Abstract
Purpose: To carry out a preliminary study examining the efficacy of long-term hot-tub therapy (HTT) in the improvement of diabetic complications on streptozotocin-induced diabetic rats.
Materials and methods: Male Wistar rats were immersed mid-sternum in a circulating water bath (42°C for 30 min) to obtain a core body temperature of 41°C; this process was repeated three times a week for 5 months. The blood was collected every month. Multiple parameters were examined for all rats including heat shock protein (Hsp70) level, serum glucose and insulin concentrations, advanced glycation end product (AGE) and glycated haemoglobin (HbA1c) formation, lipid profile and antioxidant defence system. Additionally, the chaperoning capacity of glycated Hsp70 was evaluated based on in vitro studies in which the refolding of denatured luciferase was compared to refolding by native Hsp70.
Results: HTT-treated diabetic rats showed a significant improvement in lipid profile, antioxidant capacity, insulin secretion and serum Hsp70 level and a significant decrease in AGE formation compared to the untreated diabetic rats. However, HTT had a borderline significant effect on weight and fasting blood glucose. Glycated Hsp70 lost its chaperoning ability to reactivate the denatured luciferase.
Conclusion: A decrease in complications in diabetic rats after hot-tub therapy is shown here. An increase in the extracellular Hsp70 level due to HTT was observed. This increase may serve to protect the structure of proteins (e.g. preventing AGE formation), and the observed beneficial effects may be related to it.
Introduction
It has been demonstrated that hyperglycaemia plays a critical role in causing diabetic complications, including: cardiovascular disease, nephropathy, neuropathy and retinopathy. High blood glucose levels eventually damage blood vessels, nerves, and other organ systems in the body, principally through increasing non-enzymatic glycation of proteins and formation of advanced glycation end-products (AGE). Due to the interaction of reducing sugars with active amino groups of proteins, and other biomolecules, Schiff base as a primary product is formed. Then it is spontaneously rearranged to form Amadori products. In the case of haemoglobin (Hb) this product is named HbA1c. Subsequent reactions including: dehydrations, oxidation-reductions and other rearrangements cause the formation of AGEs. The ability of AGEs to form the covalent crosslink between proteins, lipids and nucleic acids is the main reason for the structural changes of biomolecules that affect their functions as basement membrane, cellular matrix, vessel wall, etc. Several AGE receptors (RAGEs) were characterised in different cell types. AGE-RAGE binding leads to generation of reactive oxygen species (ROS) and inducing oxidative stress. In addition, ROS is generated by other reactions in the process of AGE formation, including Schiff base pathway. The above-mentioned processes are the main mechanisms involved in glycated protein toxicity that results in the diabetes complications Citation[1–4]. There is clinical evidence that a reduction in AGE formation can significantly decrease the risk of diabetic complications Citation[5], Citation[6]. There are different protocols for prevention of pathogenesis of glycated proteins, such as strategies to prevent protein glycation due to hyperglycaemia and/or induction of protein chaperones to protect the structure and function of proteins.
Heat shock proteins (HSPs), sometimes referred to as stress proteins; belong to a group of proteins known as molecular chaperones that are present in all cells in most forms of life. These proteins are induced in response to a variety of stresses (such as heat shock, chemical agents, and pathophysiological stresses) and play central roles in protein folding. Hsp70 is one of the major stress protein families found in a variety of organisms. The induction of intracellular Hsp70 protein requires the heat shock factor 1 (HSF1) be bound to the heat shock element (HSE) in the promoter region of the Hsp70 gene Citation[7], where it can then be released into the extracellular media Citation[8], Citation[9].
Recent studies have shown that expression of HSPs is required to maintain the integrity of protein structure, and when HSP levels reduced in the rat model of streptozotocin-induced type 1 diabetes Citation[10], in human subjects with diabetes Citation[11], and in people with insulin resistance Citation[12], the onset of complications of diabetes may result.
Due to protein glycation and structural changes, elevation of chaperone capacity in diabetic patients is a reasonable goal for protection of proteins against these harmful changes and conservation of their native structure. Therefore, medications and lifestyles changes that raise HSP levels lessen diabetic complications and slow the progression of diabetes. In addition, it has been reported that exercise increases HSP levels and may therefore offset compromised heat shock protein-mediated tissue defences in diabetes Citation[13].
We decided to examine whether long-term hot-tub therapy (HTT) can increase HSP concentration and thereby reduced the diabetic complications in type I diabetes in rats. In this study, we have investigated the effect of HTT using streptozotocin (STZ)-induced diabetic rat model. We tested whether Hsp70 and AGE formation were affected and correlated this with several disease state endpoints, such as glucose and insulin levels, HbA1c formation, lipid profile, and the status of the antioxidant defence system over a 5-month treatment period.
Methods
Animal care and treatment
Male Wistar rats, 8 weeks old and weighing 240 ± 20 g, were housed under controlled temperature conditions with a 12 h light and 12 h dark cycle. After 3 weeks, they were randomly divided into four groups. Two groups (G2 & G4) were given intraperitoneal injection of streptozotocin (50 mg/kg body weight in sodium citrate buffer, pH 4.5) Citation[14]. If the blood glucose level was <15 mmol/L after 4 days, the injection was repeated once, and only rats with blood glucose (Glc) levels ≥15 mmol/L were included in our experiments. Control animals (G1 & G3) were injected with vehicle alone. The number of rats in each group was between seven and nine animals. The experimental protocol was approved by the Animal Ethical Committee in accordance with the guidelines for the care and use of laboratory animals prepared by Tarbiat Modares University.
For hot-tub therapy (HTT), the treated groups (control (G3) and diabetic rats (G4)) were put in a restraint and immersed mid-sternum in a circulating water bath, set at 42°C for 30 min. This procedure resulted in a core body temperature–monitored by a rectal temperature probe–of 41°C after about ten minutes and would remain for 20 min Citation[15]. The restraint was used in order to avoid bias of the results from exercise or anaesthesia Citation[15–19]. The untreated groups, G1 & G2 (control and diabetic rats, respectively), were retained in the restraint in air, at room temperature (about 25°C) Citation[15]. The treatment began after one week of diabetes induction (time zero in the Figures and Tables), was repeated three times a week and continued up to five months. All animals were subsequently sacrificed and their organs were stored at −70°C for further studies.
Blood samples were collected from the orbit vein every 30 days for 5 months. Ethylene diamine tetra acetic acid (EDTA)-treated whole blood samples were saved for HbA1c determination and serum samples were prepared by 15 min centrifugation of blood at 5000 × g; serum was collected and stored at −70°C for further studies.
Measurement of lipid profile, glucose and insulin
Serum glucose, total cholesterol (TC) and triglyceride (TG) levels were measured by enzymatic colorimetric methods (Pars Azmune, Tehran), using Autoanalyzer Model Selectera 2. After precipitation of non-HDL lipoproteins by phosphotungstic acid and magnesium chloride in the sera, HDL cholesterol (HDL-c) was determined by an enzymatic colorimetric method (Pars Azmune). Low density lipoprotein cholesterol (LDL-c) was calculated according to the Friedewald formula for samples containing less than 400 mg/dL TG Citation[20]. Serum insulin levels were measured using a rat insulin enzyme linked immuno assay (ELISA) kit (Mercodia Corporation, Uppsala, Sweden).
Glycation measurements: HbA1c, AGE and FRAP
In this part the products of proteins glycation and their effect on the antioxidant defence system were investigated.
HbA1c (glycated haemoglobin) was measured by ion exchange chromatography method (Biosystems, Barcelona). AGE determination was performed according to the method of Kalousova et al. Citation[21]. Blood serum was diluted 1:50 with phosphate buffered saline (PBS) pH 7.4 and fluorescence intensity was recorded at the emission maximum (440 nm) upon excitation at 350 nm using the spectrofluorometer Shimadzu, Model RF 5000. Fluorescence intensity was expressed as a percentage of fluorescent emission (F%). The reducing ability of biological samples was determined by ferric reducing ability of plasma (FRAP) assay Citation[22]. FRAP assay measures the changes in the absorbance of FRAP reagent at 593 nm (Spectrophotometer Shimadzu, Model 3101) due to the formation of a blue coloured Fe II- tripyridyltriazine (TPTZ) complex from colourless oxidised Fe III formed by the action of electron donating antioxidants in the serum.
Determination of serum Hsp70 level
Serum Hsp70 was measured using a commercially available ELISA kit (StressGen Biotechnologies, USA). The standard curve was plotted between 0.78 and 50 ng/mL of protein in the solution. The sensitivity of the assay was 0.2 ng/mL. The concentrations of the Hsp70 protein in the serum were determined using the standard curve.
In vitro glycation of Hsp70
The stock solution of Hsp70 was prepared by dissolving it in PBS, pH 7.4. This solution was subsequently diluted with glucose solution made in the same buffer to form incubation mixtures of 1.25 µM of protein with 50 mmol/L glucose Citation[23]. After being sterilised by filtration (0.22 µm filters, Millipore, USA) the solutions were incubated at 37°C for 45 days in capped vials.
Refolding experiments
Firefly luciferase (FL) (25 µM) was denatured using buffer A (6 M guanidinium hydrochloride (GnHCl) in 30 mM tris HCl, pH 7.4, 5 mM dithiothreitol) by incubation for 30 min at room temperature. The denatured luciferase was diluted 1:100 to 0.25 µM (final concentration) using buffer B (10 mM 3-(N-morpholino)propanesulphonic acid (MOPS), pH 7.2, 50 mM KCl) at 25°C containing Mg2+ATP (5 mM MgCl2, 1 mM ATP) Citation[24]. The denatured luciferase was then incubated with or without normal or glycated Hsp70 (1.25 µM). At specific time intervals, luciferase activity from each of the above samples was measured using a Cary-Eclipse luminescence spectrophotometer (Varian, Victoria, Australia).
Statistical analysis
All data were analysed by SPSS and were expressed as mean ± SD. For multiple comparisons, data were analysed using the one-way or two-way repeated-measures ANOVA with treatment and time as the two factors. The non-parametric tests (Fridman and Kruskal-Wallis) were also used for the analysis of data with heterogeneous SD such as insulin.
All animals in the control groups, with or without HTT, survived up to the end of experiment; but 6 of 8 and 3 of 8 rats lived up to the end of experiment in the diabetic groups with or without HTT, respectively. Thus, to estimate the effect of HTT on the survival of animals, the Kaplan-Meier survival (univariate) analysis was used.
Results
indicate the induction of diabetes by STZ in rats (changes in the serum glucose level), its effect on animals’ weight and AGE formation; as well as the effect of HTT on these parameters. As seen in , the glucose levels in the sera of both diabetic groups are higher than that in the control groups (P < 0.01). The average serum glucose level during 5 months of experiment in groups 2 and 4 were 352.2 ± 27.3 mg/dL and 338.4 ± 23.2 mg/dL, respectively.
Figure 1. Changes in the (A) glucose (Glc) concentration, (B) weight and (C) fluorescence intensity (F%) of advanced glycated end products (AGEs) during the time course of the experiment in different groups of rats. In all figures (except ), the same symbols are used. ♦ Group 1 Control Room Temperature Air, ▪ Group 2 Diabetic Room Temperature Air, ▴ Group 3 Control group with HTT and □ Diabetic group with HTT. Data are means ± SD (n = 5–7). *P < 0.05, **P < 0.01 and ***P < 0.001 show the significant changes between two diabetic groups with or without HTT. ♣ shows the borderline of significance (0.05 < p < 0.1) in weight and glucose (P = 0.077 and P = 0.062, respectively).
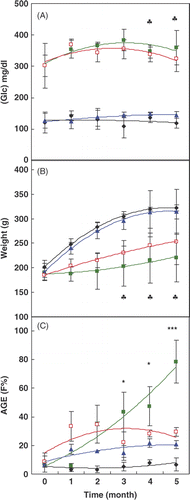
shows the changes in the weight of the rats under experiment. The results indicate that the weights of two diabetic groups were significantly (P < 0.01) lower than those of the two control groups.
These results indicate a decrease in the serum Glc level and an increase in the weight of diabetic animals due to HTT, but these changes were not significant (P = 0.077 and P = 0.062, respectively).
indicates that the AGE formation in the diabetic group treated in room temperature air increased gradually; however, in the diabetic group under treatment with HTT, it remained near the normal value. During the intervention period, there was a time × treatment interaction for AGE (P = 0.036). The significant differences (p < 0.05) in AGE formation between the diabetic group under treatment with HTT and those held in room temperature air began at the third month of the experiment.
HbA1c was also determined in the blood of rat groups. It was increased due to diabetes induction and decreased in the diabetic group with HTT, but these changes were not significant (P = 0.552) (data not shown).
indicates the serum insulin level in rats during the course of study. Serum insulin levels were significantly decreased in diabetic rats (G2 and G4) compared to control rats (G1 and G3) by Kruskal Wallis test (P < 0.05). In response to HTT, the Fridman test showed significant (P < 0.05) increases in the serum insulin levels in the diabetic group receiving HTT (within G4).
Table I. Comparison of serum insulin levels in control and diabetes rats with or without hot-tub therapy (HTT).
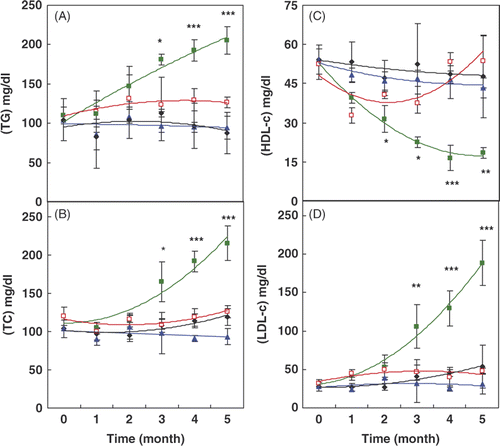
In the study of the lipid profile, our results () showed that the TG, TC and LDL-c levels increased significantly in untreated diabetic rats compared to the control groups. However, in rats receiving HTT, these parameters significantly decreased during the course of the experiment. In addition, the HDL-c that decreased significantly in diabetic rats significantly increased in the diabetic group treated with HTT.
Figure 2. Changes in the lipid profile, including: (A) triglyceride (TG), (B) total cholesterol (TC), (C) high density lipoprotein cholesterol (HDL-c) and (D) low density lipoprotein cholesterol (LDL-c) during the time course of the experiment in different groups of rats (the same indicators are used as in ). Data are means ± SD (n = 5–7). *P < 0.05, **P < 0.01 and ***P < 0.001 show the significant changes between two diabetic groups with or without HTT.
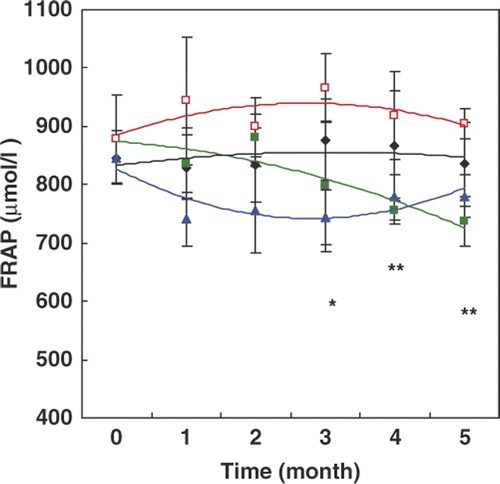
The results presented in indicate that the FRAP decreased gradually in the diabetic group in comparison with the control group and reached the minimum at month 5 of the experiment (p < 0.05). Interestingly, this decrease did not occur in diabetic rats receiving HTT, and levels in this group were comparable to the control rats.
Figure 3. Changes of the ferric reducing ability of plasma (FRAP) during the time course of the experiment in different groups of rats. In this figure the same indicators are used as in . Data are means ± SD (n = 5–7). *P < 0.05, **P < 0.01 and ***P < 0.001 show the significant changes between two diabetic groups with or without HTT.
demonstrates a gradual decrease in the serum Hsp70 level of diabetic rats. The data showed a significant increase of Hsp70 in the diabetic rats after 2 months of treatment with HTT, in comparison to the diabetic rats without treatment (P < 0.05) and this level exceeded that of non-diabetic rats.
Table II. Comparison of serum Hsp70 levels in control and diabetic rats with or without hot-tub therapy (HTT).
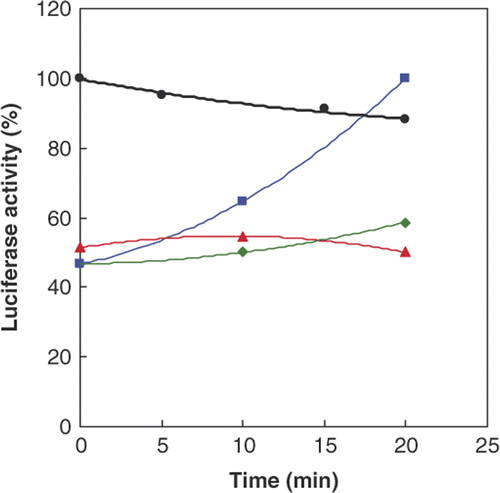
shows a very low activity for denatured luciferase with guanidinium hydrochloride (GnHCl) compared to that normal luciferase. Activity of the denatured enzyme increased (about two times) upon incubation with native Hsp70, but the glycated Hsp70 (produced by the preincubation of Hsp70 with Glc) was not able to refold the denatured protein and reactivate it. This result indicates that although the Hsp70 is able to refold the protein, non-enzymatic glycation (maybe due to its conformational change) causes Hsp70 to lose the ability to reactivate luciferase. It was observed that, in the time course of the experiment, the activity of wild type luciferase gradually decreased.
Figure 4. Activity of native (•, black) and denatured Firefly luciferase by GnHCl, in the absence (♦, green) or presence (▪, blue) of native and glycated Hsp70 (▴, red) at different time intervals. The activity of native enzyme (luciferase) was compared to the original activity and its activity in the other samples was compared to the maximum recovery by normal Hsp70 at 20 min.
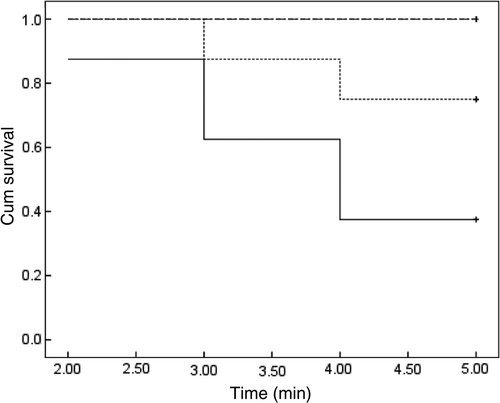
A Kaplan-Meier analysis was used to compare the survival of diabetic rats, with or without HTT treatment. reveals the beneficial effect of heat treatment on the diabetic rats. As seen, all rats in the control groups with or without HTT survived up to the end of the experiment; but in the diabetic groups the numbers of surviving animals with or without treatment at the end of experiment was 5 and 3 from 8, respectively. Statistical analysis showed significant differences between four groups of rats (p = 0.02). The Log Rank test indicated no significant differences between the survival in the HTT treated diabetic group with diabetic rats (p = 0.129), control group (p = 0.247) and control rats with HTT (p = 0.205). However, a significant difference was observed between diabetic rats with the control group (p = 0.036), and the control group with HTT (p = 0.023).
Figure 5. Plot of rats survival (cumulative survival) analysed by the Kaplan-Meier method on all groups of rats. All animals in the control groups with or without HTT (dashed line) survived up to the end of experiment. The number of rats surviving up to the end in diabetic groups were 5 and 3 from 8 (in each group) with (dotted line) or without HTT (continuous line), respectively. The results of statistical analysis are explained in the text.
Discussion
Recent studies in our lab have demonstrated the beneficial effects of small molecules (including chemical chaperones) on diabetes complications and Hsp70 induction Citation[25–27]. Our aim here was to investigate the effect of hot-tub therapy (HTT), on diabetic rats. In fact, our particular interest in hyperthermia began after examining papers on the effect of daily hot water immersion in type 2 diabetic patients Citation[28], as well as the inducibility of Hsp70 proteins in some diseases Citation[15–17] and its benefits in animal models Citation[16]. Another recent paper using the non-obese diabetic (NOD) mouse model has shown the prevention of type 1 diabetes through repeated whole body hyperthermia treatments and in that study Citation[29] an immunological effect was postulated.
The experimental methods that have been used to increase temperature in model animals include whole body hyperthermia (WBH) by warm water immersion of anaesthetised animals Citation[15–19], heating pad application Citation[15], Citation[16], preheated cages in the dry incubator Citation[19], warm air heating Citation[19], fever-like heat treatment in non-anaesthetised animals Citation[19], Citation[29] and an insulated room under far-infrared rays Citation[30]. Here, to avoid the anaesthetic agents since they may affect protein folding and function Citation[31], Citation[32], exercise which was known as an Hsp70 inducer Citation[15] and direct heating of the animals’ heads (to increase the life time) Citation[15], the restraint was used to hold the rats in the warm water bath (42°C). All rats were put in separate restrainers and after 3 min, rats in the treatment group were suspended to mid-sternum in the water bath while the control groups were held in the air, at room temperature Citation[15]. While this method does control for the effect of stress due to the restraint, it did not control for the effect of psychological stress, which is known to occur in animals that are immersed in water Citation[15]. Thus, in future studies, a water bath set to maintain normothermic core body temperatures should be used for the control non-hyperthermia groups.
There is growing evidence to show the importance of AGEs and their complexes with specific receptors (RAGEs), as well as the oxidative stress in the development and progress of diabetic micro- and macrovascular complications. AGEs may be produced by both non-oxidative and oxidative (glycoxidation) pathways of sugars, but non-oxidative AGEs may also catalyse the secondary auto-oxidation process. Thus, the antioxidant defence system has an important role to prevent AGE formation and hence diabetic complications (nephropathy, neuropathy, angiopathy, etc.). In addition, diabetes and insulin resistance are associated with stiffer, less fluid membranes, thought to be a result of glycation, oxidative stress, and insulin deficiency Citation[31].
In the present study, various parameters, in addition to Hsp70, were selected for monitoring the beneficial effects of HTT on diabetic rats. These include serum Glc concentration and weight of rats as the basic parameters; insulin secretion to evaluate β-cell improvement; HbA1c and AGE determination to investigate the extent of protein glycation; lipid profile as a measure of cardiovascular protection; and FRAP assay to study the antioxidant capacity of plasma. Furthermore, effect of glycation on Hsp70 potential to refold/reactivate a denatured protein was investigated in an in vitro experiment. Whether this is occurring in vivo in this model was not addressed in this study, but is clearly an important next step in confirming the hypothesis presented here.
indicates a slight decrease in the serum glucose level of diabetic rats due to HTT. However, a significant reduction of serum Glc has been reported previously in human subjects with type 2 diabetes under HTT Citation[28], in the mouse model with type 1 diabetes treated with fever-range whole body hyperthermia Citation[29] and obesity-induced insulin resistance in diabetic mice treated with whole body hyperthermia Citation[30]. Heat itself makes membranes more fluid Citation[12] and hyperthermia causes an increase in blood flow to all organs. Thus, it increases Glc delivery to the tissues Citation[33].
Our results about the serum insulin level, which were analysed by non-parametric test (Fridman), indicate a significant increase in the serum insulin level of diabetic rats treated by HTT during the time course of study. However, heat alone had no effect on insulin secretion in control (normal) animals (). It was previously reported that hyperthermia protects pancreatic β-cells from warm ischaemic injury in tissue culture Citation[34] and in NOD mice Citation[29]. Thus, the protective effect of hyperthermia can cause more insulin secretion, even in type I diabetes.
Heat shock proteins, especially Hsp70, are inducible proteins whose expression and release increase due to hyperthermia Citation[8]. HSPs were initially thought to be intracellular proteins, but current literature indicates that they are released into the extracellular space and are functional proteins in this media Citation[8], Citation[35–40]. The major roles of HSPs in the extracellular media are the immunomodulatory Citation[35], Citation[38], Citation[39] increasing ion conductance through their binding to membrane Citation[36] and entering the cell and promoting mitosis of dividing cells Citation[40]. In addition, the role of Hsp70 as a quality control of extracellular proteins was indicated in both health and chronic diseases Citation[37] and the effects of exogenous HSC/Hsp70 on renaturation of heat denatured luciferase Citation[40] have been reported. Our results () also indicated a non-significant decrease in Hsp70 due to diabetes induction that was significantly increased after HTT. This increase was accompanied by a significant decrease in AGE formation. These events may be explained by a possible reduction of glucose binding (glycation) to serum proteins due to the protective effect of induced Hsp70 on protein structure. In an in vitro experiment we investigated the effect of glycation on functionality of Hsp70 (). The results showed that glycated Hsp70 lack the activity to renature/refold the denatured luciferase by GnHCl. Thus, the observed reduction in serum Hsp70 level after diabetes induction may be due to its glycation and inactivation, which led to its removal from the circulation and degradation.
Our results also showed a significant reduction in the increased levels of total cholesterol, triglyceride and LDL-c in addition to a significant elevation in the decreased HDL-c level () in the diabetic group treated with HTT compared to diabetic rats without treatment. These indicate the modification of lipid profile and lipoprotein pattern in diabetic rats by heat treatment. This is in direct relation to the cardiovascular protection and prevention of angiopathy as one of the most important consequences of diabetes.
The capability of the antioxidant activity through a significant increase in the FRAP () that are revealed in this study and anti-inflammatory effect through NFκB and MAPK/p38 signalling pathways are in accordance with earlier reports Citation[9]. Hooper also reported reduced neuropathic symptoms due to hyperthermia, without considering the HSP function Citation[28].
At the end of the experiment (after 5 months), our results indicate that the mortality in diabetic rats without treatment would be about 40% more than that of the treated group. The Kaplan-Meier analysis method was used for generating the survival curve Citation[41]. shows that the survival probability increased from 37.5% in the diabetic group to 75% in the diabetic group treated with HTT. However, it is not statistically significant (a higher number of rats is needed for survival study), but the significant differences in the survival of the diabetic group in comparison with both control groups changed to the non-significant changes after HTT. The reduction in mortality may be related to the role of extracellular Hsp70 proteins that regulate cell function Citation[42], the improvement in the lipid profile and the antioxidant defence system.
Conclusion
The results of our study reveal that long-term HTT can lead to significant changes in several markers of disease severity in diabetic rats. These observations provide the basis for more in-depth investigation into the beneficial effects of HTT for the treatment of diabetes.
Acknowledgements
The authors are grateful to S. Faghihzadeh, A. Kezemnejad and A. Ghasemi for their help with the statistical analysis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Brownlee M. The pathological implications of proteins glycation. Clin Inv Med 1995; 18: 275–281
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation-end products: A review. Diabetologia 2001; 44: 129–146
- Peppa M, Uribarri J, Vlassara H. Glucose, advanced glycation end products, and diabetes complications: What is new and what works. Clin Diabetes 2003; 21: 4,186
- Rahbar S, Figarola JL. Novel inhibitors of advanced glycation endproducts. Arch Biochem Biophys 2003; 419: 63–79
- Agardh CD, Stenram U, Torffvit O, Agardh E. Effects of inhibition of glycation and oxidative stress on the development of diabetic nephropathy in rats. J Diabetes Complications 2002; 16: 395–400
- Hammes HP, Martin S, Federlin K, Geisen K, Brownlee M. Aminoguanidine treatment inhibits the development of experimental diabetic retinopathy. Proc Natl Acad Sci 1991; 88: 11555–11558
- Craig EA, Weissman JS, Horwich AL. Heat shock proteins and molecular chaperones: Mediators of protein conformation and turnover in the cell. Cell 1994; 78: 365–372
- Fleshner M, Johnson JD. Endogenous extra-cellular heat shock protein 72: Releasing signal(s) and function. Int J Hyperthermia 2005; 21: 457–471
- Mortaz E, Redegeld FA, Bloksma N, Dunsmore K, Denenberg A, Wong HR, Nijkamp FP, Engels F. Induction of Hsp70 is dispensable for anti-inflammatory action of heat shock or NSAIDs in mast cells. Exp Hematol 2006; 34: 414–423
- Yamagish N, Nakayama K, Wakatsuki T, Hatayama T. Characteristic changes of stress protein expression in streptozotocin-induced diabetic rats. Life Sci 2001; 69: 2603–2609
- Kurucz I, Morva A, Vaag A, Eriksson KF, Huang X, Groop L, Koranyi L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes 2002; 51: 1102–1109
- Hooper PL, Hopper JJ. Loss of defense against stress: Diabetes and heat shock proteins. Diabetes Thechnol Ther 2005; 7: 204–208
- Atalay M, Oksala NK, Laaksonen DE, Khanna S, Nakao C, Lappalainen J, Roy S, Hanninen O, Sen CK. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol 2004; 97: 605–611
- Mendez JD, Ramos GHB. Animal models in diabetes research. Arch Med Res 1994; 25: 367–375
- Tolson JK, Roberts SM. Manipulating heat shock protein expression in laboratory animals. Methods 2005; 35: 149–157
- King Y-T, Lin C-S, Lin J-H, Lee W-C. Whole-body hyperthermia-induced thermotolerance is associated with the induction of Heat Shock Protein 70 in mice. J Experiment Biol 2002; 205: 273–278
- Ohno S, Siddik ZH, Baba H, Stephens LC, Strebel FR, Wondergem J, Khokhar AR, Bull JMC. Effect of carboplatin combined with whole body hyperthermia on normal tissue and tumor in rats. Cancer Res 1991; 51: 2994–3000
- Yamashita N, Hoshida S, Otsu K, Taniguchi N, Kuzuya T, Hori M. Involvement of cytokines in the mechanism of whole-body hyperthermia-induced cardioprotection. Circulation 2000; 102: 452–457
- Pritchard MT, Ostberg JR, Evans SS, Burd R, Kraybill W, Bull JM, Repasky EA. Protocols for simulating the thermal component of fever: Preclinical and clinical experience. Methods 2004; 32: 54–62
- Fridewald WJ, Levy RJ, Fredrikson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502
- Kalousova M, Skrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res 2002; 51: 597–604
- Benzie IF, Stain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal Biochem 1996; 239: 70–76
- Shaklai N, Garlick RL, Bunn HF. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J Biol Chem 1984; 259: 3812–3817
- Szabo A, Langer T, Schroder H, Flanagan J, Bukau B, Hartl U. The ATP hydrolysis dependent reaction cycle of the Escherichia coli Hsp70 system–DnaK, DnaJ, and GrpE. Proc Natl Acad Sci USA 1994; 91: 10345–10349
- Jafarnejad A, Bathaie SZ, Nakhjavani M, Hassan MZ, Banasadegh S. The improvement effect of L-Lys as a chemical chaperone on STZ-induced diabetic rats, protein structure and function. Diabetes Metab Res Rev 2008; 24: 64–73
- Jafarnejad A, Bathaie SZ, Nakhjavani M, Hassan MZ. Investigation of the mechanisms involved in the high-dose and long-term acetyl salicylic acid therapy of type I diabetic rats. J Pharmacol Exp Ther 2008; 324: 850–857
- Jafarnejad A, Bathaie SZ, Nakhjavani M, Hassan MZ. Effect of spermine on lipid profile and HDL functionality in the streptozotocin-induced diabetic rat model. Life Sci 2008; 82: 301–307
- Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med 1999; 341: 924–925
- Capitano ML, Ertel BR, Repasky EA, Ostberg JR. Fever-range whole body hyperthermia prevents the onset of type 1 diabetes in non-obese diabetic mice. Int J Hyperthermia 2008; 24: 141–149
- Kokura S, Adachi S, Manabe E, Mizushima K, Hattori T, Okuda T, Nakabe N, Handa O, Takagi T, Naito Y, et al. Whole body hyperthermia improves obesity-induced insulin resistance in diabetic mice. Int J Hyperthermia 2007; 23: 259–265
- Tanner JW, Eckenhoff RG, Liebman PA. Halothane, an inhalational anesthetic agent, increases folding stability of serum albumin. Biochim Biophys Acta 1999; 1430: 46–56
- Li X, Friedman AB, Roh M-S, Jope RS. Anesthesia and post-mortem interval profoundly influence the regulatory serine phosphorylation of glycogen synthase kinase-3 in mouse brain. J Neurochem 2005; 92: 701–704
- Horsman MR. Tissue physiology and the response to heat. Int J Hyperthermia 2006; 22: 197–203
- Perdrizet GA, Rewinski MJ, Schweizer RT, Scharp DW. Heat shock and recovery protects pancreatic islets from warm ischemic injury. Transplant Proc 1994; 26: 3477–3478
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular Hsp70. Role of toll-like receptor (TLR)2 and TLR4. J Biol Chem 2002; 277: 15028–15034
- Arispe N, Doh M, Simakova O, Kurganov B, De Maio A. Hsc70 And Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEB J 2004; 18: 1636–1645
- Yerbury JJ, Stewart EM, Wyatt AR, Wilson MR. Quality control of protein folding in extracellular space. EMBO Reports 2005; 6: 1131–1136
- Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: Repercussions in cancer therapy. J Leukoc Biol 2007; 81: 15–27
- Multhoff G. Heat shock protein 70 (Hsp70): Membrane location, export and immunological relevance. Methods 2007; 43: 229–237
- Browne CL, Swan JB, Rankin EE, Calvert H, Griffiths S, Tytell M. Extracellular heat shock protein 70 has novel functional effects on sea urchin eggs and coelomocytes. J Exp Biol 2007; 210: 1275–1287
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481
- Wilson MR, Easterbrook-Smith SB. Clusterin is a secreted mammalian chaperone. Trends Biochem Sci 2000; 25: 95–98
