Abstract
Purpose: Optimal treatment for liver metastases from gastric cancer remains a matter of debate. The aim of our study is to evaluate the efficacy of radiofrequency ablation (RFA) for the treatment of liver-only metastases from gastric adenocarcinoma.
Materials and methods: We retrospectively reviewed medical records of 29 patients who developed liver-only metastases from gastric adenocarcinoma and subsequently underwent gastric resection and RFA (n = 20) or gastric resection and systemic chemotherapy (n = 9) between January 1995 and February 2008. Overall survival was estimated using the Kaplan-Meier method, and was compared using the log rank test to evaluate RFA efficacy.
Results: Twenty patients who underwent RFA showed a median overall survival of 30.7 months (range: 2.9 to 90.9 months), a median progression-free survival of 6.8 months (range: 0.8 to 45.2 months), and median overall one-, three-, and five-year survival rates were 66.8%, 40.1%, and 16.1% respectively. The RFA group showed a 76% decreased death rate compared to the chemotherapy-only group (30.7 months versus 7 months, hazard ratio, 0.24; p = 0.004). Most patients tolerated RFA well, and complications were found to be minor (transient fever (20%) and/or right upper quadrant pain (25%)). One case of treatment-related death occurred due to sepsis that originated from a liver abscess at the ablation site.
Conclusions: The data suggest that a use of RFA as a liver-directed treatment may provide greater survival benefit than chemotherapy and is an alternative option for the treatment of liver-only metastases from gastric cancer.
Introduction
The liver is one of the most common distant sites for metastasis of gastric cancer. Approximately 5–14% of patients who undergo gastric cancer surgery develop liver metastases synchronously or metachronously Citation[1–7]. Although this metastasis is limited to the liver, liver-only metastases from gastric cancer are usually considered a systemic disease, and are therefore treated with systemic chemotherapy. Despite an improvement in chemotherapy regimens over the last decade, patients with liver metastases from gastric cancer consistently show a poor prognosis with a median survival of 3–13 months) Citation[8–19]. Therefore, a novel therapeutic strategy is required to improve the survival of patients with liver metastases, especially those with metastases limited to the liver.
In colorectal liver metastases, hepatic resection has been validated as the curative modality, and has been established as the standard treatment Citation[4], Citation[20], Citation[21]. Recent reports have shown that radiofrequency ablation (RFA) could increase the overall survival of colorectal cancer patients with liver metastases Citation[22–25], suggesting that RFA might be an alternative option for patients where surgical resection is unfeasible Citation[25–27]. Moreover, RFA has shown beneficial treatment outcomes in some specific cases. In addition, the process of RFA is minimally invasive compared to liver resection.
Applying this rationale from treatment strategies for colorectal metastases, several reports have evaluated the efficacy of hepatic resection for liver-only metastases from gastric carcinoma Citation[3], Citation[4], Citation[6], Citation[35–37]. Our previous study also showed that hepatic resection had benefits for overall and recurrence-free survival, providing the rationale for liver-directed treatment of liver metastases from gastric cancer Citation[1]. However, there are a few anecdotal case studies that reported on the use of RFA as a treatment option for liver metastasis from gastric cancer Citation[35–37].
The aim of this study was to assess both the feasibility and efficacy of RFA with respect to overall survival and progression-free survival in 20 patients with liver-only metastases from gastric cancer. We also investigated the recurrence pattern after RFA treatment. To the best of our knowledge, this is the first reported comprehensive review on patients who underwent RFA for gastric cancer with liver-only metastases.
Materials and methods
This retrospective study was approved by the Severance Hospital Institutional Review Board. All patients signed a written informed consent for participation in the study.
Patient selection
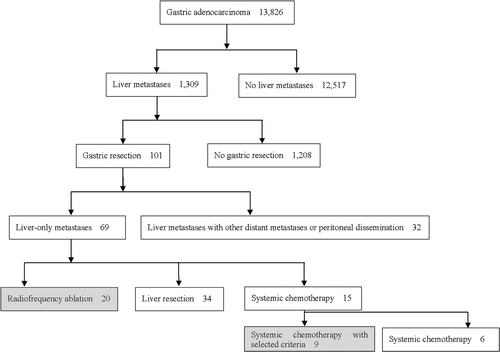
Between January 1995 and February 2008, 13,826 patients were diagnosed with gastric adenocarcinoma at the Yonsei Cancer Center (YCC) of Severance Hospital, Yonsei University Health System (YUHS) in Korea. Among them, 1,309 (9.4%) were shown to have synchronous or metachronous liver metastases. Gastrectomy was performed on 101 of the 1,309 patients. Of the 1,309 patients, 69 had liver-only metastases, 20 patients were treated with RFA, 34 with liver resection, and 15 with systemic chemotherapy (). We included 20 patients who underwent gastric resection for the primary cancer and received RFA for liver metastases either simultaneously or after gastrectomy treatment, and nine patients who underwent gastric resection and received systemic chemotherapy.
All patients treated with RFA had lesions proven to be liver metastases based on contrast-enhanced three-phase computed tomography (CT) (100%, 29/29), dynamic contrast-enhanced magnetic resonance imaging (MRI) (48.3%, 14/29), hypermetabolic lesions on positron emission tomography (PET) (20.7%, 6/29), or positive biopsy findings (41.3%, 12/29). All patients were categorised as Eastern Cooperative Oncology Group (ECOG) 0-1 for performance status, and the median follow-up period was 14.4 months (range; 2.9 to 90.9 months).
Among the 15 patients who received chemotherapy without liver-directed treatment, nine patients with liver metastases characteristics similar to the RFA group were included for a comparative analysis of RFA efficacy. Liver metastases in those nine were treatable by RFA (see Materials and methods section).
Indications for use of RFA treatment
Indications for the use of RFA treatment were as follows: (1) no sign of peritoneal dissemination or any other extrahepatic systemic metastases on imaging studies at the time of liver metastases diagnosis; (2) size of the largest liver metastasis was ≤5 cm and the number of liver metastases was less than four; (3) patients were inoperable either due to the location of liver metastases or an increase in post-operative morbidity and mortality was expected; (4) RFA was performed when the patients wished to avoid surgery. Eligibility was clinically determined by the physicians based on the number, size, location, and the timing (synchronous or metachronous) of liver metastasis.
Modality of RFA (intraoperative versus percutaneous)
We performed simultaneous gastric resection and intraoperative RFA in synchronous metastatic liver lesions. For metachronous liver lesions, the choice of intraoperative versus percutaneous RFA was dependent upon the lesion location. Visibility by percutaneous ultrasound and the possibility of injury to adjacent organs were the major determinants for access choice. RFA was performed as percutaneous (n = 11) or intraoperative (n = 9) using ultrasonographic guidance.
RFA technique
Single tip 17-G internally cooled electrodes (Cool-tip™ RF ablation system, Valleylab, Boulder, Colorado, USA) were used for 12 minutes at 100°C per ablation. The ablative margin was intended to be at least 0.5 cm, so single puncture (n = 12) was performed in tumours no greater than 2 cm in size. Double puncture (n = 2) was used for tumours between 2 and 3 cm in size. And triple puncture (n = 6) was performed in the tumours that measure 3 to 5 cm. After the electrode was connected to the generator (Valleylab), RF energy was applied for 12 minutes at each site of the tumour using an impedance control algorithm for the Cool-tip™ RF ablation system.
Chemotherapy treatment
All patients treated with RFA were recommended to receive post-RFA chemotherapy because of metastatic setting even though there have been no established data about the role of post-RFA chemotherapy in this setting.
Nine patients who underwent gastric resection without liver-directed treatment received systemic chemotherapy within four weeks after gastric resection. Systemic chemotherapy was administered until disease progression or intolerance of the treatment.
Evaluation of clinical characteristics
The following demographic and clinicopathological information was retrospectively obtained from the medical records: age, gender, status of primary gastric cancer and liver metastases, treatment after RFA, and recurrence pattern. Primary gastric cancer was characterised by TNM stage, pathologic type, tumour differentiation, tumour location, and primary gastric cancer tumour size (). Characteristics of liver metastases included number, size, lobar distribution, and timing of liver metastases (synchronous or metachronous). In this study, liver metastasis diagnosed at the time of gastric tumour resection was considered synchronous, while metastasis detected after gastric cancer resection was considered metachronous. Pathological staging was determined according to criteria defined by the American Joint Committee on Cancer Citation[38].
Table I. Patient characteristics.
Follow-up
Post-treatment contrast-enhanced three-phase CT was performed three to four weeks after RFA to assess the treatment response. Complete ablation of macroscopic tumour was defined as no viable portion in the remaining lesion on CT scan. Patients who were confirmed as complete ablation underwent follow-up imaging at three to six months intervals for the first two to three years after RFA. Local tumour progression was defined as the recurrence of tumour at the site of ablation Citation[39]. Disease progression was defined as the evidence of new tumour located outside the treated region, and included intrahepatic and extrahepatic sites. Evidence of recurrence was documented as the date on which a recurrence was noted on CT scan. Overall survival was defined as the time from liver metastases treatment to either death from any cause or to the last follow-up date. Progression-free survival was defined as the time from the liver metastasis treatment to either the first documentation of recurrence or progression or death from any cause. In chemotherapy-only patients, CT was performed every two cycles. An evaluation of the response to chemotherapy was performed during the entire course of chemotherapy treatment using response evaluation criteria in solid tumours (RECIST) Citation[40].
Treatment after recurrence
Patients who had incomplete ablation or local tumour progression over follow-up without new intra- or extrahepatic metastasis after RFA underwent repetitive RFA where possible. Patients who had only single site recurrence in the liver underwent RFA if possible, whereas those who had multiple liver recurrences or distant metastases received systemic chemotherapy.
Morbidity classification
Complications were categorised using the Society of Interventional Radiology (SIR) classification system for complications by outcome Citation[39], Citation[41] into minor and major complications.
Statistics
Analysis of the patient's clinicopathological characteristics was completed by either Fisher's exact test or a χ2 test. Survival was calculated by the Kaplan-Meier method and was compared using the log rank test. All statistical analyses were conducted using SPSS 13.0 statistical software. A p-value less than 0.05 was considered significant.
Results
Patient characteristics
summarises the baseline clinicopathological characteristics of patients who underwent RFA as a liver-directed treatment and those that received only chemotherapy. Detailed information for the 20 individual patients who received RFA is presented in . The RFA group included 15 men and 5 women with a median age of 57 years (range 33–77). Unilobar distribution was observed in most patients (80%), and hepatic metastases were detected synchronously in 6 patients (30%) and metachronously in 14 patients (70%). In patients who had metachronous liver metastases, the median interval between gastric resection and the diagnosis of hepatic metastases was 13.6 months (range 0.5–40.7 months). The percentage of patients who subsequently received chemotherapy after RFA was 75 % (15 patients).
Table II. Treatment outcome of individual patients.
There were no major differences between the two groups in terms of primary gastric cancer characteristics except the number of hepatic metastases, where a single metastasis was observed in 13 out of 20 patients (65%) in the RFA group and in only one patient (11%) in the chemotherapy-only group (p = 0.02). In the chemotherapy-only group, the number of liver metastases was as follows: one in one patient (11%), two in six patients (67%), and three in two patients (22%). The total number of liver metastases was less than four due to the selection criteria for the purpose of comparison (see Materials and methods section).
RFA treatment
Regarding the modality of RFA, nine patients (patients 1–9) received intraoperative RFA while the remaining 11 patients received percutaneous RFA (patients 10–20). All six synchronous liver metastases were treated with intraoperative RFA. Since percutaneous RFA was preferred to intraoperative RFA to avoid re-operation after gastrectomy for primary gastric cancer, 78% (11/14) of the patients with metachronous-type liver metastases received percutaneous RFA. Three patients received intraoperative RFA since their tumour locations were technically inaccessible to percutaneous RFA (). These three patients underwent laparoscopic surgery to perform RFA by the surgeon's and interventional radiologist's collaborative medical decision.
Chemotherapy treatment
Of the 20 patients received RFA, 15 patients (75%) underwent sequential chemotherapy, and the remaining five (patients 9, 15–17, and 20; 25%) did not for issues related to liver abscess (patient 15), patient refusal (patient 9, 16, and 20), or follow-up loss (patient 17) respectively.
Recurrence
Complete ablation was achieved in 95% (19/20) of the patients treated with RFA, indicating the feasibility of the use of RFA in the treatment of liver metastasis. Incomplete ablation three weeks after RFA was detected in one patient (patient 16). Local tumour progression was absent, while all were disease progression including new foci of intrahepatic metastasis or distant metastasis. The overall recurrence rate after RFA was 70% (14/20): eight patients (40%; 8/20) showed intrahepatic recurrence only in the remnant liver without any recurrence at the site of ablation, whereas six patients (30%; 6/20) showed both intra- and extrahepatic recurrences (). Recurrence was commonly observed in the liver (100%), but there was no patient with only extrahepatic recurrence. Among the eight patients with intrahepatic recurrence, a single site recurrence was observed in five patients. Among them, four patients underwent RFA treatment for the second time, and one received systemic chemotherapy. After repetitive RFA at the recurred single site, there was no tumour recurrence at 4.1, 2.1, 89.3, and 3.2-month follow-ups in patients 1, 9, 10, and 11 respectively. Overall survival of these patients was 48.1, 30.7, 90.9, and 27.3 months respectively.
Table III. Treatment modality in 14 recurred patients after RFA.
Survival
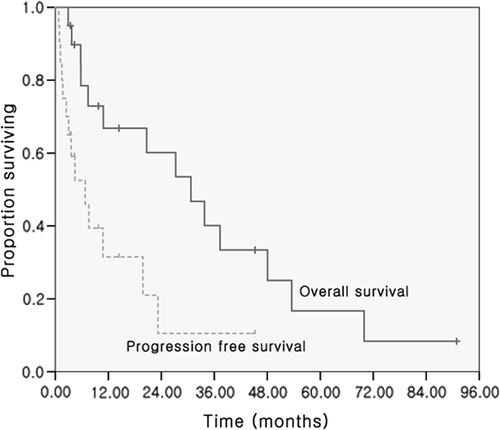
Progression-free survival was 6.8 months (range 0.8 to 45.2 months), and the median overall survival was 31 months (range 2.9 to 90.9 months) in all patients treated with RFA. The survival rates in the RFA group were 66.8% for one year, 40.1% for three years, and 16.7% for five years (). Interestingly, two out of 20 patients (10%) survived more than five years (patients #4 and 10).
Complication and morbidity of RFA
Most patients tolerated RFA treatment, due to its predominantly minor side effects. The most common complications by RFA were transient fever (4/20; 20%) and/or right upper quadrant pain (5/20; 25%). These complications were all categorised as SIR minor, B complications. One patient developed a liver abscess the ablation site and died due to sepsis (patient 15, ). This patient had no underlying co-morbidity issues such as diabetes mellitus or hepatobilliary infection/bypass procedure, which are risk factors for post-RFA complications.
Comparison of RFA and chemotherapy treatment
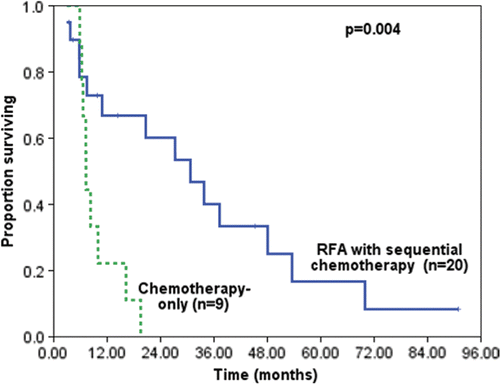
To evaluate whether RFA had a direct benefit for the treatment of liver metastases, we compared the median overall survival of RFA with the systemic chemotherapy-only group (n = 9). The patients in the chemotherapy-only were selected from the same retrospective group, and had similar pretreatment risks to the reference group. The RFA group showed a 76% decreased death rate compared to the chemotherapy-only group (31 months versus 7 months, hazard ratio, 0.24; p = 0.004) (). In this regard, the data show that RFA treatment enhances the survival rate of patients.
Discussion
Given the dismal treatment outcome of conventional systemic chemotherapy for gastric cancer with liver metastases, alternative treatment options are urgently required. The management of these patients with such a dismal prognosis is a therapeutic challenge for both surgeons and oncologists. Recently, liver-directed treatment such as hepatic resection and RFA has been applied to patients with liver-only metastases. The rationale for liver-directed treatment of gastric cancer with liver metastases is as follows. First, when overt metastases are isolated to a specific organ, cytoreduction enables chemotherapy to be more effective and is helpful for good prognosis Citation[42], Citation[43]. Second, removal of the isolated metastatic deposit can prevent further dissemination of the disease to other sites Citation[44].
Until now, there has been no report showing the benefit of RFA for the treatment of liver metastases arising from gastric adenocarcinoma. Even though systemic chemotherapy is recommended as the current standard treatment for gastric cancer with liver-only metastases, the use of systemic chemotherapy alone made long-term survival difficult to achieve, as the treatment outcome was not satisfactory with a median survival of 13 months or less. Citation[8–19]. Considering these historical data, as well as the data presented here on chemotherapy-only treatment without RFA, our RFA treatment followed by systemic chemotherapy showed a better treatment outcome with a median overall survival of 31 months, suggesting that RFA could be a potential treatment option for liver metastases originated from gastric adenocarcinoma.
Generally, RFA has several benefits including both safety and easy accessibility Citation[45], Citation[46]. RFA is less invasive and can be easily repeated when applied in a percutaneous manner. This can be especially important in metachronous metastasis, where re-operative hepatic resections after previous gastrectomy can increase postoperative morbidity and mortality. As shown in our study, most metachronous metastases were treatable with RFA. In addition, RFA might be an alternative treatment option when tumours are unresectable or inoperable due to either poor liver function or difficulty in surgical approach Citation[26], Citation[45], Citation[47–52]. Therefore, careful selection of patients with reasonable indications of RFA success is important for improving patient outcome.
In this study, indications for RFA did not always include unresectable patients. Even when the hepatic lesion was resectable, both clinicians and patients tended to prefer the less invasive RFA treatment to the more aggressive resection. A point that requires attention is whether RFA can be used for a patient eligible for surgical resection because an efficacious liver-directed treatment of gastric liver metastases has not yet been established.
In our study, chemotherapy after RFA was performed in 75% of patients as an adjuvant treatment. In actual clinical circumstances, many medical oncologists believe that post-operative chemotherapy is an available and helpful treatment option for metastatic cancer after resection, because developing liver metastases is considered a systemic disease. There is no phase III study comparing local therapy accompanied with systemic chemotherapy versus systemic chemotherapy. However, there are some reports that show adjuvant chemotherapy after hepatic resection Citation[1], Citation[3–5], Citation[7], Citation[29].
After completion of RFA treatment, the dominant site of recurrence was the liver (), suggesting that the liver should be under surveillance even after the initial completion of metastases. The rate of local recurrence in the liver without any systemic recurrence was 57.1%. Such high recurrence rate in the remnant liver can be explained by micrometastases that are defined as cancer cell clusters of 1 mm or less separated from cancer by normal liver parenchyma, around liver metastases suggested by Nomura et al. Citation[53]. However, these high recurrence rates in the liver do not indicate the local failure of RFA treatment because most local recurrences involve the development of new lesions in the remnant liver and not at the RFA site. Incomplete ablation was observed in only one patient (patient 16 in ). The cause of RFA local failure may be due to bilateral distribution and large numbers of tumours.
Some studies pertaining to hepatic resection demonstrated the number or size of liver metastases as prognostic factors for survival Citation[4], Citation[5], Citation[7], Citation[54], whereas there was no significant prognostic factor for survival identified in our study. We compared our results with previous reports and reviewed Shirabe's reports Citation[54]. ()
Table IV. Comparison of reports regarding liver-directed treatment for gastric adenocarcinoma with liver-only metastases.
Our study had some limitations. One was that patients enrolled in this study may have had undetected extrahepatic disease. Since peritoneal metastases are usually found in almost 40% of gastric cancer patients with liver metastases, an invasive laparoscopic procedure would have been required to diagnose new peritoneal disease. Also, this was a retrospective study including a very small subset of patients. With the possibility of selection bias, we are limited in applying these results to all patients with liver-only metastases from gastric cancer.
Despite these limitations, our results show that RFA followed by chemotherapy can be considered a treatment option because it was shown to be more beneficial to prolonged survival than chemotherapy alone, and feasible for patients who underwent gastrectomy. Based upon our data, we suggest RFA for the treatment of liver metastatic lesions if the liver metastases are up to 5 cm in diameter, less than four in number, and metachronous. For future study, a well-controlled prospective study would be more helpful for a detailed evaluation of RFA efficacy in gastric cancer with liver metastases.
Declaration of interest: This work was funded by the Korea Science and Engineering Foundation (KOSEF) grant awarded by the Korean government (MOST) (R11-2000-082-03006-0). The authors have no conflicting financial interests.
References
- Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, Ahn JB, Roh JK, Noh SH, Chung HC. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol 2008; 19: 1146–1153
- Marrelli D, Roviello F, De Stefano A, Fotia G, Giliberto C, Garosi L, Pinto E. Risk factors for liver metastases after curative surgical procedures for gastric cancer: A prospective study of 208 patients treated with surgical resection. J Am Coll Surg 2004; 198: 51–58
- Okano K, Maeba T, Ishimura K, Karasawa Y, Goda F, Wakabayashi H, Usuki H, Maeta H. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg 2002; 235: 86–91
- Sakamoto Y, Ohyama S, Yamamoto J, Yamada K, Seki M, Ohta K, Kokudo N, Yamaguchi T, Muto T, Makuuchi M. Surgical resection of liver metastases of gastric cancer: An analysis of a 17-year experience with 22 patients. Surgery 2003; 133: 507–511
- Sakamoto Y, Sano T, Shimada K, Esaki M, Saka M, Fukagawa T, Katai H, Kosuge T, Sasako M. Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol 2007; 95: 534–539
- Zacherl J, Zacherl M, Scheuba C, Steininger R, Wenzl E, Mühlbacher F, Jakesz R, Längle F. Analysis of hepatic resection of metastasis originating from gastric adenocarcinoma. J Gastrointest Surg 2002; 6: 682–689
- Koga R, Yamamoto J, Ohyama S, Saiura A, Seki M, Seto Y, Yamaguchi T. Liver resection for metastatic gastric cancer: Experience with 42 patients including eight long-term survivors. Jpn J Clin Oncol 2007; 37: 836–842
- Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol 2008; 9: 215–221
- Pozzo C, Barone C. Is there an optimal chemotherapy regimen for the treatment of advanced gastric cancer that will provide a platform for the introduction of new biological agents?. Oncologist 2008; 13: 794–806
- Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006; 24: 2903–2909
- Yonemura Y, Matuki N, Sakuma H, Katayama K, Sawa T, Fujimura T, Ohyama S, Miwa K, Miyazaki I, Tanaka M, et al. Effect of intra-hepatoarterial infusion of MMC and CDDP for gastric cancer patients with liver metastases. Surg Today 1992; 22: 253–259
- Tiberio GA, Coniglio A, Marchet A, Marrelli D, Giacopuzzi S, Baiocchi L, Roviello F, de Manzoni G, Nitti D, Giulini SM. Metachronous hepatic metastases from gastric carcinoma: A multicentric survey. Eur J Surg Oncol 2009; 35: 486–491
- Kumada T, Arai Y, Itoh K, Takayasu Y, Nakamura K, Ariyoshi Y, Tajima K. Phase II study of combined administration of 5-fluorouracil, epirubicin and mitomycin-C by hepatic artery infusion in patients with liver metastases of gastric cancer. Oncology 1999; 57: 216–223
- Ohtsu A, Shimada Y, Shirao K, Boku N, Hyodo I, Saito H, Yamamichi N, Miyata Y, Ikeda N, Yamamoto S, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol 2003; 21: 54–59
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol 2006; 24: 4991–4997
- Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, Planker M, Santos JG, Piedbois P, Paillot B, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol 2000; 18: 2648–2657
- Yonemura Y, Ohyama S, Kamata T, Fujimura T, Kimura H, Matsuki N, Sakuma H, Sawa T, Katayama K, Hasegawa H, et al. Multivariate analysis of gastric cancer patients with liver metastases. Gan To Kagaku Ryoho 1990; 17: 2063–2069
- Ajani JA. Evolving chemotherapy for advanced gastric cancer. Oncologist 2005; 10: 49–58
- Kang GH, Kim GS, Lee HR, Yuh YJ, Kim SR. A Phase II trial of paclitaxel, 5-fluorouracil (5-FU) and cisplatin in patients with metastatic or recurrent gastric cancer. Cancer Res Treat 2008; 40: 106–110
- Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, Curley SA, Zorzi D, Abdalla EK. Solitary colorectal liver metastasis: Resection determines outcome. Arch Surg 2006; 141: 460–466, discussion 466–467
- Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg 1995; 19: 59–71
- Kornprat P, Jarnagin WR, DeMatteo RP, Fong Y, Blumgart LH, D'Angelica M. Role of intraoperative thermoablation combined with resection in the treatment of hepatic metastasis from colorectal cancer. Arch Surg 2007; 142: 1087–1092
- Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg 2003; 90: 1240–1243
- Garrean Sean JH, Saied Abdul, Helton WS, Espat NJ. Radiofreqency ablation of primary and metastatic liver tumors: A critical review of the literature. Am J Surg 2008; 195: 508–520
- Bremers AJ, Ruers TJ. Prudent application of radiofrequency ablation in resectable colorectal liver metastasis. Eur J Surg Oncol 2007; 33: 752–756
- Abitabile P, Hartl U, Lange J, Maurer CA. Radiofrequency ablation permits an effective treatment for colorectal liver metastasis. Eur J Surg Oncol 2007; 33: 67–71
- Rhim H. Review of Asian experience of thermal ablation techniques and clinical practice. Int J Hyperthermia 2004; 20: 699–712
- Imamura H, Matsuyama Y, Shimada R, Kubota M, Nakayama A, Kobayashi A, Kitamura H, Ikegami T, Miyagawa SI, Kawasaki S. A study of factors influencing prognosis after resection of hepatic metastases from colorectal and gastric carcinoma. Am J Gastroenterol 2001; 96: 3178–3184
- Ambiru S, Miyazaki M, Ito H, Nakagawa K, Shimizu H, Yoshidome H, Shimizu Y, Nakajima N. Benefits and limits of hepatic resection for gastric metastases. Am J Surg 2001; 181: 279–283
- Bines SD, England G, Deziel DJ, Witt TR, Doolas A, Roseman DL. Synchronous, metachronous, and multiple hepatic resections of liver tumors originating from primary gastric tumors. Surgery 1993; 114: 799–805, discussion 804–795
- Miyazaki M, Itoh H, Nakagawa K, Ambiru S, Shimizu H, Togawa A, Shiobara M, Ohtsuka M, Sasada K, Shimizu Y, et al. Hepatic resection of liver metastases from gastric carcinoma. Am J Gastroenterol 1997; 92: 490–493
- Elias D, Cavalcanti de Albuquerque A, Eggenspieler P, Plaud B, Ducreux M, Spielmann M, Theodore C, Bonvalot S, Lasser P. Resection of liver metastases from a noncolorectal primary: Indications and results based on 147 monocentric patients. J Am Coll Surg 1998; 187: 487–493
- Foster JH. Survival after liver resection for secondary tumors. Am J Surg 1978; 135: 389–394
- Harrison LE, Brennan MF, Newman E, Fortner JG, Picardo A, Blumgart LH, Fong Y. Hepatic resection for noncolorectal, nonneuroendocrine metastases: A fifteen-year experience with ninety-six patients. Surgery 1997; 121: 625–632
- An JY, Kim JY, Choi MG, Noh JH, Choi D, Sohn TS, Kim S. Radiofrequency ablation for hepatic metastasis from gastric adenocarcinoma. Yonsei Med J 2008; 49: 1046–1051
- Kosaka T, Imaizumi H, Kamei K, Usami K, Nakano Y, Ueno K, Takashima S. A case of gastric cancer patient with liver metastasis treated by radiofrequency ablation therapy combined with intra-arterial chemotherapy. Gan To Kagaku Ryoho 2004; 31: 1737–1739
- Carditello A, Scisca C, Stilo F, Parisi A, Basile M. The possible role of radiofrequency as complementary treatment of locally advanced gastric cancer. Annali italiani di chirurgia 2005; 76: 39–41
- AJCC Cancer Staging Manual. 6th edn. New York, NY: Springer, 2002, pp. 99–106.
- Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, III, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT, Jr, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria. J Vasc Interv Radiol 2009; 20: S377–390
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–216
- Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 2003; 14: S199–202
- Fumoleau P. Treatment of patients with liver metastases. Anticancer Drugs 1996; 7: S21–23
- Frezza EE, Wachtel MS, Barragan B, Chiriva-Internati M, Cobos E. The role of radiofrequency ablation in multiple liver metastases to debulk the tumor: A pilot study before alternative therapies. J Laparoendosc Adv Surg Tech A 2007; 17: 282–284
- Schildberg FW, Meyer G, Piltz S, Koebe HG. Surgical treatment of tumor metastases: General considerations and results. Surg Today 1995; 25: 1–10
- Ribeiro MA, Jr, Rodrigues JJ, Habr-Gama A, Chaib E, D'Ipolitto G, Fonseca AZ, Saad WA, Jr, Saad WA. Radiofrequency ablation of primary and metastatic liver tumors–4 years experience. Hepatogastroenterology 2007; 54: 1170–1175
- Mertyna P, Dewhirst MW, Halpern E, Goldberg W, Goldberg SN. Radiofrequency ablation: The effect of distance and baseline temperature on thermal dose required for coagulation. Int J Hyperthermia 2008; 24: 550–559
- Sorensen SM, Mortensen FV, Nielsen DT. Radiofrequency ablation of colorectal liver metastases: Long-term survival. Acta Radiol 2007; 48: 253–258
- Siperstein AE, Berber E, Ballem N, Parikh RT. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg 2007; 246: 559–565, discussion 565–557
- Mulier S, Ni Y, Jamart J, Michel L, Marchal G, Ruers T. Radiofrequency ablation versus resection for resectable colorectal liver metastases: Time for a randomized trial?. Ann Surg Oncol 2008; 15: 144–157
- Leen E, Horgan PG. Radiofrequency ablation of colorectal liver metastases. Surg Oncol 2007; 16: 47–51
- Rasmussen F. Radiofrequency ablation of liver metastases improves the survival rate of patients with metastatic colorectal disease. Acta Radiol 2007; 48: 250–251
- Pereira PL. Actual role of radiofrequency ablation of liver metastases. Eur Radiol 2007; 17: 2062–2070
- Nomura T, Kamio Y, Takasu N, Moriya T, Takeshita A, Mizutani M, Hachiya O, Hirai I, Kimura W. Intrahepatic micrometastases around liver metastases from gastric cancer. J Hepatobiliary Pancreat Surg 2009; 16: 493–501
- Shirabe K, Wakiyama S, Gion T, Watanabe M, Miyazaki M, Yoshinaga K, Tokunaga M, Nagaie T. Hepatic resection for the treatment of liver metastases in gastric carcinoma: Review of the literature. HPB (Oxford) 2006; 8: 89–92