis of the knee: Randomized prospective study. J Orthop Sci 2008; 13: 187–191
- Takahashi K, Kubo T, Goomer RS, Amiel D, Kobayashi K, Imanishi J, Teshima R, Hirasawa Y. Analysis of heat shock proteins and cytokines expressed during early stages of osteoarthritis in a mouse model. Osteoarthritis Cart 1997; 5: 321–329
- Kubo T, Towle CA, Mankin HJ, Treadwell BV. Stress-induced proteins in chondrocytes from patients with osteoarthritis. Arthritis Rheum 1985; 28: 1140–1145
- Takahashi K, Kubo T, Arai Y, Imanishi J, Kawata M, Hirasawa Y. Localization of heat shock protein in osteoarthritic cartilage. Scand J Rheumatol 1997; 26: 368–375
- Takahashi K, Kubo T, Kobayashi K, Imanishi J, Takigawa M, Arai Y, Hirasawa Y. Hydrostatic pressure influences mRNA expression of transforming growth factor-beta 1 and heat shock protein 70 in chondrocyte-like cell line. J Orthop Res 1997; 15: 150–158
- Nakamura S, Arai Y, Takahashi KA, Terauchi R, Ohashi S, Mazda O, Imanishi J, Inoue A, Tonomura H, Kubo T. Hydrostatic pressure induces apoptosis of chondrocytes cultured in alginate beads. J Orthop Res 2006; 24: 733–739
- Arai Y, Kubo T, Kobayashi K, Takeshita K, Takahashi K, Ikeda T, Imanishi J, Takigawa M, Hirasawa Y. Adenovirus vector-mediated gene transduction to chondrocytes: In vitro evaluation of therapeutic efficacy of transforming growth factor-beta 1 and heat shock protein 70 gene transduction. J Rheumatol 1997; 24: 1787–1795
- Kubo T, Arai Y, Takahashi K, Ikeda T, Ohashi S, Kitajima I, Mazda O, Takigawa M, Imanishi J, Hirasawa Y. Expression of transduced Hsp70 gene protects chondrocytes from stress. J Rheumatol 2001; 28: 330–335
- Terauchi R, Takahashi KA, Arai Y, Ikeda T, Ohashi S, Imanishi J, Mazda O, Kubo T. Hsp70 prevents nitric oxide-induced apoptosis in articular chondrocytes. Arthritis Rheum 2003; 48: 1562–1568
- Grossin L, Etienne S, Gaborit N, Pinzano A, Cournil-Henrionnet C, Gerard C, Payan E, Netter P, Terlain B, Gillet P. Induction of heat shock protein 70 (Hsp70) by proteosome inhibitor MG 132 protects articular chondrocytes from cellular death in vitro and in vivo. Biorheology 2004; 41: 521–534
- Tonomura H, Takahashi KA, Mazda O, Arai Y, Inoue A, Terauchi R, Shin-Ya M, Kishida T, Imanishi J, Kubo T. Glutamine protects articular chondrocytes from heat stress and NO-induced apoptosis with Hsp70 expression. Osteoarthritis Cartilage 2006; 14: 545–553
- Etienne S, Gaborit N, Henrionnet C, Pinzano A, Galois L, Netter P, Gillet P, Grossin L. Local induction of heat shock protein 70 (Hsp70) by proteosome inhibition confers chondroprotection during surgically induced osteoarthritis in the rat knee. Biomed Mater Eng 2008; 18: 253–260
- Grossin L, Cournil-Henrionnet C, Pinzano A, Gaborit N, Dumas D, Etienne S, Stoltz JF, Terlain B, Netter P, Mir LM, et al. Gene transfer with Hsp70 in rat chondrocytes confers cytoprotection in vitro and during experimental osteoarthritis. FASEB J 2006; 20: 65–75
- Hojo T, Fujioka M, Otsuka G, Inoue S, Kim U, Kubo T. Effect of heat stimulation on viability and proteoglycan metabolism of cultured chondrocytes: Preliminary report. J Orthop Sci 2003; 8: 396–399
- Tonomura H, Takahashi KA, Mazda O, Arai Y, Shin-Ya M, Inoue A, Honjo K, Hojo T, Imanishi J, Kubo T. Effects of heat stimulation via microwave applicator on cartilage matrix gene and Hsp70 expression in the rabbit knee joint. J Orthop Res 2008; 26: 34–41
- Guillemin F, Virion JM, Escudier P, De Talancé N, Weryha G. Effect on osteoarthritis of spa therapy at Bourbonne-les-Bains. Joint Bone Spine 2001; 68: 499–503
- Li GC, Mivechi NF, Weitzel G. Heat shock proteins, thermotolerance, and their relevance to clinical hyperthermia. Int J Hyperthermia 1995; 11: 459–488
- Otaka M, Yamamoto S, Ogasawara K, Takaoka Y, Noguchi S, Miyazaki T, Nakai A, Odashima M, Matsuhashi T, Watanabe S, et al. The induction mechanism of the molecular chaperone Hsp70 in the gastric mucosa by geranylgeranylacetone (HSP-inducer). Biochem Biophys Res Commun 2007; 353: 399–404
- Kato K, Ito H, Kamei K, Iwamoto I. Stimulation of the stress-induced expression of stress proteins by curcumin in cultured cells and in rat tissues in vivo. Cell Stress Chaperones 1998; 3: 152–160
