Abstract
Purpose: The goal of this study was to determine whether short-duration (15 s–3 min) high-temperature (50°C) heat shocks inhibit the repair of DNA damage.
Materials and methods: Cultured HeLa cells were used. DNA damage was measured after UV exposure or X-irradiation. Three methods were used to measure DNA damage: alkaline comet assay with the endonuclease, UVDE, for single strand breaks and UV photoproducts, antibodies specific for cyclo-pyrimidine dimers (CPD) or for 6-4 photoproducts (64PP), and the appearance-resolution of γ-H2AX foci for DNA double strand breaks.
Results: Heat shocks of 15–30 s at 50°C inhibited repair of DNA damage after UV exposure or X-irradiation detected by the alkaline comet assay (after UV) or by persistence of γ-H2AX foci (after X-rays). The phosphorylation of histone, H2AX, induced by 1 or 4 Gy of X-rays was inhibited in a time-dependent manner after 15–45 s at 52°C. When the excision of UV-induced PP was measured, heat shocks of more than 60 s at 50°C were required to show measurable inhibition.
Conclusion: Severe (50°C) short-duration (15 s or greater) heat shocks inhibit repair of UV-induced DNA damage. The ability to detect the inhibitory effects of very short, 15–60 s, heat shocks was assay dependent. The comet assay could detect repair inhibition after a 15-s heat shock. Detection of DNA damage by specific antibodies could only detect repair inhibition after 1–3-min heat shocks. Using the γ-H2AX foci method 30 s at 50°C induced a significant delay in the repair of DNA damage after 1 Gy of X-rays.
Introduction
Recent work has shown a renewed interest in the effects of high-temperature (48–52°C), short-duration (<5–10 min) hyperthermia, i.e. severe heat shocks Citation1–11. Renewed interest in severe heat shocks is due to two developments. First is the medical application of thermal ablation Citation1–8. Although thermal ablation uses heat to destroy the target tissue, the surrounding tissue can be subjected to a range of heat shocks up to and including severe heat shock. Thus, thermal ablation could be combined with radiation therapy Citation9. Second is the application of millimetre wave (MMW) technology in both civilian and military application Citation10–11. MMW has the potential to increase the skin temperature rapidly (+15°C in 1–3 s), which makes its use as a non-lethal deterrent attractive. For more detail on the use of MMW as a non-lethal deterrent see www.janes.com, search ‘active denial’. However, the biological effects of these exposures remain uncertain. Since the only established effect of MMW exposures is thermal, one approach is to consider these exposures as simple heat shocks. Thus it is conceivable that current knowledge of the effects of hyperthermia on DNA repair would allow us to predict the effects of short-duration severe heat shocks. However, the published data covers only the 43–47°C range Citation12–13. Questions arise as to whether one can extrapolate from our knowledge of 43–47°C heat shocks to the very short-duration 50°C heat shocks. Therefore we have undertaken a study to determine the effects of very short-duration 50°C heat shocks on DNA repair. Because skin exposed to MMW in the field would also likely be exposed to UV at the same time we chose UV as the DNA damaging agent in this study.
It has long been recognised that heat shock inhibits DNA repair Citation12–15. Any thermal effect on DNA repair would be of relevance to both clinical application and regulatory concerns because incomplete or defective DNA repair is associated with the increased genomic instability and increased carcinogenic potential. Thus hyperthermic inhibition of DNA repair could increase the risk of cancer due to exposure to DNA damage agents such as UV light. On the other hand, inhibiting DNA repair in a thermal ablation setting may increase the potential effects on surrounding tissue and alter strategies for adjuvant therapies. Therefore, knowledge of the effects of short-duration severe heat shocks on DNA repair should be relevant to both risk assessment and medical application. To this end we have undertaken a study to determine whether short-duration severe heat shocks inhibit the repair of DNA damage.
For the present study we have emphasised the risk assessment aspects of the issue by focusing on the thermal dose that is imparted by MMW exposure and its effects on the repair of UV-induced DNA damage. One potential hazard from MMW exposure in the field could come from exposure to sunlight if the thermal dose imparted to the skin could inhibit the repair of UV-induced DNA damage. Further, the effects of hyperthermia on that pathway have been studied less extensively than those of other pathways. While UV exposure causes a wide variety of DNA lesions, among the numerous are cyclobutane pyrimidine dimers (CPD) and 64-photoproducts (64PP) Citation16–17. We used two assays for DNA damage after UV exposure; one measures CPD and 64PP directly using antibodies to these photoproducts, and the second measures their presence indirectly using a specific endonuclease (UVDE) to introduce strand breaks at the DNA sites which contain photoproducts and measuring the resulting single strand DNA breaks measured by the alkaline comet assay. In addition, because thermal ablation can be combined with radiotherapy, we included one study with X-rays by measuring γ–H2AX formation and resolution.
Materials and methods
Cell lines, culture methods and viability measurements
HeLa cells were routinely cultured in a-MEM supplemented with 10% newborn calf serum. RKO human colon carcinoma cells were obtained from ATCC. They were grown at 37°C at 5% CO2 in MEM containing 10% fetal calf serum and antibiotics. Cells were subcultured into 100 mm culture dishes 24–48 h prior to heating and/ or UV exposure. Cell counts and cell viability (i.e. fraction of trypan blue negative cells) were routinely measured using a Vi-cell from Beckman Coulter according to the manufacturer's direction.
UV exposure
An UVP Black-Ray Lamp, model XX-15M, (UVP, Upland, CA) was used as the UVB source. Prior to UV exposure, each day the UV flux was measured with a NIST traceable photometer, model IL1400A, (International Light, Newburyport, MA). Monolayer HeLa cells, growing in a 100 mm culture dish were prepared for exposure by washing the monolayer with PBS and scraping the cells from the outer edge of the plate to ensure that that no cells received a reduced exposure due to diffraction or shadows from the dish wall. A small volume of warm 37°C PBS (1.5 mL) was added to the monolayer to keep the cells moist but not deeply covered, while the UV exposure was delivered with the dish cover absent. After UV exposure some of the dishes were processed immediately for DNA damage assays (see below). Warm (37°C culture media) was added to the remaining dishes, which were returned to the incubator for the indicated repair time, after which the plates were prepared for DNA assay, as described below.
Hyperthermia exposure and temperature monitoring
Heating of the cell solutions was performed in covered plastic Petri dishes placed on a heating plate. The heating plate had built-in means to automatically keep the plate temperature at a preset value.
Routine heating was done as follows. Media was quickly aspirated from the plate and replaced with media at 50°C and placed on the heating block. After the heating time, the media was aspirated. The indicated heating time is the time between the addition of 50°C media and the end of aspiration. Note that the rapid cooling after media aspiration can be seen in , line t3.
Figure 1. Temperature profiles in typical experiments. The heating system was set to test four different target temperatures: 38°C (t1), 49°C (t2), 50°C (t3) and 56°C (t4). All curves used media preheated to the indicated target temperature. The 50°C (t3) line shows the temperature transients that occurred when preheated culture medium was aspirated and replaced with 37°C culture media. In view of the long temperature transient, all experiments used preheated culture media.
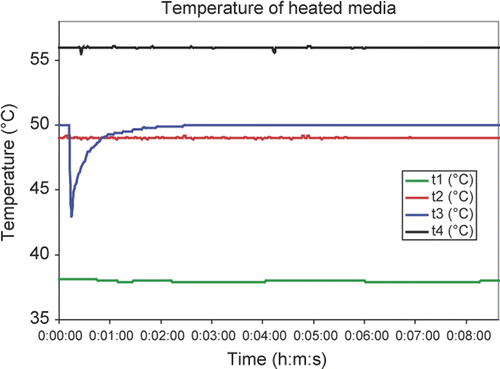
For temperature monitoring the Petri dishes were modified to allow additional monitoring of temperature directly in the solution. A small hole of 0.3 mm in diameter was drilled into the side of two dishes and a thin needle thermocouple (0.2 mm, HYP-0, OMEGA Engineering, Stamford, CT) was pulled through the dish wall. The tip of the thermocouple was placed to the centre and about 1 mm above the bottom of the dish. This assured temperature measurement in the centre of the solution volume assuming a 2-mm solution layer. Silicon adhesive was applied to the drilled opening to seal the volume around the thermocouple. The thermocouple was connected to a PC-controlled thermometer (DatAq, DI-1000TC, Akron, OH) for data collection. Temperature in the solution was measured and recorded in 2 s intervals, as shown in for several temperatures, from below to above the temperatures studied and for time intervals greater than those to which cells were heat shocked. These results show that with the addition of preheated media, good temperature control was achieved.
DNA damage assays
We used two assays for DNA damage after UV exposure. One assay, the alkaline comet assay was used because of its reported sensitivity and potential to respond to a variety of damage products Citation18–20. The second assay, an antibody detection method was used because this method is specific for individual lesions.
Alkaline comet assay with endonuclease digestion
Cells in100 mm plates were prepared for the comet assay as follows. After removing the culture media, 5 mL of Hank's BBS with 20 mM EDTA was added to the monolayer and the cells scraped from the dish, suspended in a larger volume of this solution and diluted 1/10 in a 1% solution of low melting-point agarose (Invitrogen, Carlsbad, CA) and layered onto pre-warmed, pre-coated flare slides. After being allowed to gel on ice for 3 min, the slides were placed in a Coplin jar filled with lysis solution (0.1 M EDTA, 30 mM NaOH, 1.2 M NaCl and 0.1% sucrose) and allowed to lyse overnight at 4°C. After lysis the slides were treated with UVDE (Trevigen and a gift from Paul Doetsch, Emory University) to introduce DNA strand breaks at the site of UV photoproducts according the manufacturer's instructions. After UVDE digestion slides were placed in the electrophoresis unit in alkali buffer (30 mM NaOH, 500 mM EDTA) and kept at 4°C for 30 min to allow DNA unwinding. Slides were electrophoresced at 18 V for 25 min. Slides were stained with a 5 mg/mL solution of propidium iodide (PI) and analysed on an Olympus inverted microscope using an Optimus image analysis system. Our software could measure four parameters reflecting the extent of DNA migration. (1) Comet length is overall length of the comet in the direction of DNA migration. (2) The DNA per comet moment is the PI intensity for each pixel summed over all of the comet pixels. (3) The fraction of DNA in the comet head is the summation of the PI intensities for the pixels in the comet's head divided by that for the entire comet. (4) The comet moment is the sum of the product of the PI intensity and pixel's distance from the comet centroid (positive in the direction of DNA migration and negative in the direction opposite that of DNA migration) divided by the DNA per comet. These parameters were analysed for their ability to reflect increasing DNA damage and decreasing DNA damage during repair (see Results).
Detection of UV photoproducts using antibodies to DNA fragments containing CPDs or 64PPs
The frequency of UV photoproducts in DNA was measured using antibodies specific for DNA containing either CPDs or 64PPs (MBL International, Woburn, MA). Basically, the assays were done according to the manufacturer's directions, except that the final antibody dilutions were optimised for sensitivity and specificity. Briefly, DNA is isolated from exposed or unexposed cells using the Qiagen DNeasy tissue kit according to the manufacturer's directions. Note that this procedure shears the DNA by vortexing, which is then denatured so that the antibody can react with the oligonucleotides containing the photoproducts. The DNA samples were placed in precoated multi-well plates and dried overnight. Antibodies to either CPD (dilution 1:1500) or 64PP (dilution 1:1000) were added to each well and the plates incubated at 37°C for 30 min. We then added to each well 100 µL 1:2000 Biotin-F(ab) fragment of anti-mouse IgG(H + L), Zymed, and incubated the plates at 37°C for 30 min. The plates were washed five times with PBS-T buffer (150 µL per well) then 100 µL per well of peroxidase-streptavidin (1:10,000) Zymed was added and incubated at 37°C for 30 min. Plates were washed five times with PBS-T buffer (150 µL per well). Plates were then washed once with 150 µL per well citrate-phosphate buffer, pH 5 (Sigma, P4922-100CAP (dissolve 1 capsule in 100 mL double distilled water)). Buffer was kept in wells until the next substrate solution was ready. The buffer was discarded and 100 µL per well of substrate solution (o-Phenylenediamine tablets; Sigma, P-5412. D 1 tablet dissolved in 50 mL of citrate-phosphate buffer) was added and incubated at 37°C for 30 min. An aliquot of 50 µL per well of 3 M H2SO4 was added to each well to stop enzymatic reaction. The absorbance at 492 nm was measured for each well.
Immunofluorescence
Cells growing on 9 × 9 mm coverslips in monolayers in a 60 mm dish were subjected to various experimental treatments, washed three times with PBS and then fixed in 3.7% formaldehyde containing 0.2% Triton X-100 for 10 min. After three washes in PBS, coverslips were incubated with a 1:200 dilution of a polyclonal antibody against γ-H2AX (Upstate Biotechnologies, Temecula, CA), washed three times with PBS, followed by incubation with a 1:500 dilution of Alexa-596 tagged anti-rabbit antibody (Molecular Probes, Eugene, OR). After three more washes with PBS, coverslips were mounted in 1% Moviol. Images were captured using a 100 × Planapo NA1.4 objective of an Olympus BX 40 microscope and a Diagnostic Instruments Spot Pursuit CCD camera.
Irradiation
Control cells or pre-heated cells were treated with ionising radiation from a Pantak (Branford, CT) model PMC1000 X-ray generator operated at 250 kV and 12 mA. Irradiation was performed in a chamber containing 5% CO2 at 37°C. For cells exposed to combined treatments, the time interval between the various treatments was less than 2 min.
Calculation of percentage DNA damage remaining and percentage repair inhibition
In order to increase the sensitivity of the comet assay, we calculated the damage level at each measured time point as a fraction of the initial damage measured by a given parameter. Then the repair data was normalised by converting the fractions to percentage. This approach allowed us to average the repair data obtained from all four measured comet parameters thereby making a more sensitive measure of any thermal effects.
Results
Four comet parameters as a function of UV exposure
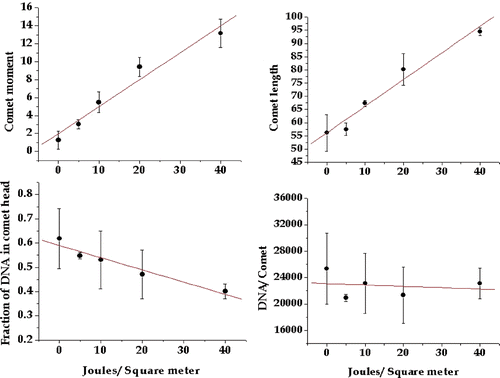
In order to evaluate the utility of the comet assay for measuring the DNA damage induced by UV we exposed HeLa cells to increasing UV fluence, immediately ran the alkaline comet assay as described above, and analysed the comet images for four parameters. The comet length is a measure of overall DNA migration and emphasises the effects of the smaller fragments. The comet moment reflects the distance migrated weighted by the DNA migrating that distance and reflects the extent of damage throughout the genome. The fraction of DNA remaining in the comet head reflects the extent of undamaged DNA. DNA per comet reflects the loss of DNA that has been broken into very small pieces. All four comet parameters changed as a function of UV exposure (); both the comet moment and the comet length increased with increasing UV exposure, while the fraction of DNA remaining in the head and DNA per comet decreased with increasing UV exposure. We concluded that all of the parameters would be useful indicators of the extent of DNA damage, even though the change in DNA per comet appeared rather modest in this study, due to what appeared to be a high value at 40 J/m2 (see below).
Figure 2. Comet parameters reflecting DNA damage as a function of UV dose. Four comet parameters were tested to determine which showed a linear response with increasing UV exposure. The comet moment, comet length, fraction of DNA in the comet head and total DNA in the comet were measured as described in the text and plotted against UV fluence. The plotted points are the means of at least three experiments and the bars are one standard error of the mean.
Four comet parameters as a function of repair and their use for detecting the effects of high-temperature–short-duration heat shocks
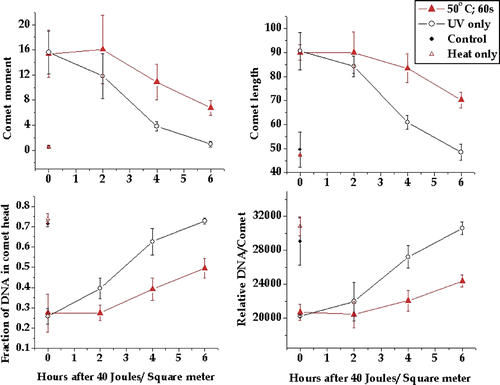
We next considered whether the four comet parameters would change significantly as the DNA damage was being repaired, and if in turn they would be sensitive enough to detect any heat-induced inhibition of DNA repair. After exposing HeLa cells to 40 J/m2, an exposure producing a level of DNA damage that requires several hours to repair, we measured the extent of DNA damage by the comet assay at 2, 4 and 6 h after exposure. All four of the comet parameters showed a significant level of DNA damage after 40 J/m2 and reduction of the damage levels could be seen at 2 and 4 h of repair time (). At 6 h of repair time, the values of all four comet parameters had returned to their respective pre-exposure levels, indicating that DNA repair was complete. In this study the DNA per comet parameter showed an excellent repair response and a significant change after 40 J/m2. This result is in contrast to that shown in . We cannot explain why this parameter showed an excellent repair response but a poor dose response. However, we believe that the more robust results in are due to improved experience with the assay. Thus, all four parameters were used in subsequent assays.
Figure 3. Inhibition of DNA damage repair by a 60-s 50°C heat shock. Four comet parameters were tested to determine which showed the effect of repair with increasing time after UV exposure. In addition, the parameters were tested to determine if they could show inhibitory effects of a 60-s 50°C heat shock on the repair of UV-induced DNA damage. The comet moment, comet length, fraction of DNA in the comet head and total DNA in the comet were measured as described in the text. The plotted points are the means of at least three experiments and the bars are one standard error of the mean. The comet parameters for cells not exposed to UV are shown on each graph. Similar results were obtained with 30-s and 15-s 50°C heat shocks except that the levels of DNA repair inhibition were progressively smaller. Data not shown.
To determine whether the test comet parameters were sensitive to the inhibitory effects of heat shock we heat shocked HeLa cells for 60 s at 50°C prior to a 40 J/m2 UV exposure. A heat shock of 60 s at 50°C clearly inhibited the repair of DNA damage as detected by all four comet parameters. In contrast to the unheated cells no detectable DNA repair was observed during the first 2 h after UV exposure (). Further DNA damage that was detectable by all four comet parameters remained at 6 h after UV exposure. Thus 60 s at 50°C induced significant inhibition of the repair of UV-induced DNA damage.
Heat dose reduction
We next determined whether 15-s or 30-s heat shocks could induce detectable inhibition of DNA repair. When HeLa cells were exposed to 40 J/m2 immediately after a 30-s 50°C heat shock, detectable inhibition of repair was apparent for each of the four comet parameters at 4 and 6 h after UV exposure (). At 2 h there was little difference between heated and unheated in terms of the DNA damage level, suggesting that any difference in the rate of repair was not observable until after 2 h (see Discussion). After a 15-s, 50°C heat shock there was little difference between the levels of DNA damage at any of the time points for each of the four comet parameters (). However, the comet moment showed a significant difference between heated and control at 6 h after UV exposure. This result suggested that the effects of 15 s at 50°C were near the limit of detection for the comet assay.
Figure 4. Inhibition of DNA damage repair by a 30-s 50°C heat shock. The comet moment, comet length, fraction of DNA in the comet head and total DNA in the comet were tested to determine if they could detect inhibitory effects of a 30-s 50°C heat shock on the repair of UV-induced DNA damage. The plotted points are the means of at least three experiments and the bars are one standard error of the mean. The comet parameters for cells not exposed to UV are shown on each graph.
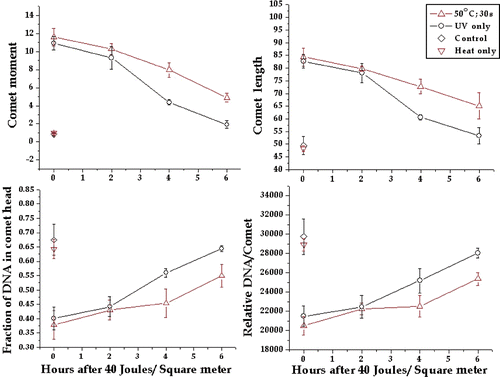
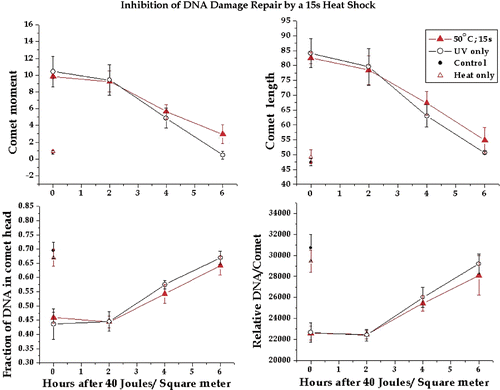
Figure 5. Inhibition of DNA damage repair by a 15-s 50°C heat shock. The comet moment, comet length, fraction of DNA in the comet head and total DNA in the comet were tested to determine if they could detect inhibitory effects of a 15-s 50°C heat shock on the repair of UV-induced DNA damage. The plotted points are the means of at least three experiments and the bars are one standard error of the mean. The comet parameters for cells not exposed to UV are shown on each graph.
Thermal dose response for DNA repair inhibition and analysis of combined comet parameters
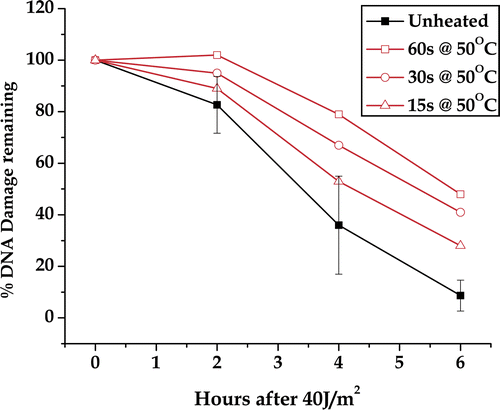
To determine a thermal dose response for DNA repair inhibition we converted the repair curves to percentage damage remaining by dividing the amount of damage measured at a given repair time by the amount of damage at zero repair time. Then the percentage repair inhibition was calculated as the reduction in the percentage repaired at each measured repair time point. This normalisation was necessary because the repair parameters had different values and direction of change. The percentage repair inhibition was essentially a linear function of heating time for each comet parameter studied (data not shown). This result suggested that additional sensitivity for the comet assay could be gained by combining the four parameters and the thermal dose response could be extrapolated to lower heating times. The percentage DNA damage remaining for all four comet parameters was averaged and plotted as a function of repair time (). Plotting each of the individual DNA repair curves for unheated cells separately shows the variation in the measurement of the DNA repair curve. Using the data points in these curves (which are means) a mean of means was calculated as well as the standard error of the mean (only shown for the unheated samples, ). Following a 60-s heat shock, all time points were beyond the 95% confidence limit (i.e., twice the standard error of the mean) of the data points on the curve for unheated cells. After the 30-s heat shock, the 4 h and 6 h repair time points were beyond the 95% confidence limit of the control data. After the 15 s heat shock only the 6 h repair point was beyond the 95% confidence limit. This result suggests that a 15-s 50°C heat shock can inhibit the repair of UV-induced DNA damage, but the extent of inhibition is at the limit of detection by the alkaline comet assay, one of the most sensitive assays for measuring DNA damage. Note that the use of four parameters averaged as above reduced variation and made the assay more sensitive.
Figure 6. DNA damage remaining as a function of time after UV exposure. The data shown in along with the results for a 30-s and 15-s heat shock at 50°C (not shown) were converted to percentage damage remaining for each comet parameter measured. The plotted points are the average of the four metrics of DNA damage. The controls have been combined from all three experiments. The error bars are 95% confidence limits. Error bars for the heated samples were omitted for clarity.
Measuring CPD and 64PP directly as a function of UV exposure
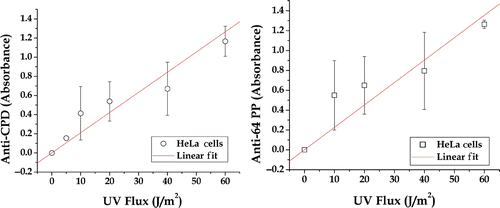
While the alkaline comet assay is a very sensitive assay, it is not specific for known DNA lesions. We therefore extended our studies to measure the effects of short-duration high-temperature heat shocks on the repair of two specific UV photoproducts; CPD and 64PP. These photoproducts can be measured in DNA isolated from cells exposed to UV light. The first step was to measure the exposure response (). The exposure responses for both photoproducts were linear, even though some of the individual points showed significant variation. It can also be seen that the antibody-based assays are less sensitive than the alkaline comet assay (compare and ). The comet moment and comet length parameters showed a larger change between 0–60 J/m2 than either antibody-based assay. Therefore we chose an exposure of 60 J/m2 as the UV exposure that gave sufficient damage so that repair kinetics could be measured.
Effects of high-temperature short-duration heat shocks on the repair of CPD and 64PP
To measure the effects of heat shock on the repair of UV-induced DNA photoproduct cells were exposed to 1 min at 50°C, then exposed to 60 J/m2, and allowed to repair for 1–6 h. While an inhibition of removal of 64PP was detectable after 1 min at 50°C heat shock, there was no detectable effect on the removal of CPD (). We next determined whether we could detect an inhibitory effect of a higher heat shock on the repair of CPDs. After a 3-min 50°C heat shock there was a clear inhibition of repair of CPD with an even greater inhibition of the repair of 64PP (). We did not extend these studies to longer heat shocks because significant levels of immediate cell death (as measured by trypan blue uptake) were observed after 5- (67 ± 15% trypan blue negative) and 7- (20 ± 13% trypan blue negative) min heat shocks. In contrast, after a 3-min 50°C 97 ± 2% of the cells were trypan blue negative). The presence of dead and disintegrating cells would confound DNA damage and repair assays. Note that the reason for doing the trypan blue viability assay was to assure that immediate cell death was not confounding any DNA repair of the assays. The viability studies show that almost 100% of the cells were intact at the time of the DNA damage assays.
Figure 8. Repair of UV-induced DNA CDP and 64PP in heat shocked and control HeLa cells. The relative fraction of the indicated UV photoproduct remaining per unit DNA as a function of repair time. The points are an average of three or more experiments ±the standard error.
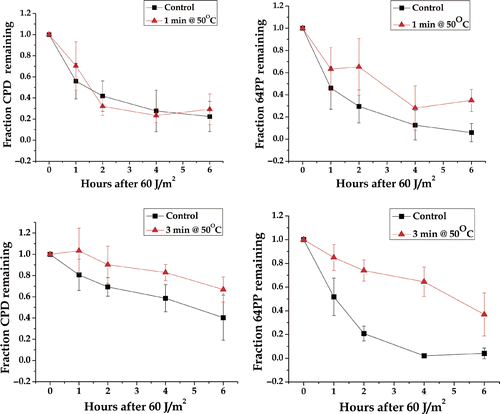
These results show that the antibody-based assays for CPD and 64 PP in are less sensitive than the comet assay at detecting the effects of heat shock on the repair of UV-induced DNA damage (compare with ). Also there is the possibility that heat shock has less of an effect on the removal than on the overall process of which the re-ligating of strand breaks would be a late step. We found no evidence that UV exposure combined with heat for less than 3 min contributed to immediate cell death.
Effects on the γ-H2AX response to DNA damage from X-irradiation
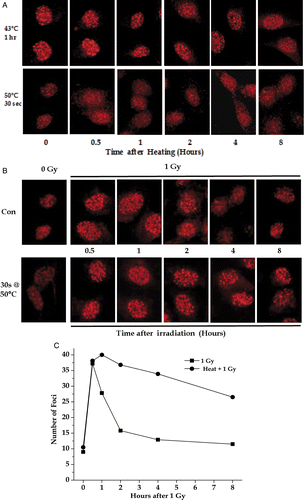
To determine whether short-duration severe heat shocks would inhibit the repair of DNA damage induced by ionising radiation, control and heated RKO cells (30 sec at 50°C) were irradiated with 1 Gy as described under Materials and methods and then analysed for γ-H2AX foci (). Unlike the observations that moderate (43–45°C) hyperthermia induced γ-H2AX foci in the absence of DNA damage Citation22–23, 30 s at 50°C did not induce γ-H2AX foci (see ). Thus γ-H2AX foci could be used to monitor irradiation-induced DNA damage as was done for irradiation without heat Citation24. γH2AX foci were counted visually under a microscope of 1000 × magnification without knowledge of the experimental history of the sample being scored. Most foci are readily distinguishable as a round or ova spot, which is ∼5-fold brighter than background. Foci are almost always separated from each other after low (1–4 Gy) radiation doses. While the initial number of γ-H2AX foci was the same after 1 Gy with or without heat shock, the resolution of the foci was delayed in the heated-irradiated cells as can be seen by the unresolved γ-H2AX foci at 2 and 4 h after 1 Gy in the heated cells (). Quantification of these images showed that only minimal resolution of γ-H2AX foci occurred even 8 h after 1 Gy of X-rays (). Delays in the resolution of γ-H2AX foci have been associated with increased radiation sensitivity Citation24–28 suggesting that 30 s at 50°C can be a radiosensitising thermal dose. Similar studies with HeLa cells (data not shown) gave results consistent with those RKO cells.
Figure 9. The effects of acute heat shock on radiation-induced γ-H2AX foci. Exponentially growing RKO cells on coverslips were heated at 43° or 50°C and allowed to recover at 37°C for various lengths of time (A) or irradiated with 1 Gy with or without a previous heat shock of 30 sec at 50°C and then allowed to recover at 37°C for various lengths of time (B). At various time points, the coverslips were stained with an anti γ-H2AX antibody (A and B). The number of γ-H2AX foci per cell after irradiation was determined (C).
Discussion
The results presented in this study clearly show that short duration, i.e. less than 1 min of severe hyperthermia can inhibit the repair of DNA damage induced by either UV or ionising radiation. Further, the time of thermal exposures that produce measurable inhibition of DNA repair can be rather short for high-temperature heat shocks. For example heat shocks as short as 15 s produced detectable, albeit small, reductions in repair rate of UV-induced DNA damage as detected by the alkaline comet assay with UVDE digestion. In contrast, methods based on the use of antibodies to detect specific UV-induced lesions, CPD and 64PP, were only able to detect an inhibition in removable rate after 1 min at 50°C. This difference can be due to the fact that these assays could only detect a single type of lesion while the comet assay was sensitive to all types of damage that lead to a single strand break in the presence of the endonuclease, UVDE. It is also possible that the antibody method is less sensitive overall than the alkaline comet method, which is considered by many researchers to be the most sensitive assay for DNA damage Citation20. Even with the differences in sensitivity, results from the two methods are consistent with the conclusion that short-duration (on the order of 15 s–60 s) exposures to severe heat shocks produce a detectable inhibition of the DNA repair. The next question is ‘what is the biological significance of such inhibition?’ While the answer is beyond the scope of this paper in that additional experiments are needed to determine whether these heat shocks would enhance UV-induced transformation or lethality. However, it can be noted that the specific assays for CPD and 64 PP only used removal of damage as an endpoint. Complete DNA repair requires several more steps, including repair synthesis and chromatin reassembly. Both of these steps in post-UV repair have been reported to be heat sensitive Citation14. In this regard, the comet assay detects unrejoined single strand breaks that occur after repair synthesis, but before chromatin reassembly, which could explain in part why the comet assay showed a greater sensitivity to heat shock. However, heat effects on the initial steps in the repair process appear to be the main effect Citation14 which would be relevant to all of the assays used.
It is also of interest to compare the results of this study after applying the Sapareto-Dewey method (or equivalent) Citation29–34 to convert the short-duration severe heat shocks to equivalent min at 43°C. Using their method, 1 min at 50°C is equivalent to 128 min at 43°C or 32 min at 45°C while 15 s at 50°C is equivalent to 32 min at 43°C or 8 min at 45°C. Clearly these heat shocks are known to inhibit DNA repair and/or radiosensitise cells Citation8. For example thermal doses as low as 7–8 min at 43°C have been reported to radiosensitise some human tumour cells Citation31. Previous studies of the cell-killing effects of severe heat shocks also found the Sapareto-Dewey method worked for this temperature range Citation32. Using this approach one can also infer the expected clonogenic survival after the heat shocks used in this study. A 1-min 50°C heat shock is equivalent to 32 min at 45°C which kills (clonogenically) 80% of the cells, while a 30-s 50°C heat shock (∼ to 16 min at 45°C) kills only 10–20% of the cells Citation35.
Since the results for DNA repair in this study along with most others are consistent with the Sapareto-Dewey method, one approach to the risk assessment would be to use it for converting thermal dose at a given temperature to an equivalent exposure at 43°C, for example, converting the thermal doses used in Bodell et al. Citation14 to 50°C would suggest that 28 s would produce a 40% reduction in the rate of repair synthesis and 30% reduction in the rate of post-repair chromatin reassembly. These numbers compare well with the 40% reduction in the rate of DNA damage removal after 30 s at 50°C observed in this study (). This result is also consistent with the assumption that the initial incision step of DNA repair is the primary inhibitory target for heat shock and the effects on the remaining steps are consequences of effects of heat on the first step, as suggested by Bodell et al. Citation14. However, the authors recommend, particularly in view of the results with γ-H2AX (see below), that the thermal dose conversion not be used as a ‘stand alone’ approach to risk assessment, but rather as a justification for investigating the hazards associated with any such thermal exposure.
The observation that 60 m at 43°C induces γ-H2AX foci while 30 s at 50°C did not induce these foci is of interest from two points of view. First, heat-shock-induced γ-H2AX formation did not follow the Sapareto-Dewey thermal dose conversion method since 30 s at 50°C should convert to 64 min at 43°C. Thus, if the Sapareto-Dewey method were correct, we would expect the two exposures to show the same level of γ-H2AX induction. The second consideration is why an equivalent heat shock did not induce the same response. One possibility is that the duration of the heat shock may be critical. The formation of γ-H2AX foci after 60 min at 43°C is ATM dependent, suggesting that the effect is dependent on a signal transduction cascade Citation21. Thus the 30-s duration of the heat shock may be too short for the effect to be induced. In any event, this result suggests that not all hyperthermic effects will follow a thermal dose response consistent with the Saperato-Dewey conversion method. Furthermore, the result suggests that the γ-H2AX foci method may be used to study DNA repair inhibition if the heat shock duration is short.
In summary, we have found that short-duration severe heat shocks do inhibit the repair of UV-induced DNA damage and that the thermal dose causing these effects is consistent with the Dewey-Sapareto thermal dose conversion method.
Acknowledgement
This work was supported by a contract from the Non-Lethal Technology Innovation Center, Durham, NH.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Boutros C, Somasundar P, Garrean S, Saied A, Espat NJ. Microwave coagulation therapy for hepatic tumors: Review of the literature and critical analysis. Surg Oncol 2009; 18: 1–11
- Gough-Palmer AL, Gedroyc WM. Laser ablation of hepatocellular carcinoma–A review. World J Gastroenterol 2008; 14: 7170–7174
- Hytonen ML, Back LJ, Malmivaara AV, Roine RP. Radiofrequency thermal ablation for patients with nasal symptoms: A systematic review of effectiveness and complications. Eur Arch Otorhinolaryngol 2009; 266: 1257–1266
- Lencioni R, Crocetti L, Pina MC, Cioni D. Percutaneous image-guided radiofrequency ablation of liver tumors. Abdom Imaging 2009; 34: 547–556
- Pennathur A, Abbas G, Schuchert M, Landreneau RJ, Luketich JD. Radiofrequency ablation for the treatment of lung neoplasm. Expert Rev Med Devices 2009; 5: 613–621
- Patel PR, Luk A, Durrani A, Dromi S, Cuesta J, Angstadt M, Dreher MR, Wood BJ, Frenkel V. In vitro and in vivo evaluations of increased effective bean width for heat deposition Musing a split focus high intensity ultrasound (HIFU) transducer. Int J Hyperthermia 2008; 24: 537–549
- Mertyna P, Dewhirst MW, Halpern E, Goldberg W, Goldberg SN. Radiofrequency ablation: The effect of distance and baseline temperature on thermal dose required for coagulation. Int J Hyperthermia 2008; 24: 550–559
- Hennings L, Kaufmann Y, Griffin R, Siegel E, Novak P, Corry P, Moros EG, Shafirstein G. Dead or alive? Autofluorescence distinguishes heat-fixed from viable cells. Int J Hyperthermia 2009; 25: 355–363
- Solazzo S, Mertyna P, Peddi H, Ahmed M, Horkan C, Nahum S, Goldberg MD. RF ablation with adjuvant therapy: Comparison of external beam radiation and liposomal doxorubicin on ablation efficacy in an animal tumor model. Int J Hyperthermia 2008; 24: 560–567
- Department of Defense Directive (USA) 3003.3. Policy for Non-Lethal Weapons. July 9, 1996.
- Mason PA, Walters TJ, DiGiovanni J, Beason CW, Jauchem JR, Dick Jr. EJ, Mahajan K, Dusch SJ, Shields BA, Merritt JH, et al. Lack of effect of 94-GHz radiofrequency radiation exposure in an animal model of skin carcinogenesis. Carcinogenesis 2001; 22: 1701–1708
- Kampinga HH, Dynlacht JR, Dikomey E. Mechanism of radiosensitization by hyperthermia (43°C) as derived from studies with DNA repair defective mutant cell lines. Int J Hyperthermia 2004; 20: 131–139
- Roti Roti JL. Cellular responses to hyperthermia (40-46°C): Cell killing and molecular events. Int J Hyperthermia 2008; 24: 3–15
- Bodell WJ, Cleaver JE, Roti Roti JL. Inhibition of hyperthermia of repair synthesis and chromatin reassembly of ultraviolet-induced damage to DNA. Radiat Res 1984; 100: 87–95
- Roti Roti JL. Heat-induced alterations of nuclear protein associations and their effects on DNA repair and replication. Int J Hyperthermia 2007; 23: 3–15
- Imoto K, Kobayashi N, Katsumi S, Nishiwaki Y, Iwamoto TA, Yamamoto A, Yamashina Y, Shirai T, Miyagawa S, Dohi Y, et al. The total amount of DNA damage determines ultraviolet-radiation-induced cytotoxicity after uniform or localized irradiation of human cells. J Invest Dermatol 2002; 119: 1177–1182
- Nishiwaki Y, Kobayashi N, Imoto K, Iwomoto TA, Yamamoto A, Katsumi S, Shirai T, Sugiura S, Nakamura Y, Sarasin S, et al. Trichothiodystrophy fibroblasts are deficient in the repair of ultraviolet-induced cyclobutane pyrimidine dimers and (6-4)photoproducts. J Invest Dermatol 2004; 122: 526–532
- Olive PL, Wlodek D, Durand RE, Banath JP. Factors influencing DNA migration from individual cells subjected to gel electrophoresis. Exp Cell Res 1992; 198: 259–267
- Olive PL, Frazer G, Banath JP. Radiation-induced apoptosis measured in TK6 human B lymphoblast cells using the comet assay. Radiat Res 1993; 136: 130–136
- Malyapa RS, Bi C, Ahern EW, Roti Roti JL. Detection of DNA damage by the alkaline comet assay after exposure to low dose gamma radiation. Radiat Res 1998; 149: 396–400
- Hunt CR, Pandita RK, Laszlo A, Higashikubo R, Agarwal M, Kitamura T, Gupta A, Rief N, Horikoshi N, Baskaran R, et al. Hyperthermia activates a subset of ataxia-telangiectasia mutated effectors independent of DNA strand breaks and heat shock protein 70 status. Cancer Res 2007; 6: 3010–3017
- Laszlo A, Fleischer I. The heat-induced γ-H2AX response does not play a role in hyperthermic cell killing. Int J Hyperthermia 2009; 25: 199–209
- Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of (125)IdU-induced DNA double-strand breaks with γ-H2AX antibody. Radiat Res 2002; 158: 486–492
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 2002; 10: 886–895
- Stucki M, Jackson SP. γH2AX and MDC1: Anchoring the DNA-damage-response machinery to broken chromosomes. DNA Repair 2006; 5: 534–543
- Olive PL, Banáth JP. Phosphorylation of histone H2AX as a measure of radiosensitivity. Int J Radiat Oncol Biol Phys 2004; 58: 331–335
- Riballo E, Kuhne M, Rief N, et al. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to γ-H2AX foci. Mol Cell 2004; 16: 715–724
- Mirzayans R, Severin D, Murray D. Relationship between DNA double-strand break rejoining and cell survival after exposure to ionizing radiation in human fibroblast strains with differing ATM/p53 status: implications for evaluation of clinical radiosensitivity. Int J Radiat Oncol Biol Phys 2006; 66: 1498–1505
- Henle KJ, Roti Roti JL. Time-temperature conversions in biological applications of hyperthermia. Radiat Res 1980; 82: 138–145
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984; 10: 787–800
- Xu M, Myerson RJ, Straube WL, Moros EG, LaGroye I, Wang LL, Lee JT, Roti Roti JL. Radiosensitization of heat resistant human tumor cells by 1 hour at 41.1°C and its effect on DNA repair. Int J Hyperthermia 2002; 18: 385–403
- Borrelli MJ, Thompson LL, Cain CA, Dewey WC. Time-temperature analysis of cell killing of BHK cells heated at temperatures in the range of 43.5°C to 57°C. Int J Radiat Oncol Biol Phys 1990; 19: 389–399
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 2009; 25: 3–20
- Dewey WC, Diederich CJ. Hyperthermia classic commentary: Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 2009; 25: 21–24
- Roti Roti J, Turkel N. Heat-shock-induced changes in nuclear protein and cell killing in thermotolerant HeLa cells. Radiat Res 1994; 138: 286–290