Abstract
This paper reviews systems and techniques to deliver simultaneous thermoradiotherapy of breast cancer. It first covers the clinical implementation of simultaneous delivery of superficial (microwave or ultrasound) hyperthermia and external photon beam radiotherapy, first using a Cobalt-60 teletherapy unit and later medical linear accelerators. The parallel development and related studies of the Scanning Ultrasound Reflector Linear Arrays System (SURLAS), an advanced system specifically designed and developed for simultaneous thermoradiotherapy, follows. The performance characteristics of the SURLAS are reviewed and power limitation problems at high acoustic frequencies (>3 MHz) are discussed along with potential solutions. Next, the feasibility of simultaneous SURLAS hyperthermia and intensity modulated radiation therapy/image-guided radiotherapy (IMRT/IGRT) is established based on published and newly presented studies. Finally, based on the encouraging clinical results thus far, it is concluded that new trials employing the latest technologies are warranted along with further developments in treatment planning.
Hyperthermia and cancer
Conventional hyperthermia in cancer therapy can be defined as the elevation of tissue temperatures to 41° ∼ 43°C for more than 30 min. Hyperthermia is typically used as an adjunct therapy to radiotherapy and/or chemotherapy Citation[1–5]. Many well-conducted clinical trials, including phase III multi-institutional trials following quality assurance guidelines, have shown that hyperthermia can significantly increase both local tumour control rates and duration of local control in tumours that recur or persist after surgery, radiotherapy and/or chemotherapy Citation[6–9]. Furthermore, hyperthermia has been shown to be beneficial in the treatment of residual microscopic disease in the management of local-regional breast cancer Citation[10], in the treatment of soft tissue sarcomas preoperatively with radiation Citation[11], and in the treatment of deep pelvic tumours Citation[12], Citation[13]. A significant survival benefit was also reported when hyperthermia was combined with brachytherapy in the treatment of glioblastoma multiforme Citation[14] and with external beam radiotherapy in the treatment of recurrent head and neck cancer Citation[15].
In fact, when clinical trials have been conducted under widely accepted quality assurance guidelines–which implies matching the heating technology to the target site along with the implementation of a reasonable thermometry strategy–radiotherapy plus hyperthermia has always resulted in statistically better outcomes than radiotherapy alone Citation[16].
Thermoradiotherapy of breast cancer
Patients with persistent and/or recurrent breast cancer and chest wall tumours have significantly benefitted when hyperthermia has been added to their radiotherapy and/or chemotherapy regimens Citation[17–26]. One of the physical reasons for the success of hyperthermia in this group of patients is the superficial location of the local disease. Superficial lesions are the least difficult to heat adequately because of their accessibility and proximity to external energy sources. The composition of the local anatomy may also contribute to better heating Citation[27], Citation[28]. Another possible reason may be that superficial lesions are more amenable to invasive thermometry and thermal mapping of temperature sensors, thus providing more temperature feedback data during treatment, which can be used to improve heat delivery and ensure treatment quality Citation[29], Citation[30].
Clinical research efforts have demonstrated that the response of cancerous tumours to sequential thermoradiotherapy (i.e. sequentially combined radiotherapy and hyperthermia) is well correlated with power deposition coverage and/or thermal dose coverage Citation[24], Citation[43–47]. These studies also point to the challenge of achieving consistently biologically meaningful thermal doses in 100% of the target volume in routine clinical practice. Therefore, it is widely accepted that the benefits of hyperthermia as an adjunct to radiotherapy can be significantly augmented with improvements in treatment delivery techniques, better heating technology, advances in treatment planning and implementation of quality assurance guidelines Citation[6], Citation[13], Citation[30], Citation[41], Citation[42]. Moreover, in vitro and animal studies have shown that when hyperthermia and radiation are administered simultaneously–rather than sequentially as it is conventionally done–heat-induced radiosensitisation (HIR) is increased at thermal doses achievable in the clinic Citation[43–47]. For instance, mild hyperthermia −41°C maintained for ∼60 minutes–produces HIR if radiation and heat are delivered simultaneously, but not sequentially. This is significant because a minimum target temperature of 41°C is more clinically achievable than the >42°C needed to produce HIR with sequential treatment Citation[44], Citation[47], Citation[48]. The above facts support the development of clinical hyperthermia devices that permit simultaneous delivery of heat and ionising radiation.
Clinically implemented approaches for simultaneous thermoradiotherapy of breast cancer
In this section a review is given of the systems and techniques clinically implemented for the delivery of simultaneous thermoradiotherapy at Washington University in St Louis. This research group has been the only one to complete several simultaneous thermoradiotherapy clinical trials with breast cancer being the site treated the most.
Microwave hyperthermia and Co-60 teletherapy
Although it has been known from in vitro and in vivo experiments that simultaneous delivery resulted in larger thermal enhancement ratios than sequential delivery, simultaneous treatments had not been tried with human patients mainly due to formidable logistical problems Citation[46]. The research group set out to overcome these problems and embark on a long-term clinical research effort. The first approach consisted of heating with 915 MHz microwave applicators and irradiating with a Co-60 teletherapy unit Citation[49]. Both technologies enjoyed extensive clinical experience, were well-understood and had been used in sequential thermoradiotherapy Citation[39], Citation[40]. Therefore, any unusual response to the new therapy approach could be attributed to the simultaneous delivery of heat and gamma radiation. Hyperthermia waveguide applicators were used, one at the time. Two clinical set-ups were characterised. In one a microwave applicator was attached to a blocking tray and inserted into the tray slot in the head of the gantry of the teletherapy unit. Thus the gamma beam travelled through a mostly hollowed waveguide and into a treatment volume after transversing a mineral oil coupling bolus. This was called the en face set-up. The other set-up was with the microwave propagation vector perpendicular to the gamma beam central axis. This was called the orthogonal set-up. Using these set-ups a 400 cGy radiation fraction of 4 to 7 min duration could be delivered in the middle of a 60-min hyperthermia treatment without interruptions. Temperatures and power level were remotely monitored and recorded outside the teletherapy room. The microwave applicator was not mounted or in contact with the teletherapy unit in the orthogonal set-up, so it did not affect the radiation dose distributions. However, for the en face set-up the dose distributions were affected by the microwave applicator. Film measurements in a solid water phantom showed uniform dose within the radiation field, except for 10–18% attenuation under the metal tuning electrodes inside the waveguide. This dose defect was clinically smoothed using feathering techniques. This approach was used successfully without technical problems in a phase I/II clinical trial. Importantly, an analysis showed that the temperature distributions achieved during simultaneous delivery had the same general characteristics as those achieved during sequential delivery, and that the steady-state distributions were maintained during the time of simultaneous irradiation. This first experience demonstrated that simultaneous superficial microwave hyperthermia and external beam radiation was technically feasible and safe Citation[49].
Ultrasound hyperthermia and Co-60 teletherapy
The waveguide microwave applicators limited the size of the lesions we could treat with hyperthermia simultaneously with gamma rays Citation[39], Citation[40]. In order to be able to treat larger lesions, we decided to adapt a commercial planar ultrasound system to deliver simultaneous treatments. This task was much more challenging because the ultrasound applicators were not hollowed like the microwave waveguides, and degassed water was needed to couple the sound waves into the treatment volume. The ultrasound applicators of the Labthermics 1000 system were also bulkier and heavier than the microwave applicators. An orthogonal set-up similar to the one used with microwaves was relatively simple to mimic, but many tumour sites would require an en face set-up for proper treatment. Since ultrasound is reflected efficiently and specularly from metal surfaces, we devised a reflecting system in which the ultrasound beam, initially directed perpendicularly to the gamma beam, was deflected 90° so that both beams travelled through the same window of entry into the tumour while the ultrasound source remained outside the radiation beam Citation[50]. The reflecting system, which was mounted on the blocking tray, was filled with degassed water and made of water-equivalent materials, except for a 1 mm sheet of polished brass used as the reflector. With this en face approach the absorbed power patterns generated with and without the reflecting system at the same extended distance from the transducer demonstrated that the hyperthermia system remained able to control heating patterns. The effect of the reflecting system on the gamma beam was minimal except for uniform attenuation, and the Co-60 beam had no effect on the performance of the thermocouples. Extensive testing followed by clinical treatments of patients on protocol demonstrated that the modifications made did not impair the ability to deliver ultrasound hyperthermia or teletherapy effectively. Again, this work showed that the implementation of ultrasound hyperthermia simultaneous with gamma irradiation was technically and clinically feasible without complications or hazards to patients.
Superficial (microwave or ultrasound) hyperthermia and megavoltage photon beam radiation using a medical linear accelerator
In 1995 the Co-60 teletherapy unit at the Mallinckrodt Institute of Radiology was decommissioned. The only option for external beam radiation was to develop the devices and techniques to allow the use of a medical linear accelerator. Although excited about the use of modern radiation technology, the transfer to megavoltage photon beam necessitated additional developments and measurements. Both the orthogonal and the en face set-ups were successfully transferred, but had to be adapted to the different geometry and dimensions of the linear accelerator (linac). Straube et al. (2001) reported on devices, techniques and dosimetry for simultaneous thermoradiotherapy delivered with a linear accelerator including detailed day-to-day simulation and treatment procedures Citation[51].
Summary of simultaneous thermoradiotherapy clinical trials
The above described developments have made possible the conduct of clinical trials since 1992. The first phase I trial was ended successfully in 1995, a second phase I/II dose escalation trial closed in 2001, and a third trial was initiated 1999. These clinical results have been summarised previously by Myerson et al. (1999, 2004) Citation[44], Citation[47]. To date, 119 patients have been enrolled on these three consecutive trials. The first two trials (60 cases) were for patients with macroscopic disease, usually recurrent after prior therapies, including radiotherapy, and escalated the number of simultaneous treatments from three to eight in a course of 30–32 Gy. The third trial was to evaluate the short- and long-term effects and local control of simultaneous thermoradiotherapy in the treatment of high risk but curative subclinical (no visible disease after surgery and/or chemotherapy) breast carcinoma in patients with no prior radiotherapy. The simultaneous administration of radiotherapy and hyperthermia is of particular potential value for this patient population because tumour cells share normal tissue physiology. Fifty-nine patients were accrued in this trial. The number of simultaneous treatments was escalated from four to eight in a course of 60 Gy; most patients receiving 400 cGy per hyperthermia treatment–approximately 26% to 53% of the radiation dose was given under hyperthermic conditions. Elective hyperthermia was given to the primary tumour bed site plus an adjacent 6 × 12 cm strip randomised to lie medial or lateral to the primary site. The adjacent strip served as an unheated but irradiated control.
The first two studies demonstrated that the per treatment average thermal dose progressively improved with the number of hyperthermia treatments. In addition, the product of radiation dose and total thermal dose was highly correlated with complete response Citation[47]. In the third trial the overall local control was 97% with no significant morbidity difference between heated and control sectors and no increase in morbidity between four and eight simultaneous treatments.
Finally, the data clearly shows that chest wall lesions (recurrent breast cancer) have been the most responsive to simultaneous thermoradiotherapy with highly significant statistical correlation with radio-thermal dose factors (p < 0.05) Citation[47]. This exciting finding points to the importance of developing thermoradiotherapy treatment planning systems and continuing efforts to improve clinical thermal dosimetry techniques.
The SURLAS: A device developed for simultaneous thermoradiotherapy
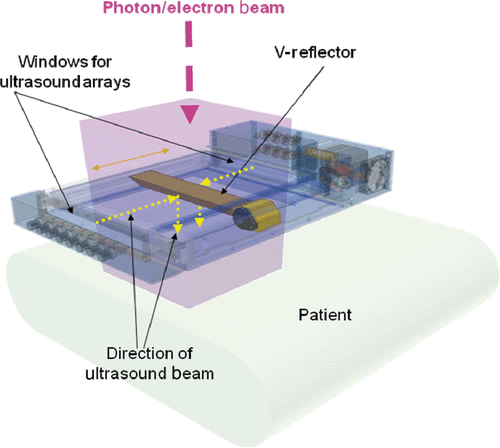
In parallel with efforts to adapt existing commercially available hyperthermia technology to deliver simultaneous hyperthermia and external photon beam radiation, ideas were being discussed for the development of a system specifically designed for this purpose. During the 1990s, electron beams were used commonly in the treatment of superficial lesions due to their limited depth of penetration, which meant sparing underlying normal tissues. However, the existing hyperthermia devices were too bulky/dense to permit the use of electron beams. This was the main reason only high energy photon beams had been used when delivering simultaneous thermoradiotherapy. Eventually, the concept of the scanning ultrasound reflector linear arrays system, or SURLAS, was born Citation[52], Citation[53]. By combining ultrasound linear arrays and scanning ultrasound reflectors, an applicator thin enough to permit the passage of high energy electron beams could be made to work using an en face approach. Obviously, photon beams would also work as in the past. The device concept is illustrated in , which shows a computer-aided solid model of the clinical grade applicator that was developed Citation[54], Citation[55]. shows the basic components of a dual-frequency SURLAS applicator. The reflecting surfaces of a scanning double-faced V-shaped reflector make 45° angles with respect to the sound propagation coming from the (high and low frequency) arrays at the side ends of the applicator head ( shows the windows for the arrays, not the arrays), thus both sound beams are deflected in parallel towards the treatment volume. As the reflector scans back and forth, the sound energy is distributed over the treatment volume producing therapeutic time-averaged temperature distributions with acceptable temperature fluctuations Citation[56], Citation[57]. Each array has several individually powered elements. Modulation of power input to the array elements as the reflector scans between the arrays provides control of the two-dimensional surface energy fluence pattern Citation[55], Citation[58]. This capability is referred to as lateral power conformability or LPC. One of the arrays operates at a low frequency (1 ∼ 2 MHz) while the other operates at a high frequency (3 ∼ 5 MHz). This arrangement allows concomitant dual-frequency insonation. Penetration depth control, or PDC, is thus achieved by varying the power input to one array relative to the other, i.e. frequency mixing Citation[59], Citation[60]. For example, for areas where the target volume is deeper, the low frequency can be weighted more heavily than the high frequency. LPC plus PDC means that the SURLAS has 3D power deposition control, which can eventually be fully exploited with the implementation of temperature feedback control strategies based on temperatures measured during treatment Citation[61].
Several laboratory prototypes of the SURLAS were developed over the years in order to demonstrate feasibility of the overall concept and of particular features such as LPC and PDC. Finally, a clinical grade system was developed that could be used under approval of the Federal Food and Drug Administration (FDA) via the Investigational Device Exemption (IDE). However, manufacturing a clinical device proved to be a much more rigorous process than assembling prototypes for laboratory studies. Important requirements concerning reliability, patient and operator safety, and quality control/assurance presented great challenges to the research group. In addition to these rigorous requirements, the following were specific design criteria for the clinical SURLAS applicator:
Capable of heating superficial tumours extending up to 30 mm deep and up to 150 × 150 mm lateral dimensions;
Capable of controlling power deposition in three dimensions;
No operational interference with radiotherapy linear accelerators;
Suitable for delivery of an external photon (en face or orthogonal set-ups) or electron (en face set-up only) beam radiation during a hyperthermia treatment;
Significantly more compact and lighter than existing commercial ultrasound applicators;
These design criteria were met for the most part as reported in a paper published in early 2005 that focused mainly on the hardware components of the clinical grade SURLAS Citation[54]. A second paper published later that same year reported on the design, development, and testing of the personal computer-based treatment delivery software that coordinated the interactions between the operator, the SURLAS applicator and several peripheral devices Citation[55]. There were two important tasks in software development worth mentioning here. The first was the coordination of the input power sequences to the elements of the high (4.9 MHz) and the low (1.9 MHz) frequency arrays (eight 15 × 20 mm elements/array) with the position of the dual-face scanning reflector. This was achieved by dividing the treatment window in up to 64 sectors (minimum size of 20 mm × 20 mm) and controlling the power delivery to each sector independently by adjusting the output power from the 16 channels of a programmable radio-frequency generator. Upon completion of the software development process it was integrated with the hardware followed by extensive testing. The second important task was safety, which was a paramount concern and design criterion. To ensure safety, a failure mode and effects analysis, or FMEA, was applied to the entire system in order to identify safety issues and rank their relative importance. The FMEA analysis led to the implementation of a software structure where each peripheral device communicated independently and directly with the controlling personal computer, so that in case of a malfunction in any component of the system or any violation of a pre-defined safety criterion, the software can terminate treatment immediately.
Performance of the SURLAS
As mentioned before, the original motivation and goal was to develop an ultrasound hyperthermia device that would enable simultaneous hyperthermia and electron beam radiation Citation[52]. Consequently, we tried to make the profile (height) of the SURLAS as small as possible in order that a high energy electron beam could travel through it and still be energetic enough to penetrate into superficial lesions as deep as 30 mm (electron beams are attenuated in water about 2 MeV/cm and the 80–90% isodose cloud is commonly used for tumour coverage). This meant that we implemented the smallest arrays possible that our previous studies indicated suitable in terms of the required emittance (W/cm2) to induce adequate hyperthermia. Note that the smaller the array the higher the required continuous wave (CW) emittance. This choice and other factors discussed below, however, created problems in practice that required time to figure out and resolve and derailed the clinical translation of the SURLAS.
The major problem was a decrease in electrical-to-acoustic efficiency with increasing transducer-to-reflector distance Citation[54]. This was particularly obvious for the high frequency array as shown in , which presents radiation force balance measurements for the low (2A) and high (2B) linear arrays. This loss of ultrasonic power with distance was determined to be non-linear propagation, a subject of much research in recent years. Essentially, non-linear propagation is the conversion of ultrasonic energy from the fundamental frequency to higher frequency harmonics as the waves are distorted while propagating from sinusoidal-like to sawtooth-like Citation[62]. The wave distortion increases with the acoustic intensity, increasing distance from the source and increasing frequency. It also depends on the characteristics of the medium. This phenomenon has been described by the shock parameter σ Citation[63],where β is the coefficient of non-linearity of the medium, ε is the mach number, k is the wave number, and x the travel distance. Based on calculations using the shock parameter, we determined that the high frequency array was suffering significant power loss due to non-linear propagation. For the clinical SURLAS, the maximum travel distance of the waves from the array to the distal water bolus membrane (including reflection) was about 230 mm. For the high frequency waves (∼4.9 MHz), non-linear propagation was calculated to occur at acoustic intensities as low as 0.7 W/cm2 at the maximum distance for σ = 1 (onset of non-linear propagation). The acoustic intensities required for hyperthermia are higher than this value Citation[53]. Moreover, note that scanning of the ultrasound energy over the treatment window requires higher CW intensities in comparison to a single transducer of comparable size to the treatment window Citation[53], Citation[64]. When σ = 3, the waveforms become sawtooth causing intensity losses of 6 dB with respect to the fundamental frequency. Assuming plane wave behaviour, a series of simulations were generated to determine (1) the CW intensity required for the clinical SURLAS to induce and maintain hyperthermic temperatures, and (2) the maximum deliverable CW intensity for a shock parameter equal to 3 (σ = 3). The frequency, the treatment window length (∼scanning distance), and height of the linear array were variables. Selected results are presented in , which shows the maximum fundamental frequency (fmax) for four treatment window lengths (WL) and three array heights (AH). The AH for the clinical SURLAS is 15 mm and the thickness of the water coupling bolus is approximately 20 mm. The developed clinical SURLAS has a maximum window length of 160 mm. indicates that to utilise a window this size fmax could not be higher than 1.89 MHz. The frequency of the high frequency array is ∼4.9 MHz, hence significant non-linear propagation effects are to be expected as per Equation 1 and as shown in . We had seen this effect before with our commercial ultrasound system for the en face set-up at the frequency of ∼3.5 MHz Citation[50], but the source-to-skin distance there was over 500 mm, and despite the ∼40% reduction of power, adequate hyperthermia treatments were possible. This indicated that other factors were contributing to low efficiencies. These were:
Reflectivity of a Styrofoam reflector relative to a brass reflector was estimated to be as 0.76 at 5 MHz Citation[52].
The first radiofrequency generator used had a drop in electrical power output for frequencies > 3.5 MHz.
Figure 2. Force balance results for the low (A) and high (B) frequency arrays. The three curves in each plot are for measurements for three different positions of the reflector with respect to an array. Distal was when the reflector was the furthest from the array in question and thus the ultrasound travelled the longest before reaching the force balance detector. Each data value was the average of five measurements. Standard deviations were too small to plot as error bars.
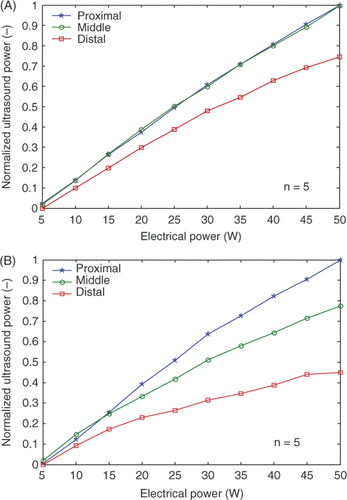
Table I. Maximum fundamental frequency (fmax) for four treatment window lengths (WL) and three array heights (AH).
The array element size in the current arrays is 15 × 20 mm (the smallest ever used). This turned out to be too small due to: (1) influence of the soldered spot and (2) damping by the mount at the edges.
Beam divergence at large reflector-to-source distance due to small misalignment between the arrays and the reflector. Computer models had ruled out this possibility Citation[53], but beam plot measurements of the clinical SURLAS suggested otherwise Citation[54].
The message from and the above listed factors is clear, to avoid non-linear propagation and improve electrical-to-acoustic efficiency, a new SURLAS must be constructed considering the following: lower frequency for the high frequency array, larger surface areas arrays (larger AH), shorter treatment window length (smaller WL), reflecting areas > array projected areas, and a more powerful radiofrequency generator with flat frequency response. The lower frequency for the high frequency array means some reduction in PDC. The smaller treatment window implies a proportional reduction in the size of the treatment area. A larger array height means a higher profile applicator–less or not suitable for electron beams. And a larger scanning reflector (possibly metallic) precludes electron beams. That leaves only photon beams for the delivery of simultaneous thermoradiotherapy with the SURLAS. As discussed in the next section, this was actually welcome news due to advances in modern radiotherapy, especially intensity modulated radiation therapy or IMRT, as it is commonly called.
SURLAS and IMRT/IGRT
Intensity modulated radiation therapy has become clinically widespread worldwide in the last decade Citation[65]. IMRT's appeal is its ability to focus the dose to the targeted volume (commonly called the planning treatment volume or PTV) while minimising the dose to surrounding normal tissues. Our first working hypothesis was that IMRT can produce clinically acceptable dose distributions on a recurrent breast cancer tumour while that same tumour receives ultrasound hyperthermia using the SURLAS. To test this hypothesis two research projects were initiated: a radiation treatment planning study to compute dose distributions in recurrent breast cancer PTVs with the SURLAS applicator in place Citation[66], and an experimental study where a radiation treatment plan was computed and delivered to a phantom with the SURLAS applicator on top of the phantom simulating a clinical set-up to evaluate the effects of the presence and potential misplacement of the SURLAS applicator on measured dose distributions Citation[67].
The above studies were carried out using the treatment planning station and treatment unit of a helical tomotherapy (HT) system (Tomotherapy, Madison, WI). This technology was chosen over conventional gantry-based medical linear accelerators (both available at our institution), because we thought that the multiplicity of beam angles and the helical motion would greatly minimise the perturbing effects of the SURLAS applicator on dose distributions. In addition, the megavoltage computed tomography (MVCT) feature of tomotherapy, which is used for image-guided radiotherapy (IGRT) to verify the patient's position right before treatment delivery, could also be used for treatment planning purposes without major image distortions or artefacts due to the metallic parts of the SURLAS applicator Citation[68]. Moreover, the MVCT images could be also used to verify the position of the SURLAS applicator with respect to the patient and from treatment to treatment. All these ideas were tested successfully Citation[66], Citation[67].
The main conclusion from the treatment planning study was that simultaneous treatment with the SURLAS and HT IMRT/IGRT is feasible as demonstrated by the clinically acceptable radiation treatment plans generated Citation[66]. Likewise, the main conclusion of the experimental study was that the delivered (measured) and planned radiation dose distributions were in excellent agreement when the SURLAS applicator was positioned as planned. Misplacement of the applicator from its planned position was found to have small effects on the measured dose distributions Citation[67]. Potentially large discrepancies between planned and delivered doses due to gross misplacement of the SURLAS can be further minimised by the implementation of routine quality assurance procedures using a position and orientation magnetic tracking device which was successful in precisely and reproducibly positioning the applicator. Moreover, it was shown that there is great potential for the use of this tracking device for monitoring the SURLAS applicator position and orientation during simultaneous treatments, and not just for patient/SURLAS set-up prior to daily treatment using MVCT Citation[67].
Do the above findings apply if a conventional medical linear accelerator is used? This is an important question to answer because gantry-based linacs remain the most commonly used technology in radiation therapy departments. Here we present new treatment planning results that indicate that linac-based IMRT can also deliver clinically acceptable radiation dose distributions with the SURLAS applicator on the patient's chest wall. The clinical set-up and planning MVCT can be seen on the axial plane of . The PTV was the superficial case in Penagaricano et al. 2008, with maximum dimensions of 10 mm deep by 115 mm wide (lateral) by 110 mm long (superior-inferior) on the lower chest/upper abdominal wall mimicking a chest wall recurrence of breast cancer Citation[66]. The results of and were generated using the Pinnacle3 inverse IMRT treatment planning system (P3IMRT, Philips, Andover, MA) to model megavoltage photon beam dose deposition delivered by a Varian 2100 EX linear accelerator with 120-leaf dynamic multi-leaf collimator (Varian Medical Systems, Palo Alto, CA). A 12-beam plan was used, eight beams were coplanar (linac central axis on the axial plane) and four beams were non-coplanar (linac central axis on the sagittal plane). Of the eight coplanar beams, six were aimed from above the SURLAS applicator and two were aimed laterally and tangentially to the chest wall. The non-coplanar beams were all aimed from above. The prescription was 50 Gy in 25 fractions to 95% of the PTV using six MV photons. It was assumed that every fraction was given under hyperthermic conditions for the purpose of illustration. Cardiac toxicity would be solely dependent on radiation dose because none of the existing or forthcoming devices would be able to induce significant hyperthermia in heart tissue due to (1) the depth of heart tissue and (2) the very high cardiac perfusion rates.
Figure 3. Twelve-beam IMRT plan dose distributions on the axial, sagittal and coronal planes through the PTV and SURLAS applicator. An MVCT of the RANDO phantom with the SURLAS on top simulating a typical clinical set-up for the treatment of chest wall recurrence of breast cancer was used as the planning CT.
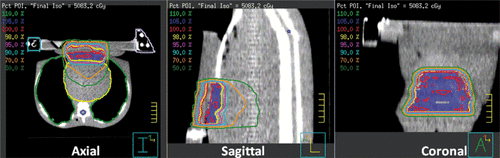
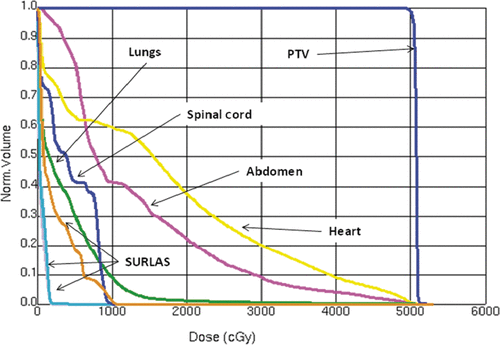
shows computed dose distributions on the axial, sagittal and coronal planes through the PTV and SURLAS applicator. It can be readily seen that the mass was nicely covered by the prescription dose with low doses to surrounding normal tissues. Dose volume histograms (DVH) for the PTV and organs at risk are shown in . These DVHs are clinically acceptable for the PTV and surrounding organs and are comparable to the ones obtained using the Tomotherapy system Citation[66]. The PTV mean dose, the PTV D95%, the heart, lung and abdomen mean doses, and the spinal cord maximum dose were 50.7, 50.4, 16.7, 3.7, 12.9 and 10 Gy, respectively. These metrics are in good agreement with those reported by Penagaricano et al. 2008 for the Tomotherapy system (Table IV in Citation[66]). In short, IMRT with either radiation therapy system is suitable for simultaneous thermoradiotherapy using the SURLAS.
Besides more conformal radiation doses, there are other advantages of using IMRT with the SURLAS. First, IMRT is expected to be highly forgiving of the modifications needed to the SURLAS applicator to minimise non-linear propagation and improve electrical-to-acoustical efficiencies. Second, the clinical experience thus far has only used one radiation beam concomitantly with hyperthermia; consequently the time of simultaneous delivery is short compared to the heating time. With IMRT and the SURLAS, the simultaneous time is expected to be longer as hyperthermia can be given during an entire multi-beam IMRT fraction. Third, the application of IGRT and magnetic position orientation tracking technologies to ensure reproducible applicator positioning prior to and during the treatments may encourage physicians to prescribe hyperthermia simultaneously with more if not every IMRT fraction. Finally, more simultaneous fractions should maximise the radiosensitising and physiological effects of hyperthermia even if IMRT is delivered in shorter times using the emerging arc therapy paradigm Citation[69].
Final remarks
The clinical implementation of simultaneous thermoradiotherapy has been challenging but proven to be feasible and safe. Clinical trial results have been encouraging and breast cancers are thus far the most responsive, with statistically significant correlations (p < 0.05) with radio-thermal dosimetric factors Citation[47]. Therefore, new technological developments such as combining the SURLAS and IMRT/IGRT with thermo-radio dose treatment planning should translate into improvements in radiothermal dose coverage and consequently in better treatment outcomes. Furthermore, several research groups have been developing advanced simultaneous heat and irradiation technologies that are moving toward clinical deployment, while others have attempted different clinical approaches using existing hyperthermia devices Citation[70–76]. In conclusion, new clinical protocols combining hyperthermia and radiotherapy simultaneously are now possible and warranted.
Acknowledgements
We thank our cancer patients for their courage and participation in the clinical research and gratefully acknowledge the contributions of many collaborators over the years as reflected in the authorship of previously published papers and abstracts.
Declaration of interest: The work reviewed in this paper was supported by NIH Grants CA 63121 and CA 71638, and by a grant from the Whitaker Foundation. We gratefully acknowledge partial support from the Central Arkansas Radiation Therapy Institute (CARTI) and a Medical Research Endowment pilot award from College of Medicine of the University of Arkansas for Medical Sciences, Little Rock.
References
- Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int J Hyperthermia 2006; 22: 191–196
- Moyer HR, Delman KA. The role of hyperthermia in optimizing tumor response to regional therapy. Int J Hyperthermia 2008; 24: 251–261
- Hildebrandt B, Wust P. The biologic rationale of hyperthermia. Cancer Treat Res 2007; 134: 171–184
- Dewhirst MW, Vujaskovic Z, Jones E, Thrall D. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia 2005; 21: 779–790
- Bergs JW, Franken NA, Haveman J, Geijsen ED, Crezee J, van Bree C. Hyperthermia, cisplatin and radiation trimodality treatment: A promising cancer treatment? A review from preclinical studies to clinical application. Int J Hyperthermia 2007; 23: 329–341
- Dewhirst MW, Prosnitz L, Thrall D, Prescott D, Clegg S, Charles C, MacFall J, Rosner G, Samulski T, Gillette E, et al. Hyperthermic treatment of malignant diseases: Current status and a view toward the future. Semin Oncol 1997; 24: 616–625
- Falk MH, Issels RD. Hyperthermia in oncology. Int J Hyperthermia 2001; 17: 1–18
- Hehr T, Wust P, Bamberg M, Budach W. Current and potential role of thermoradiotherapy for solid tumours. Onkologie 2003; 26: 295–302
- Issels RD. Regional hyperthermia in high-risk soft tissue sarcomas. Curr Opin Oncol 2008; 20: 438–443
- Kapp DS, Cox RS, Barnett TA, Ben-Yosef R. Thermoradiotherapy for residual microscopic cancer: Elective or post-excisional hyperthermia and radiation therapy in the management of local-regional recurrent breast cancer. Int J Radiat Oncol Biol Phys 1992; 24: 261–277
- Prosnitz LR, Maguire P, Anderson JM, Scully SP, Harrelson JM, Jones EL, Dewhirst M, Samulski TV, Powers BE, Rosner GL, et al. The treatment of high-grade soft tissue sarcomas with preoperative thermoradiotherapy. Int J Radiat Oncol Biol Phys 1999; 45: 941–949
- van der Zee J, Gonzalez GD. The Dutch Deep Hyperthermia Trial: Results in cervical cancer. Int J Hyperthermia 2002; 18: 1–12
- van der Zee J. Heating the patient: A promising approach?. Ann Oncol 2002; 13: 1173–1184
- Sneed PK, Stauffer PR, McDermott MW, Diederich CJ, Lamborn KR, Prados MD, Chang S, Weaver KA, Spry L, Malec MK, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/- hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys 1998; 40: 287–295
- Valdagni R, Amichetti M. Report of long-term follow-up in a randomized trial comparing radiation therapy and radiation therapy plus hyperthermia to metastatic lymph nodes in stage IV head and neck patients. Int J Radiat Oncol Biol Phys 1994; 28: 163–169
- Dewhirst MW, Sneed PK. Those in gene therapy should pay closer attention to lessons from hyperthermia. Int J Radiat Oncol Biol Phys 2003; 57: 597–599, author reply 599-600
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005; 23: 3079–3085
- Jones EL, Prosnitz LR, Dewhirst MW, Marcom PK, Hardenbergh PH, Marks LB, Brizel DM, Vujaskovic Z. Thermochemoradiotherapy improves oxygenation in locally advanced breast cancer. Clin Cancer Res 2004; 10: 4287–4293
- Feyerabend T, Wiedemann GJ, Jager B, Vesely H, Mahlmann B, Richter E. Local hyperthermia, radiation, and chemotherapy in recurrent breast cancer is feasible and effective except for inflammatory disease. Int J Radiat Oncol Biol Phys 2001; 49: 1317–1325
- Hehr T, Lamprecht U, Glocker S, Classen J, Paulsen F, Budach W, Bamberg M. Thermoradiotherapy for locally recurrent breast cancer with skin involvement. Int J Hyperthermia 2001; 17: 291–301
- Jones EL, Marks LB, Prosnitz LR. Point: Hyperthermia with radiation for chest wall recurrences. J Natl Compr Canc Netw 2007; 5: 339–344
- Wahl AO, Rademaker A, Kiel KD, Jones EL, Marks LB, Croog V, McCormick BM, Hirsch A, Karkar A, Motwani SB, et al. Multi-institutional review of repeat irradiation of chest wall and breast for recurrent breast cancer. Int J Radiat Oncol Biol Phys 2008; 70: 477–484
- Welz S, Hehr T, Lamprecht U, Scheithauer H, Budach W, Bamberg M. Thermoradiotherapy of the chest wall in locally advanced or recurrent breast cancer with marginal resection. Int J Hyperthermia 2005; 21: 159–167
- Lee HK, Antell AG, Perez CA, Straube WL, Ramachandran G, Myerson RJ, Emami B, Molmenti EP, Buckner A, Lockett MA. Superficial hyperthermia and irradiation for recurrent breast carcinoma of the chest wall: Prognostic factors in 196 tumors. Int J Radiat Oncol Biol Phys 1998; 40: 365–375
- Kapp DS, Barnett TA, Cox RS, Lee ER, Lohrbach A, Fessenden P. Hyperthermia and radiation therapy of local-regional recurrent breast cancer: Prognostic factors for response and local control of diffuse or nodular tumors. Int J Radiat Oncol Biol Phys 1991; 20: 1147–1164
- Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD, Gonzalez Gonzalez D, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 1996; 35: 731–744
- Leon SA, Asbell SO, Edelstein G, Arastu HH, Daskal I, Sheehan S, Plunkett DM, Guttmann GG, Packel AJ, Leon O. Effects of hyperthermia on bone. I. Heating rate patterns induced by microwave irradiation in bone and muscle phantoms. Int J Hyperthermia 1993; 9: 69–75
- Moros EG, Novak P, Straube WL, Kolluri P, Yablonskiy DA, Myerson RJ. Thermal contribution of compact bone to intervening tissue-like media exposed to planar ultrasound. Phys Med Biol 2004; 49: 869–886
- Hand JW. Guidelines for thermometry in clinical hyperthermia. Front Med Biol Eng 1992; 4: 99–104
- Dewhirst MW, Phillips TL, Samulski TV, Stauffer P, Shrivastava P, Paliwal B, Pajak T, Gillim M, Sapozink M, Myerson R, et al. RTOG quality assurance guidelines for clinical trials using hyperthermia. Int J Radiat Oncol Biol Phys 1990; 18: 1249–1259
- Cox RS, Kapp DS. Correlation of thermal parameters with outcome in combined radiation therapy-hyperthermia trials. Int J Hyperthermia 1992; 8: 719–732
- Hand JW, Machin D, Vernon CC, Whaley JB. Analysis of thermal parameters obtained during phase III trials of hyperthermia as an adjunct to radiotherapy in the treatment of breast carcinoma. Int J Hyperthermia 1997; 13: 343–364
- Sherar M, Liu FF, Pintilie M, Levin W, Hunt J, Hill R, Hand J, Vernon C, van Rhoon G, van der Zee J, et al. Relationship between thermal dose and outcome in thermoradiotherapy treatments for superficial recurrences of breast cancer: Data from a phase III trial. Int J Radiat Oncol Biol Phys 1997; 39: 371–380
- Kapp DS, Cox RS. Thermal treatment parameters are most predictive of outcome in patients with single tumor nodules per treatment field in recurrent adenocarcinoma of the breast. Int J Radiat Oncol Biol Phys 1995; 33: 887–899
- Leopold KA, Dewhirst M, Samulski T, Harrelson J, Tucker JA, George SL, Dodge RK, Grant W, Clegg S, Prosnitz LR, et al. Relationships among tumor temperature, treatment time, and histopathological outcome using preoperative hyperthermia with radiation in soft tissue sarcomas. Int J Radiat Oncol Biol Phys 1992; 22: 989–998
- Leopold KA, Dewhirst MW, Samulski TV, Dodge RK, George SL, Blivin JL, Prosnitz LR, Oleson JR. Cumulative minutes with T90 greater than Tempindex is predictive of response of superficial malignancies to hyperthermia and radiation. Int J Radiat Oncol Biol Phys 1993; 25: 841–847
- Oleson JR, Samulski TV, Leopold KA, Clegg ST, Dewhirst MW, Dodge RK, George SL. Sensitivity of hyperthermia trial outcomes to temperature and time: Implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys 1993; 25: 289–297
- Oleson JR, Dewhirst MW, Harrelson JM, Leopold KA, Samulski TV, Tso CY. Tumor temperature distributions predict hyperthermia effect. Int J Radiat Oncol Biol Phys 1989; 16: 559–570
- Myerson RJ, Perez CA, Emami B, Straube W, Kuske RR, Leybovich L, Von Gerichten D. Tumor control in long-term survivors following superficial hyperthermia. Int J Radiat Oncol Biol Phys 1990; 18: 1123–1129
- Myerson RJ, Emami BN, Perez CA, Straube W, Leybovich L, Von Gerichten D. Equilibrium temperature distributions in uniform phantoms for superficial microwave applicators: Implications for temperature-based standards of applicator adequacy. Int J Hyperthermia 1992; 8: 11–21
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002; 3: 487–497
- Roemer RB. Engineering aspects of hyperthermia therapy. Annu Rev Biomed Eng 1999; 1: 347–376
- Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 1994; 10: 457–483
- Myerson RJ, Straube WL, Moros EG, Emami BN, Lee HK, Perez CA, Taylor ME. Simultaneous superficial hyperthermia and external radiotherapy: Report of thermal dosimetry and tolerance to treatment. Int J Hyperthermia 1999; 15: 251–266
- Steeves RA, Tompkins DT, Nash RN, Blair JR, Gentry LL, Paliwal BR, Murray TG, Mieler WF. Thermoradiotherapy of intraocular tumors in an animal model: Concurrent vs. sequential brachytherapy and ferromagnetic hyperthermia. Int J Radiat Oncol Biol Phys 1995; 33: 659–662
- Overgaard J. The current and potential role of hyperthermia in radiotherapy. Int J Radiat Oncol Biol Phys 1989; 16: 535–549
- Myerson RJ, Roti Roti JL, Moros EG, Straube WL, Xu M. Modelling heat-induced radiosensitization: Clinical implications. Int J Hyperthermia 2004; 20: 201–212
- Xu M, Myerson RJ, Straube WL, Moros EG, Lagroye I, Wang LL, Lee JT, Roti Roti JL. Radiosensitization of heat resistant human tumour cells by 1 hour at 41.1 degrees C and its effect on DNA repair. Int J Hyperthermia 2002; 18: 385–403
- Moros EG, Straube WL, Klein EE, Maurath J, Myerson RJ. Clinical system for simultaneous external superficial microwave hyperthermia and cobalt-60 radiation. Int J Hyperthermia 1995; 11: 11–26
- Straube WL, Moros EG, Low DA, Klein EE, Willcut VM, Myerson RJ. An ultrasound system for simultaneous ultrasound hyperthermia and photon beam irradiation. Int J Radiat Oncol Biol Phys 1996; 36: 1189–1200
- Straube WL, Klein EE, Moros EG, Low DA, Myerson RJ. Dosimetry and techniques for simultaneous hyperthermia and external beam radiation therapy. Int J Hyperthermia 2001; 17: 48–62
- Moros EG, Straube WL, Klein EE, Yousaf M, Myerson RJ. Simultaneous delivery of electron beam therapy and ultrasound hyperthermia using scanning reflectors: A feasibility study. Int J Radiat Oncol Biol Phys 1995; 31: 893–904
- Moros EG, Straube WL, Myerson RJ. A reflected-scanned ultrasound system for external simultaneous thermoradiotherapy. Ieee T Ultrason Ferr 1996; 43: 441–449
- Novak P, Moros EG, Straube WL, Myerson RJ. SURLAS: A new clinical grade ultrasound system for sequential or concomitant thermoradiotherapy of superficial tumors: Applicator description. Med Phys 2005; 32: 230–240
- Novak P, Moros EG, Straube WL, Myerson RJ. Treatment delivery software for a new clinical grade ultrasound system for thermoradiotherapy. Med Phys 2005; 32: 3246–3256
- Moros EG, Fan X, Straube WL, Myerson RJ. Numerical and in vitro evaluation of temperature fluctuations during reflected-scanned planar ultrasound hyperthermia. Int J Hyperthermia 1998; 14: 367–382
- Moros EG, Fan X, Straube WL. Ultrasound power deposition model for the chest wall. Ultrasound Med Biol 1999; 25: 1275–1287
- Moros EG, Straube WL, Myerson RJ. Potential for power deposition conformability using reflected-scanned planar ultrasound. Int J Hyperthermia 1996; 12: 723–736
- Moros EG, Fan X, Straube WL. An investigation of penetration depth control using parallel opposed ultrasound arrays and a scanning reflector. J Acoust Soc Am 1997; 101: 1734–1741
- Moros EG, Fan X, Straube WL. Experimental assessment of power and temperature penetration depth control with a dual frequency ultrasonic system. Med Phys 1999; 26: 810–817
- Moros EG, Straube WL, Myerson RJ, Fan X. The impact of ultrasonic parameters on chest wall hyperthermia. Int J Hyperthermia 2000; 16: 523–538
- Harpen MD. Basic nonlinear acoustics: An introduction for radiological physicists. Med Phys 2006; 33: 3241–3247
- Hamilton MF, Blackstock DT. Nonlinear Acoustics. Academic Press, London 1997
- Moros EG, Myerson RJ, Straube WL. Aperture size to therapeutic volume relation for a multielement ultrasound system: Determination of applicator adequacy for superficial hyperthermia. Med Phys 1993; 20: 1399–1409
- Veldeman L, Madani I, Hulstaert F, De Meerleer G, Mareel M, DN W. Evidence behind use of intensity-modulated radiotherapy: A systematic review of comparative clinical studies. Lancet Oncology 2008; 9: 367–375
- Penagaricano JA, Moros E, Novak P, Yan Y, Corry P. Feasibility of concurrent treatment with the scanning ultrasound reflector linear array system (SURLAS) and the helical tomotherapy system. Int J Hyperthermia 2008; 24: 377–388
- Novak P, Penagaricano JA, Nahirnyak V, Corry P, Moros EG. Influence of the SURLAS applicator on radiation dose distributions during simultaneous thermoradiotherapy with helical tomotherapy. Phys Med Biol 2008; 53: 2509–2522
- Meeks SL, Harmon JF, Jr, Langen KM, Willoughby TR, Wagner TH, Kupelian PA. Performance characterization of megavoltage computed tomography imaging on a helical tomotherapy unit. Med Phys 2005; 32: 2673–2681
- Palma DA, Verbakel WF, Otto K, Senan S. New developments in arc radiation therapy: A review. Cancer Treat Rev
- Armour EP, Raaphorst GP. Long duration mild temperature hyperthermia and brachytherapy. Int J Hyperthermia 2004; 20: 175–189
- Fuwa N, Nomoto Y, Shouji K, Kodaira T, Kamata M, Ito Y. Therapeutic effects of simultaneous intraluminal irradiation and intraluminal hyperthermia on oesophageal carcinoma. Br J Radiol 2001; 74: 709–714
- Geiger M, Strnad V, Lotter M, Sauer R. Pulsed-dose rate brachytherapy with concomitant chemotherapy and interstitial hyperthermia in patients with recurrent head-and-neck cancer. Brachytherapy 2002; 1: 149–153
- Gelvich EA, Klimanov VA, Kramer-Ageev EA, Mazokhin VN. Computational evaluation of changes in ionizing radiation dose distribution in tissues caused by EM applicators when external radiation and hyperthermia act simultaneously. Int J Hyperthermia 2006; 22: 343–352
- Juang T, Stauffer PR, Neuman DG, Schlorff JL. Multilayer conformal applicator for microwave heating and brachytherapy treatment of superficial tissue disease. Int J Hyperthermia 2006; 22: 527–544
- Sakurai H, Mitsuhashi N, Tamaki Y, Kurosaki H, Akimoto T, Ishikawa H, Saitoh J, Muramatsu H, Yamakawa M, Hayakawa K, et al. Clinical application of low dose-rate brachytherapy combined with simultaneous mild temperature hyperthermia. Anticancer Res 2001; 21: 679–684
- Diederich CJ, Khalil IS, Stauffer PR, Sneed PK, Phillips TL. Direct-coupled interstitial ultrasound applicators for simultaneous thermobrachytherapy: A feasibility study. Int J Hyperthermia 1996; 12: 401–419