Abstract
Purpose: We employed a grp170-secreting tumour cell system to determine whether tumour cells engineered to secrete grp170 generate an antitumour-specific immune response. Further, we examine the possibility that secreted grp170 can bind to and co-transport out of tumour cells full-length tumour antigens that may play a role in the anti-tumour immune response.
Materials and methods: Wild type Colon-26 and Colon-26 engineered to secrete grp170 were subcutaneously inoculated into BALB/c mice. Tumour growth was monitored, and variations in immunoregulatory mechanisms were evaluated using immunohistochemistry, lymphocyte depletion, ELISpot assays, and Western blot analysis.
Results: Immunisation of animals with grp170-secreting tumour cells results in rejection of the tumour by induction of antigen-specific, CD8-dependent immune responses. The secreted grp170 is able to deliver full-length tumour antigens to the tumour microenvironment, thus making them available for uptake by antigen presenting cells (APCs) to initiate tumour-specific immune responses.
Conclusions: These data parallel our studies showing that hsp110 or grp170 are able to chaperone full-length proteins, and when complexed with protein antigens and used as vaccines, these complexes elicit immune responses in vivo against the protein antigens. This cell-based approach has the potential to be utilised as a tumour-specific vaccine in tumours of various histological origins.
Introduction
Stress proteins are molecular chaperones that, either alone or in concert with other chaperones and co-chaperones can inhibit the aggregation of proteins and refold and reactivate damaged proteins that form during cellular stress. Molecular chaperones also participate in numerous other cellular processes such as protein folding, transport, and trafficking of full-length proteins Citation[1]. Grp170 is a major stress protein/molecular chaperone resident in the ER Citation[2–4] and it is induced by conditions such as hypoxia, ischaemia and interference in calcium homeostasis Citation[5], Citation[6]. In addition, grp170 is associated with the folding/processing of secretory proteins such as thyroglobulin and immunoglobulin chains Citation[2], Citation[7] and may also be involved in protein/peptide import into the ER Citation[8], Citation[9].
While it has been reported that elevated levels of a particular type of stress protein, often referred to as heat shock proteins (HSP), can promote cancer by inhibiting programmed cell death and by promoting autonomous growth Citation[10], several others have shown that HSPs, including hsc70, grp94/gp96, Calreticulin, hsp110 and grp170 can serve as tumour rejection antigens Citation[11–15], Citation[16]. Further, it has been shown that some stress proteins can interact with various receptors on APCs leading to their uptake along with associated peptides that stimulate specific immune responses Citation[17–20], secretion of pro-inflammatory cytokines Citation[21], Citation[22], and maturation of dendritic cells Citation[17]. There is also an abundance of literature reporting a role for hsp70 in the activation of natural killer (NK) cells Citation[23]. Thus, the adjuvant activity of some HSPs/stress proteins appears to be multi-fold in that they induce both innate and adaptive immunity by stimulation of pro-inflammatory responses and priming of T cells Citation[24]. These immunostimulatory advantages have made heat shock proteins promising candidates in various immunotherapeutic approaches. For detailed reviews regarding the utility of HSPs as immunotherapeutic agents, refer to Manjili et al. Citation[25] and Wang et al. Citation[26].
Murine tumours of several histological backgrounds exhibit decreased tumourigenicity and increased immunogenicity in vivo when transfected to secrete gp96 Citation[27], Citation[28]. Similarly, mice inoculated with tumours engineered to secrete hsp70 display strong CTL and NK cell responses, which impair tumour growth and increase survival Citation[29]. In the studies presented here we use a grp170 secreting tumour cell system to determine whether tumour cells engineered to secrete grp170 generate an antitumour-specific immune response. We also explore the possibility that secreted grp170 can bind to and co-transport out of tumour cells a full-length tumour antigen that may play a role in the anti-tumour immune response.
Materials and methods
Construction of sgrp170- pcDNA expression vector
Murine grp170 cDNA is amplified by PCR using Taq polymerase, a forward primer 5′ACCATCTCGCAAATAAATAGT-3′, and a reverse primer 5′GTACAGTCTAGATTAATGGTGATGGTGATGATGTGAAGGCCGCTTCTGTCC3′. The PCR product which encodes for grp170 with deletion of KNDEL sequence (ER retention signal) Citation[30], Citation[31] and addition of a six histidine-tag at the C-terminus was then inserted into BamH I and Xba I restriction sites of a plasmid vector pcDNA3.1 (Invitrogen, Carlsbad, CA) which contains human cytomegalovirus immediate-early (CMV) promoter and Neomycin resistance gene.
Tumour cells transfection and selection
Colon-26 murine colon adenocarcinoma cells originally derived from BALB/c mice treated with N-nitroso-N-methylurethane (ATCC, Rockville, MD) were transfected with sgrp170-pcDNA or mock-transfected with pcDNA3.1 using Lipofectamine reagent (Invitrogen). Stable transfectants were selected using a limiting dilution culture with 1 mg/ml of Geneticin (G418) (Sigma-Aldrich, St Louis, MO) Citation[32]. Cells were maintained in RPMI 1640, supplemented with 10% foetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin, and 100 g/mL streptomycin.
Luciferase aggregation assay
The assay was performed as described previously Citation[33]. Briefly, 150 nM Luciferase (Sigma-Aldrich) was incubated in 25 mM HEPES (pH 7.9), 5 mM magnesium acetate, 50 mM KCl and 5 mM β-mercaptoethanol at 43°C for 30 min with or without an equal molar of sgrp170 purified from culture supernatant of sgrp170-transfected Colon-26 cells by nickel-nitrilotriacetic acid (Ni-NTA) chromatography (Qiagen, Valencia, CA). Protein aggregation was monitored by the increase of optical density at 320 nm.
Tumour rejection assay
Six- to eight-week-old female BALB/c mice were inoculated subcutaneously into the left groin with 2 × 106 wild-type Colon-26, pcDNA3.1-transfected Colon-26 or sgrp170-pcDNA Colon-26. All mice used in these experiments were bred and cared for in accordance with the regulations of the Department of Laboratory Animal Resources at Roswell Park Cancer Institute. Tumour growth was monitored by measurements of the shortest diameter (A) and the longest diameter (B); tumour volume is then calculated using the formula V = (A2B)/2.
Immunohistochemistry (IHC)
For IHC, 5 µm sections of formalin-fixed, paraffin-embedded tumour specimens taken on day 8 of growth were deparaffinised in xylene, rehydrated and blocked for endogenous peroxidase in 3% H2O2 in methanol. High temperature antigen retrieval was carried out by steam heating the slides in DakoCytomation Target Retrieval Solution in a Black and Decker vegetable steamer for 20 min, followed by a 20-min cool-down period. Sections were incubated at room temperature for 1 h with either anti-CD3 (DAKO rabbit anti-human) or isotype control antibodies, washed in PBS and visualised with the Zymed SuperPicture HRP polymer system for detection of rabbit primary antibodies.
In vivo lymphocytes depletion
The GK1.5 (anti-CD4) and 2.43(anti-CD8) antibodies were kindly provided by Drew Pardoll (John Hopkins University, Baltimore, MD). CD4+ and/or CD8+ cells were depleted in vivo using i.p. injections of 200 g of GK1.5 and/or 200 g of 2.43 antibodies administered every day for 3 days before immunisation and once per week thereafter. Depletion of each subpopulation was verified by FACScan analysis of splenocytes after the last antibody injection using isotype-matched antibodies as a control for specific staining.
ELISPOT assay
The T cell responses against the AH-1 peptide of gp70 were evaluated using ELISPOT assay as described previously Citation[34]. Briefly, 96-well filtration plates were coated with anti-mouse IFN-γ antibody, and then blocked with RPMI-1640 containing 10% foetal bovine serum. Effector splenocytes from individual mice (3 per group) taken at 21 days post-inoculation were prepared separately by lysing red blood cells and then added into wells. A 17-mer extended version of the AH1 (SPSYVYHQF) peptide was added to each well, followed by washing and staining with another biotinylated IFN-γ antibody and alkaline phosphate avidin. Spots are developed by adding BCIP/NBT solution and counted using an inverted microscope.
Western blot
Secreted grp170 was purified from the spent media of transfected Colon-26 cell culture under native conditions using Ni-NTA metal affinity chromatography (Qiagen) performed according to the manufacturer's instructions. The resulting eluant fraction was designated (T). The spent media of mock-transfected Colon-26 cells treated in the same way served as a negative control (E). Proteins in eluants (T) and (E) were resolved on reducing 10% Tris-HCl SDS-polyacrylamide gel electrophoresis Citation[35]. Proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA) which was then blocked extensively with 5% non-fat milk in tris-buffered saline with 0.5% Tween-20 (TBST), and then incubated with anti-gp70 from hybridoma 83A25 (a kind gift of Frank Malik, National Institute of Allergy and Infectious Diseases, Hamilton, Montana). Specific anti-gp96 and anti-hsp70 (Stressgen, Victoria, BC, Canada), anti-H-2Kd (BD Pharmingen, San Diego, CA), anti-proteasome 26S (Calbiochem, San Diego, CA) or anti-Large T Antigen (Lab Vision, Fremont, CA) were also used as primary antibodies. Specific antisera to horseradish peroxidase–conjugated corresponding secondary antibodies were added and immunoreactivity was detected by chemiluminescence (ECL) (PerkinElmer Life Sciences, Boston, MA).
Statistical analysis
Unpaired Student t-tests were used to compare different groups of mice presented in and , and to compare different sets of splenocytes from individual mice in . P values <0.05 were considered statistically significant.
Results
Expression and characterisation of secreted grp170 (sgrp170)
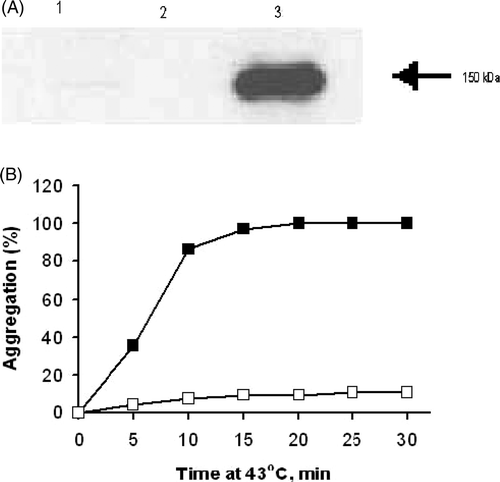
To design a secretory form of grp170 we used an approach that had been applied in other laboratories to gp96 Citation[24], Citation[27]. This involves removal of the KDEL or analogous C-terminal retention sequence that characterises resident endoplasmic reticulum proteins. In this case, grp170 has KNDEL at its C-terminus Citation[3]. For this purpose a sgrp-pcDNA3.1 expression vector was constructed by insertion of the cDNA of murine grp170 with the C-terminal ER retention signal (KNDEL) replaced by a six histidine tag into pcDNA3.1 plasmid (Invitrogen). sgrp-pcDNA3.1 was then used to transfect the Colon-26 cell line, an aggressive murine colon carcinoma that can be grown in vivo and ex vivo. Expression of a secreted form of grp170 (sgrp170) was detected in the spent culture media of transfected cells using an anti-grp170 polyclonal antibody. A stable clone with the highest level of expression of sgrp170 was isolated by limiting dilution culture in the presence of the selective antibiotic G-418 (Sigma) Citation[32]. Empty plasmid (mock)-transfected Colon-26 did not secrete grp170 as determined by Western blotting (). Intracellular levels of grp170 were similar between wild type and transfected Colon-26 as measured by Western blot indicating that sgrp170 did not accumulate in the cells (data not shown). The chaperoning function of sgrp170 was examined using a well-established in vitro aggregation assay Citation[33] to verify that it possessed chaperoning function. Purified sgrp170 proved to be highly efficient in inhibiting the heat-induced aggregation of a substrate protein (Luciferase) at 43°C at a 1 : 1 substrate to chaperone molar ratio (). Thus, protein substrates associated with sgrp170 described in the studies to follow are likely to be products of molecular chaperoning.
Figure 1. Characterisation of sgrp170. (A) Western blot with polyclonal anti-grp170 on the spent culture media of wild type Colon-26 (lane 1), mock-transfected Colon-26 (lane 2) and sgrp170-transfected Colon-26 (lane 3). (B) The effect of sgrp170 on the inhibition of protein aggregation in vitro. Luciferase alone (filled squares) or with equal molar ratio of sgrp170 (open squares) was incubated at 43°C for 30 min. The light scattering was measured by optical density change at 320 nm every 5 min.
Secreted grp170 results in tumour rejection accompanied by a specific anti-tumour immune response
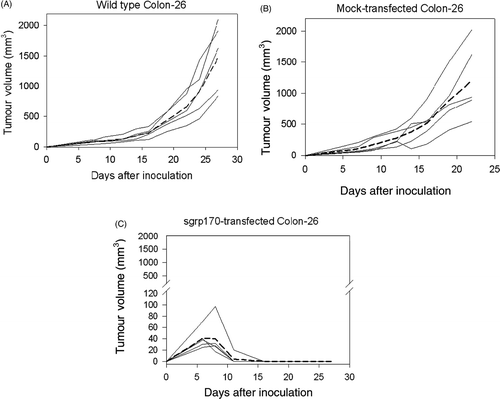
All mice inoculated with wild-type Colon-26 developed rapidly growing tumours until they had to be sacrificed when tumour size reaches 2000 mm3 starting at day 27 after tumour inoculation (). Mice inoculated with mock-transfected Colon-26 developed tumours in a pattern similar to wild-type Colon-26 (). However, all mice inoculated with sgrp170-transfected Colon-26 exhibited initial tumour growth followed by tumour regression and rejection (P = 0.000197, comparing wild-type to sgrp170-transfected Colon-26 tumour volumes at day 22). The tumour regression was first noticed on day 8 and continued until all tumours disappeared at day 16 (). The data presented in represents one of two independent experiments that were consistent with regression of the sgrp170-transfected, but not mock-transfected Colon-26 tumours.
Figure 2. Transfection of Colon-26 with secreted grp170 results in tumour rejection in vivo. Balb/c mice were inoculated with wild type or transfected tumour cells as indicated (n = 5 per group), and the tumour growth measured as described in materials and methods. Tumour growth curves for wild-type Colon-26 (A), mock-transfected Colon-26 (B) and sgrp170-transfected Colon-26 (C) are shown. Growth curves for individual mice are represented by solid lines and the average growth curve is represented by a dotted line.
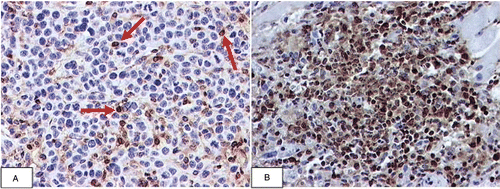
To determine the underlying immunological mechanisms for tumour rejection, a T lymphocyte subset depletion study was performed. In vivo depletion of both CD4+ and CD8+ T-lymphocyte subpopulations in BALB/c mice with GK 1.5 and 2.43 antibodies respectively abrogated the rejection of sgrp170-transfected Colon-26 tumours (P = 0.00314, comparing tumour volumes at day 22 of sgrp170-transfected Colon-26 in immunocompetent mice and mice depleted of both CD4+ and CD8+ cells) (). Similarly, the depletion of CD8+ cells alone abrogated the rejection of sgrp170-transfected Colon-26 tumours (P = 0.005617, comparing tumour volumes of sgrp170-transfected Colon-26 in immunocompetent mice and mice depleted of CD8+ cells at day 22) (). On the other hand, depletion of the CD4+ subpopulation had no effect on tumour rejection in BALB/c mice (). These results support the notion that the inhibitory effect on Colon-26 growth induced by sgrp170 is a result of an adaptive immune response. While tumours arising from mock-transfected colon-26 cells (M) show few CD3+ cells by immunohistochemical staining (), significant numbers of CD3+ cells were found in the tumours originating from sgrp170-transfected cells at day 8, which showed a dense aggregation of CD3+ cells and few viable tumour cells (). However, we have not shown that these infiltrating cells are specifically cytolytic. Nevertheless, we have previously shown that CD8+ T-cells are involved in the antitumour responses elicited by grp170-secreting tumour cells in vivo Citation[36]. The present results are consistent with our earlier studies and support the conclusion that CTLs are responsible for tumour rejection in the Colon-26 model.
Figure 3. The effect on the growth of sgrp170-transfected Colon-26 tumours by in vivo depletion of both CD8+ and CD4+ T-lymphocytes (A), depletion of the CD8+ subpopulation alone (B) or depletion of the CD4+ subpopulation alone (C). Tumour growth curves for individual mice are represented by solid lines (n = 5 per group), and the average growth curve is represented by the dotted line.
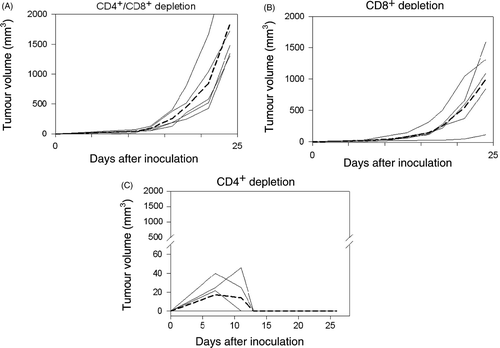
Figure 4. Immunohistochemical localisation of CD3+ immune cells in Colon-26 tumours. On the eighth day of growth, mock-transfected Colon-26 tumour contained a number of CD3 positive immune cells (red arrows) in close proximity to viable tumour cells (blue nuclei) (A). In contrast, after eight days, the site of inoculation with the sgrp170 transfected tumour cells consists of a dense aggregation of CD3 positive immune cells and few viable tumour cells. (20 × original magnification) (B).
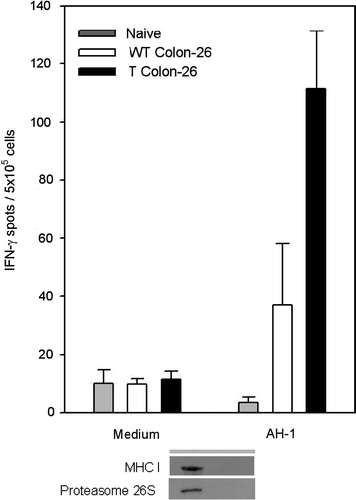
To pursue this further, we examined the extent of the cellular immune response against the H-2d restricted (AH1) epitope of the envelope protein gp70 of the murine leukemia virus (MuLV), which is a known tumour antigen in Colon-26 Citation[37] using ELISPOT assay. Splenocytes isolated from mice bearing sgrp170-transfected tumours showed significantly higher frequency of AH1-specific cells compared to the mice bearing wild-type Colon-26 tumours (P = 0.03) or naïve BALB/c mice (P = 0.002) (). Background stimulation with media alone was minimal and Con A stimulation showed competent T-lymphocytes in all mice tested (Data not shown). We conclude therefore that targeting grp170 to the extracellular environment leads to an enhanced cellular immune response against the tumour-specific antigen of Colon-26.
Figure 5. Splenocytes from mice bearing sgrp170 transfected tumours have a significantly higher frequency of AH-1 reactive cells. Splenocytes isolated from naïve BALB/c mice (grey bars), mice bearing wild-type Colon-26 (white bars) or sgrp170-transfected Colon-26 tumours (black bars) were assayed for the frequency of IFN-γ-producing cells by ELISPOT in response to medium alone or the AH-1 epitope of gp70 of MuLV. The error bars indicate the standard error of the measurements from individual mice (n = 3 per group).
sgrp170 chaperones the full-length tumour antigen, gp70, and other proteins into the tumour microenvironment
Since grp170 and other stress proteins/molecular chaperones have been long recognised to bind full-length protein substrates in situ, we hypothesised that the secreted grp170 chaperones full-length protein substrates. We also hypothesised that this includes full length tumour antigens and that these antigens are delivered into the tumour microenvironment, thus making them available for uptake by APCs. To test this hypothesis the histidine-tagged secreted grp170 was purified from the spent media of transfected Colon-26 cell culture using nickel-metal affinity chromatography (T). Purification of the spent media of mock-transfected Colon-26 cells served as a negative control (E). Both eluants (T) and (E) were initially analysed by SDS-PAGE and several protein bands were observed. Bands that were present in T, but not E, were excised, in gel digested with trypsin and the proteins identified by liquid chromatography coupled to tandem mass spectrometry. Our preliminary analysis (data not shown) identified hsp70, gp96, MHC Class I and the proteasome 26s subunit as being present in these bands. Importantly, gp70, the envelope protein of the MuLV was also identified. This protein is known to be present in Colon 26 cells and gives rise to the Colon 26-associated tumour-specific epitope AH1. Three tryptic peptides of gp70 were identified by LC-MS/MS: 266–267, VPIGPNPVLSDR; 242–256, LYVSGHDPGLIFGIR; 450–464, VTYHSPSYVYHQFKR. Peptide 450–464 contains the H-2d-restricted AH1 epitope (shown in bold) which we used in ELISPOT assays to quantify the immune response.
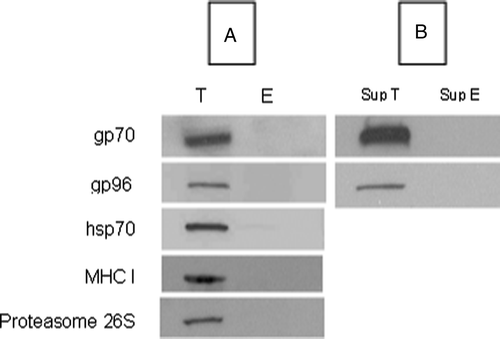
In order to validate the presence of this protein, SDS-PAGE gels were immunoblotted with anti-gp70 specific antibody. Gp70 was detected at the correct molecular size region on the gel in the purified sgrp170 fraction (T) but not in the control (E) (). To investigate whether the secreted grp170 binds its substrate (gp70) intracellularly or extracellularly the spent supernatants of sgrp170-transfected Colon-26 (Sup T) and mock-transfected Colon-26 cells (Sup E) were compared for the presence of gp70 (). Gp70 was detected only in the supernatant of sgrp170-transfected Colon-26 cells suggesting that sgrp170 binds its substrate inside the cell and co-transports it into the extracellular environment by association with sgrp170. The chaperones gp96 and hsp70, and MHC Class I and the proteasome 26s subunit were also found by immunoblotting in fraction T and not E, suggesting that these proteins are also found in association with sgrp170 ().
Figure 6. Western blots of the eluants from nickel-chromatography of cell culture supernatants of Colon-26 cells transfected with sgrp170 (T) or mock-transfected Colon 26 (E). Western blot of the T and E fractions using specific antibodies to gp70, gp96, MHC Class 1, proteasome 26S and hsp70 (A). Western blot of the unfractionated culture supernatants of sgrp170-transfected Colon-26 cells (Sup T) and mock-transfected Colon-26 (Sup E) using antibodies specific for gp70 and gp96 (B).
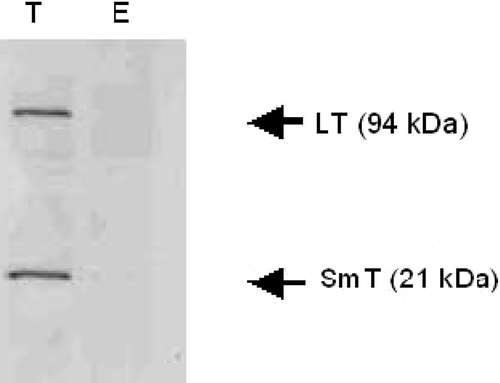
To verify this finding in an independent cellular system, monkey Cos-7 cells were stably transfected either with sgrp170-pcDNA3.1 or mock-transfected with pcDNA3.1. Spent cell culture media from both transfectants were run over nickel-metal affinity chromatography. Eluants were checked for the presence of the small and large T-antigens (both are well studied tumour antigen in Cos-7 cells) using Western blotting with an antibody specific to the small and large T-antigens (). Both the large and small T-antigens were found associated with sgrp170. These results, similar to those obtained with gp70 of Colon-26 cells, further strengthen our conclusion that secreted grp170 chaperones full-length protein substrates, including tumour antigens, into the tumour microenvironment.
Discussion
Grp170 is a major molecular chaperone/stress protein resident in the endoplasmic reticulum (ER). It is distantly related in sequence to both the hsp110 and hsp70 families, but represents its own grp170 stress protein family Citation[3], Citation[4]. Studies have suggested that grp170 is an important element of the protein processing machinery of the ER Citation[2], Citation[7], Citation[8] and the molecular chaperoning properties of grp170 have been recently defined Citation[38].
Although most chaperones are intracellular proteins they can be modified to be secreted. Chaperones secreted into the tumour microenvironment can recruit professional APCs and promote antigen presentation and priming of T-lymphocytes. Impaired tumour growth, increased immunogenicity and specific CTL responses have been shown for several murine tumour cell lines transfected to secrete gp96 Citation[24], Citation[27] or hsp70 Citation[29]. We demonstrated here that targeting grp170 to the extracellular environment has immunological consequences resulting in tumour rejection that is CD8+ dependent. The stable clone we selected (T) grows at the same rate as mock-transfected (E) Colon-26 in vivo in mice depleted of CD8+ lymphocytes (), as well as in SCID mice (data not shown). This indicates that the selected clone is growth-competent and that the regression of the sgrp170 transfected tumour was due to a CD8+ mediated immune response.
We also showed that the envelope protein gp70 of the MuLV, a tumour-specific antigen is associated with grp170 secreted from Colon-26 cells. Indeed, the precursor of gp70, gp90, is known to be present in the ER and to be processed in the Golgi, and this provides a venue for sgrp170 to bind to gp70 and co-transport it into the extracellular environment Citation[39]. Similarly, small and large T-antigens co-purified with grp170 secreted from Cos-7 cells; we speculate that grp170-associated tumour-specific antigens may serve as a source of full-length antigen for cross-priming in APCs. Because these are full length proteins harbouring multiple MHC Class I epitopes there is the potential for priming across a variety of MHC haplotypes, a phenomenon observed when using tumour-derived heat shock protein vaccine preparations Citation[40]. However, further studies are needed to determine whether the tumour-specific responses are triggered by the chaperone itself, its associated tumour antigens or peptide fragments of these antigens.
Chaperones such as gp96 and hsp70 were also found associated with secreted grp170. The presence of chaperones in multi-protein complexes is well-documented Citation[1], Citation[7]. For example, grp170 has been previously shown to associate in situ with grp78 and gp96 Citation[2]. It is nonetheless unclear how other chaperones could escape the ER despite possessing an ER-retention signal (gp96) or being cytosolic chaperones (hsp70). However, there are several reports on the secretion or cell surface expression of cytosolic and ER chaperones in immature thymocytes, rat exocrine pancreatic cells, a thyroid cell line and human prostate cell lines in a physiological state or following a forced overexpression Citation[41]. It should also be noted that these chaperones may also contribute to the immune response to colon-26 tumour cells observed in these studies. Also of possible interest and importance to the immune responses to Colon-26 is that parts of the intrinsic antigen processing and presentation machinery such as MHC class I molecules and the proteasome 26s subunit were also found associated with sgrp170.
It has been postulated that the immunogenicity of HSPs stems from their ability to bind a diverse array of peptides in the cell including immunogenic peptides generated from proteasomal degradation of tumour antigens in a tumour cell. For the past several years attempts were made to identify peptides associated with chaperones using highly sensitive techniques such as mass spectrometry. However, most of these studies focus on a defined T-cell epitope sequence from tumour cells that are virally infected such as vesicular stomatitis virus (VSV) Citation[42] and Hepatitis B virus (HBV) Citation[43] or over-express a certain tumour antigen (e.g. OVA and c-Akt) Citation[44], Citation[45]. Recently, Binder et al. Citation[46] provided evidence that that the specific immunogenicity of gp96 preparations isolated from β-galactosidase-expressing cells derives from gp96-chaperoned peptides rather than from full-length β-galactosidase itself. However, Kunisawa and Shastri Citation[47] reported that hsp90 binds to large proteolytic intermediates or truncated proteins (10 ∼ 30 kDa) containing an immunogenic OVA epitope, although there is some debate whether these peptide precursors are extended at both the N- and C- termini Citation[48]. Our observations in the present study support the idea that HSP is involved in antigen presentation by directly carrying proteins/or peptide precursors, rather than precisely processed MHC-binding peptides.
From the studies described here, grp170 chaperones full-length proteins and, when engineered to be secreted from the cell, grp170 accompanies/carries immunologically relevant, full-length proteins into the extracellular compartment. The broader question of where and how cytoplasmic proteins such as gp70 encounter sgrp170 for co secretion is unclear. Although we conclude in our studies that grp170 chaperones full-length proteins, we cannot exclude the possibility that grp170 also chaperones small immunogenic peptides in vivo, and this remains to be more fully examined. Furthermore, although we use the anti AH-1 response as an indication of the immune response to sgrp170-transfected Colon-26 cells, there may well be other as yet unidentified epitopes involved in the response.
Conclusion
Together, these results indicate that immunisation of animals with grp170-secreting tumour cells results in rejection of the tumour by induction of antigen-specific, CD8-dependent immune responses. Furthermore, the secreted grp170 is able to deliver full-length tumour antigens to the tumour microenvironment, thus making them available for uptake by antigen presenting cells to initiate a tumour-specific immune response. These data parallel our studies showing that hsp110 or grp170 are able to chaperone full-length proteins and when complexed with protein antigens and used as vaccines, the complexes elicit immune responses in vivo against the protein antigens Citation[13], Citation[14]. The cell-based approach described here has the potential of being exploited as a tumour-specific vaccine in tumours of various histological origins.
Acknowledgements
This work was supported in part by the National Institutes of Health grants PO1 CA094045 and RO1 CA099326, and the Roswell Park Cancer Institute Cancer Center Support Grant P30 CA 016056-29. The authors have no conflicting financial interest.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins. Ann Rev Biochem 1993; 62: 349–384
- Lin HY, Masso-Welch P, Di YP, Cai JW, Shen JW, Subjeck JR. The 170-kDa glucose-regulated stress protein is an endoplasmic reticulum protein that binds immunoglobulin. Mol Biol Cell 1993; 4: 1109–1119
- Chen X, Easton D, Oh HJ, Lee-Yoon DS, Liu X, Subjeck J. The 170 kDa glucose regulated stress protein is a large hsp70-, hsp110-like protein of the endoplasmic reticulum. FEBS Lett 1996; 380: 68–72
- Easton DP, Kaneko Y, Subjeck JR. The hsp110 and Grp1 70 stress proteins: Newly recognized relatives of the hsp70s. Cell Stress Chaperones 2000; 5: 276–290
- Shen J, Hughes C, Chao C, Cai J, Bartels C, Gessner T, Subjeck J. Coinduction of glucose-regulated proteins and doxorubicin resistance in Chinese hamster cells. Proc Natl Acad Sci USA 1987; 84: 3278–3282
- Cai JW, Henderson BW, Shen JW, Subjeck JR. Induction of glucose regulated proteins during growth of a murine tumor. J Cell Physiol 1993; 154: 229–237
- Kuznetsov G, Chen LB, Nigam SK. Multiple molecular chaperones complex with misfolded large oligomeric glycoproteins in the endoplasmic reticulum. J Biol Chem 1997; 272: 3057–3063
- Dierks T, Volkmer J, Schlenstedt G, Jung C, Sandholzer U, Zachmann K, Schlotterhose P, Neifer K, Schmidt B, Zimmermann R. A microsomal ATP-binding protein involved in efficient protein transport into the mammalian endoplasmic reticulum. Embo J 1996; 15: 6931–6942
- Spee P, Subjeck J, Neefjes J. Identification of novel peptide binding proteins in the endoplasmic reticulum: ERp72, calnexin, and grp170. Biochemistry 1999; 38: 10559–10566
- Calderwood SK, Ciocca DR. Heat shock proteins: Stress proteins with Janus-like properties in cancer. Int J Hyperthermia 2008; 24: 31–39
- Parmiani G, De Filippo A, Pilla L, Castelli C, Rivoltini L. Heat shock proteins gp96 as immunogens in cancer patients. Int J Hyperthermia 2006; 22: 223–227
- Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science 1997; 278: 117–120
- Park JE, Facciponte J, Chen X, MacDonald I, Repasky EA, Manjili MH, Wang XY, Subjeck JR. Chaperoning function of stress protein grp170, a member of the hsp70 superfamily, is responsible for its immunoadjuvant activity. Cancer Research 2006; 66: 1161–1168
- Wang XY, Chen X, Manjili MH, Repasky E, Henderson R, Subjeck JR. Targeted immunotherapy using reconstituted chaperone complexes of heat shock protein 110 and melanoma-associated antigen gp100. Cancer Res 2003; 63: 2553–2560
- Basu S, Srivastava PK. Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity. J Exp Med 1999; 189: 797–802
- Udono H, Srivastava PK. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J Immunol 1994; 152: 5398–5403
- Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee H-G, Toes REM, Schild H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol 2000; 30: 2211–2215
- Calderwood SK, Theriault JR, Gong J. How is the immune reponse affected by hyperthermia and heat shock proteins?. Int J Hyperthermia 2005; 21: 713–716
- Facciponte JG, Wang XY, Subjeck JR. Hsp110 and Grp170, members of the hsp70 superfamily, bind to scavenger receptor-A and scavenger receptor expressed by endothelial cells-I. Eur J Immunol 2007; 37: 2268–2279
- Binder RJ, Han DK, Srivastava PK. CD91: A receptor for heat shock protein gp96. Nat Immunol 2000; 1: 151–155
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. Hsp70 stimulates cytokine production through a CD14-dependent pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med 2000; 6: 435–442
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 2000; 12: 1539–1546
- Multhoff G. Activation of natural killer cells by heat shock protein 70. Int J Hyperthermia 2009; 25: 169–175
- Hilf N, Singh-Jasuja H, Schild H. The heat shock protein Gp96 links innate and specific immunity. Int J Hyperthermia 2002; 18: 521–533
- Manjili MH, Wang X-Y, Park J, MacDonald IJ, Li Y, Van Schie RCAA, Subjeck JR. Cancer immunotherapy: Stress proteins and hyperthermia. Int J Hyperthermia 2002; 18: 506–520
- Wang X-Y, Li Y, Yang G, Subjeck JR. Current ideas about applications of heat shock proteins in vaccine design and immunotherapy. Int J Hyperthermia 2005; 21: 717–722
- Yamazaki K, Nguyen T, Podack ER. Cutting edge: Tumor secreted heat shock-fusion protein elicits CD8 cells for rejection. J Immunol 1999; 163: 5178–5182
- Baker-LePain JC, Sarzotti M, Fields TA, Li CY, Nicchitta CV. GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J Exp Med 2002; 196: 1447–1459
- Massa C, Guiducci C, Arioli I, Parenza M, Colombo MP, Melani C. Enhanced efficacy of tumor cell vaccines transfected with secretable hsp70. Cancer Res 2004; 64: 1502–1508
- Lewis MJ, Pelham HR. Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell 1992; 68: 353–364
- Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell 1987; 48: 899–907
- Southern PJ, Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet 1982; 1: 327–341
- Schumacher RJ, Hansen WJ, Freeman BC, Alnemri E, Litwack G, Toft DO. Cooperative action of hsp70, hsp90, and DnaJ proteins in protein renaturation. Biochemistry 1996; 35: 14889–14898
- Miyahira Y, Murata K, Rodriguez D, Rodriguez JR, Esteban M, Rodrigues MM, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods 1995; 181: 45–54
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685
- Wang X-Y, Arnouk H, Chen X, Kazim L, Repasky EA, Subjeck JR. Extracellular targeting of endoplasmic reticulum chaperone glucose-regulated protein 170 enhances tumor immunity to a poorly immunogenic melanoma. J Immunol, 2006; 177: 1543–1551
- Huang AY, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, Engelhard VH, Pasternack G, Cotter R, Hunt D, et al. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc Natl Acad Sci USA 1996; 93: 9730–9735
- Park J, Easton DP, Chen X, MacDonald IJ, Wang XY, Subjeck JR. The chaperoning properties of mouse grp170, a member of the third family of hsp70 related proteins. Biochemistry 2003; 42: 14893–14902
- Golgher D, Korangy F, Gao B, Gorski K, Jaffee E, Edidin M, Pardoll DM, Elliott T. An immunodominant MHC class II-restricted tumor antigen is conformation dependent and binds to the endoplasmic reticulum chaperone, calreticulin. J Immunol 2001; 167: 147–155
- Arnold D, Faath S, Rammensee H, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med 1995; 182: 885–889
- Beachy SH, Kisailus AJ, Repasky EA, Subjeck JR, Wang XY, Kazim AL. Engineering secretable forms of chaperones for immune modulation and vaccine Development. Methods 2007; 4: 184–193
- Nieland TJ, Tan MC, Monne-van Muijen M, Koning F, Kruisbeek AM, van Bleek GM. Isolation of an immunodominant viral peptide that is endogenously bound to the stress protein GP96/GRP94. Proc Natl Acad Sci USA 1996; 93: 6135–6139
- Meng SD, Gao T, Gao GF, Tien P. HBV-specific peptide associated with heat-shock protein gp96. Lancet 2001; 357: 528–529
- Breloer M, Marti T, Fleischer B, von Bonin A. Isolation of processed, H–2Kb-binding ovalbumin-derived peptides associated with the stress proteins hsp70 and gp96. Eur J Immunol 1998; 28: 1016–1021
- Ishii T, Udono H, Yamano T, Ohta H, Uenaka A, Ono T, Hizuta A, Tanaka N, Srivastava PK, Nakayama E. Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins hsp70, hsp90, and gp96. J Immunol 1999; 162: 1303–1309
- Binder RJ, Kelly JB, III, Vatner RE, Srivastava PK. Specific immunogenicity of heat shock protein gp96 derives from chaperoned antigenic peptides and not from contaminating proteins. J Immunol 2007; 179: 7254–7261
- Kunisawa J, Shastri N. Hsp90alpha chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity 2006; 24: 523–534
- Callahan MK, Garg M, Srivastava PK. Heat-shock protein 90 associates with N-terminal extended peptides and is required for direct and indirect antigen presentation. Proc Natl Acad Sci USA 2008; 105: 1662–1667