Abstract
Objective: The aim is to analyse a modified standardised HILP procedure regarding the response rates, local recurrences and complication rates.
Patients and methods: 152 patients (101 females, 51 males) with an average age of 62 years and locoregionally metastasised malignant melanoma underwent HILP using melphalan and dactinomycin between 1992 and 2007. Using M.D. Anderson's classification at the time of the perfusion 51 patients presented in stage IIIA, 43 patients in stage IIIAB and 58 patients in stage IV. If indicated, lymph node dissection was performed simultaneously just before perfusion of the extremity.
Results: Complete remission was observed in 91 (62.7%) of 145 patients, partial remission in 26 (17.9%) patients. 28 (19.3%) patients showed no response. The overall response rate was 80.7% (117 of 145 patients). Severe complications (Wieberdink IV/V) were seen in eight cases. The average recurrence-free survival was 17 months. The median survival was 39 months; the five-year overall survival rate was 38%. The overall survival rate was significantly influenced by the stage of the disease.
Conclusion: HILP is an efficient therapy for multiple or recurrent in-transit metastases of malignant melanoma of the lower extremities. The efficiency increased by improving the technique of the perfusion. Long-term survival can be observed in patients without regional lymph node metastases or distant metastases.
Introduction
Hyperthermic isolated limb perfusion is a well-known concept in the therapy of locoregionally metastasised malignant melanoma. In 1957, isolated limb perfusion was introduced as a clinical procedure by Creech et al. Citation1. Costs and complexity of the procedure have restricted HILP to a few specialised centres. Since the beginning, important issues in HILP have been temperature of the perfusate, duration of perfusion, type and dose of chemotherapeutic agents used. These technical details have been considered responsible for the different results which have been found in the past Citation2. The first HILP in Germany was performed at the Department of General Surgery in Erlangen in 1975. Since then, our institution has performed over 650 perfusions. The HILP procedure underwent various changes during this period of time. Reasons for these changes were results from our own experimental and clinical studies and experience Citation2, Citation3. Since 1992, the procedure has not been modified and is performed under standardised conditions. Thus, it was of interest to examine our results using this standardised method regarding the response and complication rates.
Patients, material and methods
Between September 1992 and December 2007 HILP was performed in 152 patients with locoregionally metastasised malignant melanoma of the extremities (upper extremity n = 10, lower extremity n = 142). A total of 51 males and 101 females between 28 and 88 years of age were included in the analysis (average 68 years). All patients had tumourous manifestations varying in size and number localised on the extremities. The tumour load was not accurately recorded. All participants had at least one visual or palpable in-transit metastasis (+/− regional lymph node metastases). Those cases presenting with only few tumours (1–3) were all patients with repeated recurrence of in-transit metastases. Most of the patients had multiple (10 or more) in-transit metastases varying in size (1 mm up to several cm). The largest diameter of a metastasis we measured in one of our patients was 8 cm.
M.D. Anderson's system was used to classify the stage of disease Citation4. At the time of the perfusion 51 patients were in stage IIIA according to M.D. Anderson's classification (in-transit metastases), 43 patients in stage IIIAB (in-transit metastasis and regional lymph node metastases) and 58 patients in stage IV (distant metastases). For the further appraisal seven patients had to be excluded because of incomplete or inconsistent records.
The perfusion technique used in our institution has been published in the past Citation3, Citation5. The external iliac vessels were used for the perfusion of the lower extremity and the axillary vessels for the perfusion of the upper extremity.
Regional lymph node dissection of the inguinal, iliac or obturator lymph nodes and axillary lymph nodes (level I-III) was performed in the same operation before the perfusion, in case it had not already been done at an earlier stage of the disease.
Melphalan (1.3 mg/kg body weight for the lower extremity; 0.7 mg/kg body weight for the upper extremity) was administered into the artery at a tissue temperature of 38.5°C for 20 min using a pump. Dactinomycin (1 mg for the lower extremity; 0.5 mg for the upper extremity) was injected as a bolus into the venous line. The subcutaneous tissue temperature was constantly held at a level of 40° to 41.5°C for a period of 90 min. Before disconnecting the system at least 2000 mL of human albumin 5% were administered to wash out the agents in the extremity.
Data was collected prospectively by the tumour registry of the Department of General Surgery, University Medical Centre, Erlangen. The median follow-up period was 26 months (range 1–192). Complication, locoregional recurrence, survival and rates were recorded.
The remission rate was assessed over a period of up to 6 months after perfusion. Complete response was defined as a complete clinical and/or histological remission of all metastases after perfusion. On the other hand, partial response was defined as clinical and/or histological tumour regression of at least 25–50% of the volume of the metastases.
SPSS (Version D 15.0) was used for the statistical analysis and the calculation of the survival rate using the Kaplan–Meier estimation (observed cumulative survival rate). P-values <0.05 calculated with the log-rank test were regarded as significant.
Results
Response rate
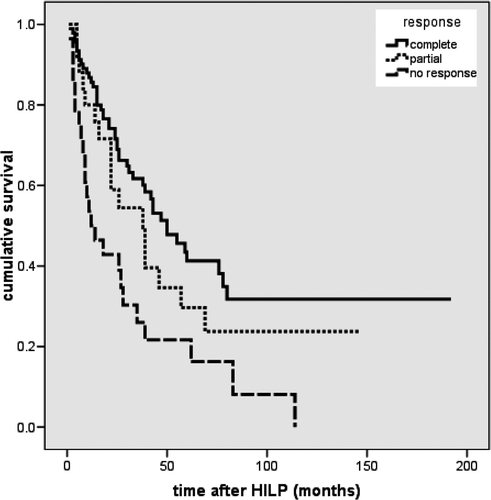
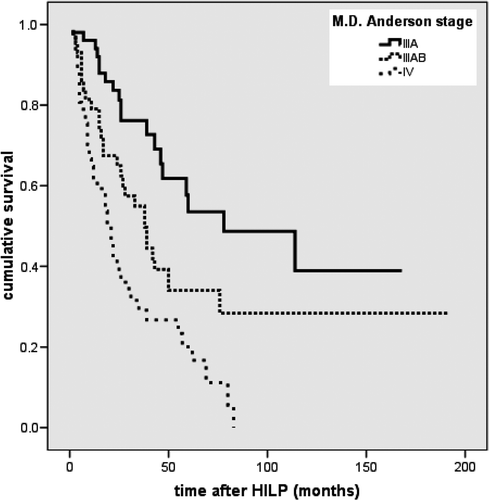
145 patients were eligible for analysis of the response rate. Altogether, 117 patients responded to the therapy (overall response rate 80.7%). In 91 (62.8%) patients a complete remission was observed. Partial remission was seen in 26 (17.9%) patients while 28 (19.3%) patients showed no tumour regression at all. demonstrates the response rate depending on the stage of the disease. In stage IIIA complete remission was observed in 39 (81.3%) of 48 patients, in stage IIIAB in 27 (65.9%) of 41 patients and in stage IV in 25 (44.6%) of 56 patients.
Table I. Local tumour control after HILP according to M.D. Anderson stage.
Pattern of recurrence
The median recurrence-free interval in 91 patients with complete remission was 17 months. Twenty-eight patients died during the follow-up period due to distant metastases without occurrence of locoregional metastases (median survival 17 months). No complete remission lasted longer than 68 months. The staging system according to M.D. Anderson showed a significant correlation (p = 0.01) with the recurrence-free interval.
The median time interval until the development of distant metastases (77 patients) during the follow-up period was eight months. The length of the interval until diagnosis of distant metastasis in patients with stage IIIA/IIIAB correlated significantly (p = 0.001) with local tumour response. After partial tumour regression the median interval free of distant metastases was nine months; after complete tumour regression 27 months (). The length of the interval until distant metastasis occurred in patients with partial vs. complete remission correlated significantly (p = 0.009).
Survival rate
After HILP the median overall survival rate was 39 months (average 67 months) (range 1–92 months). The five-year survival rate was 34%. The survival rate showed significant differences depending on the stage of disease (p < 0.001). The best prognosis was observed in stage IIIA with a five-year survival rate of 54% compared to 47% in stage IIIAB and 34% in stage IV (). The local tumour response rate had a significant influence on the survival rate (p = 0.001).
Complications
Local toxicity was classified according to the Wieberdink scale () Citation6. Eight patients developed severe toxic reactions (Wieberdink IV) necessitating fasciotomy in six and major amputation (above knee) in two cases. One of the amputated patients had peripheral vascular disease. The other participants showed toxicity grades II–III.
Table II. Wieberdink classification Citation6.
An 82 year-old patient with breast cancer, malignant pleural effusion and multiple, ulcerated in-transit metastases of the upper extremity died on day 14 after surgery due to multiple organ dysfunction syndrome.
The most common complication was persistent lymph secretion in 49 cases. In these cases we left the drains in the wound for longer than 10 days, up to four weeks. Six patients developed a lymphocele after removal of the drains. These lymphoceles were either punctured or drained again.
Seventeen patients showed signs of bone marrow depression which recovered spontaneously: anaemia (n = 12, probably also due to intraoperative loss of blood), leucopenia (n = 8), leucopenia and thrombopenia (n = 1).
Deep wound infections occurred in eight patients whereas 18 patients had superficial infections ().
Table III. Postoperative complications.
Discussion
HILP has proved to be a good therapeutic option in patients with recurrent multiple in-transit metastases of malignant melanoma of the extremities. The comparison of data collected in different centres is difficult. This is not only due to heterogeneity in the groups of patients treated and the lack of standardised surgical procedures but also to the difficulty of quantifying the exact tumour load.
Recent data in international literature shows that HILP is superior to isolated limb infusion (ILI) Citation7. For isolated limb infusion, a complete response rate in 30% and a partial response rate of 14% was reported in patients with intransit metastasis of malignant melanoma. Furthermore, data show that the median recurrence-free interval of complete response rate after isolated limb infusion is 12 months Citation7. Furthermore, the efficiency of HILP was improved by modification of the procedure. In our institution we were able to raise the complete remission rate from 49% Citation3 to 62.7%. It was not possible to confirm the initially reported good response rates after administration of TNF-alpha during perfusion in long-term studies Citation8–10. The results using melphalan (+/− Actinomycin D) in the perfusate can be compared with the TNF results Citation11. One can infer from this comparison that TNF has no advantage over the chemotherapeutic agents used so far, although a perfusion regimen including TNF does seem to have a positive effect in patients with bulky disease Citation12.
In our study we were also able to show that the local response rate after HILP depends on the stage of disease (M.D. Anderson) at the time of perfusion. The best results were achieved in patients who presented with in-transit metastases only (stage IIIA). The local response rate also had a significant influence on the survival rate.
Furthermore, a significant correlation of local response and the time interval until occurrence of distant metastases could be demonstrated. In the case of a complete remission the time till occurrence of distant metastases was significantly prolonged. In consequence, the local tumour response rate could have an indirect effect on the prognosis, which is dependent on the systemic spread of the disease. There is still a lack of statistical proof for this hypothesis.
Locoregional recurrence is still a problem after complete remission. In the literature there are reports of recurrence rates up to 70% and local recurrence-free intervals of 9.5–16 months Citation13, Citation14. Repeated perfusions of recurrent locoregional metastases with melphalan and/or TNF have also been discussed, but on the account of a potentially increased local toxicity. This approach is left to personal preference. Adjuvant therapy after complete remission is not established yet and should be a task for further studies.
The extent of locoregional tumour load has an influence on the local response rate as well as on the survival rate. Our five-year survival rate of 34% correlates with those found in the international literature, which vary from 28% to 73% Citation15, Citation16. Synchronous regional lymph node metastases significantly deteriorate the prognosis. Still an acceptable number of these patients can reach a five-year overall survival rate or even longer. In these cases it is advisable to perform HILP in combination with lymph node dissection, albeit possible side effects such as persistent postoperative fistulae, lymphatic congestion or oedema and wound infections.
In five patients with stage IV disease it was possible to achieve a survival of over five years after HILP. These were patients with distant metastases in lymph nodes (iliac or obturator lymph nodes) which were dissected during surgery. In these cases literature reports five-year survival rates of up to 20% Citation17.
The toxicity of the perfusion is tolerable in the face of the prognosis of the disease. Data in the literature show that neurological impairment of the affected limb is rare and usually reversible Citation18. The long-term functional outcome after HILP has already been published Citation19. Peripheral vascular disease must be excluded beforehand to ensure adequate perfusion and oxygenation of the limb. From our experience it is advisable to keep the ischaemic time as short as possible. Also, the haematocrit in the perfusate should be checked regularly and corrected if necessary. The haematocrit should be kept beyond 25–30%, because the risk of compartment syndrome increases with lower values. In three of six patients with grade IV toxicity the haematocrit of the perfusate was less than 20%.
Conclusion
In summary, HILP is an efficient therapy for multiple or recurrent in-transit metastases of malignant melanoma of the lower extremities. The procedure was made more efficient by improving the technique of the perfusion. Prophylactic HILP is not advisable according to a large, randomised multicentre study Citation20. Palliative HILP to save a limb affected by distant metastases may be indicated in selected patients. Long-term survival can be achieved in patients who present without regional lymph node metastases or distant metastases and who respond well to the treatment.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Creech OJ, Jr, Krementz ET, Ryan RF, Winbald JN. Chemtherapy of cancer: Regional perfusion utilizing an extracorporal circuit. Ann Surg 1958; 148: 616–632
- Hohenberger W. Isolation perfusion for malignant melanomas: Established facts and parameters to be clarified. Progress in regional cancer therapy, R Jakesz, H Rainer. Springer, Berlin, Heidelberg, New York 1990; 182
- Meyer T, Göhl J, Haas C, Hohenberger W. Hyperthermic isolated limb perfusion–23 years’ experience and improvement of results by modification of technique. Onkologie 1998; 21: 198–202
- Smith JL. Histopathology and biological behaviour of melanoma. Neoplasms of skin and malignant melanoma, JL Smith. Year Book Medical Publishers, Chicago 1976; 293
- Knorr C, Meyer T, Janssen T, Goehl J, Hohenberger W. Hyperthermic isolated limb perfusion (HILP) in malignant melanoma. Experience with 101 patients. Eur J Surg Oncol 2006; 32: 224–227
- Wieberdink J, Benckhuijsen C, Braat RP, van Slooten EA, Olthuis GA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol 1982; 18: 905–910
- Beasley GM, Petersen RP, Yoo J, McMahon N, Aloia T, Petros W, Sanders G, Cheng TY, Pruitt SK, Seigler H, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: A well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol 2008; 15: 2195–2205
- Lienard D, Eggermont AM, Kroon BR, Koops HS, Lejeune FJ. Isolated limb perfusion in primary and recurrent melanoma: Indications and results. Semin Surg Oncol 1998; 14: 202–209
- Lienard D, Lejune FJ, Ewalenko P. In transit metastases of malignant melanoma treated by high dose rTNFα in combination with interferon-γ and melphalan in isolation perfusion. World J Surg 1992; 16: 234–240
- Vaglini M, Belli F, Ammatuna M, Inglese MG, Manzi R, Prada A, Persiani L, Santinami M, Santoro N, Cascinelli N. Treatment of primari or relapsing limb cancer by isolation perfusion with high-dose alpha-tumor necrosis factor, gamma-interferon and melphalan. Cancer 1994; 73: 483–492
- Cornett WR, McCall LM, Petersen RP, Ross MI, Briele HA, Noyes RD, Sussman JJ, Kraybill WG, Kane JM, 3rd, Alexander HR, et al. American College of Surgeons Oncology Group Trial Z0020. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol 2006; 24: 4196–4201
- Di Filippo F, Cavaliere F, Garinei R, Anzà M, Di Angelo P, Psaila A, Piarulli L, Callopoli A, Bruno P, Di Filippo S, et al. TNFα-based isolated hyperthermic limb perfusion (HILP) in the treatment of limb recurerent melanoma: Update 16 years after its first clinical application. J Chemother 2004; 16: S62–65
- Rossi CR, Foletto M, Pilati P, Mocellin S, Lise M. Isolated limb perfusion in locally advanced cutaneous melanoma. Semin Oncol 2002; 29: 400–409
- Thompson JF, Hunt JA, Shannon KF, Kam PC. Frequency and duration of remission after isolated limb perfusion for melanoma. Arch Surg 1997; 132: 903–907
- Krementz ET, Sutherland CM, Muchmore JH. Isolated hyperthermia chemotherapy perfusion for limb melanoma. Surg Clin North Am 1996; 76: 1313–1330
- Di Filippo F, Calabrò A, Giannarelli D, Carlini S, Cavaliere F, Moscarelli F, Cavaliere R. Prognostic variables in recurrent limb melanoma treated with hyperthermic antiblastic perfusion. Cancer 1989; 63: 2551–2561
- Meyer T, Merkel S, Goehl J, Hohenberger W. Surgical therapy for distant metastases of malignant melanoma. Cancer 2000; 89: 1983–1991
- Pace M, Gattai R, Matteini M, Mascitelli EM, Bechi P. Toxicity and morbility after isolated lower limb perfusion in 242 chemo-hyperthermal treatments for cutaneous melanoma: The experience of the Tuscan Reference Centre. J Exp Clin Cancer Res 2008; 27: 67
- Knorr C, Melling N, Goehl J, Drachsler T, Hohenberger W, Meyer T. Long-term functional outcome after hyperthermic isolated limb perfusion (HILP). Int J Hyperthermia 2008; 24: 409–414
- Koops HS, Vaglini M, Suciu S, Kroon BB, Thompson JF, Göhl J, Eggermont AM, Di Filippo F, Krementz ET, Ruiter D, et al. Prophylactic isolated limb perfusion for localized, high-risk limb melanoma: Results of a multicenter randomized phase III trial. European Organization for Research and Treatment of Cancer Malignant Melanoma Cooperative Group Protocol 18832, the World Health Organization Melanoma Program Trial 15, and the North American Perfusion Group Southwest Oncology Group-8593. J Clin Oncol 1998; 16: 2906–2912