Abstract
Purpose: To compare the local tumour control, survival, and acute mucous toxicity of nasopharyngeal cancer (NPC) patients treated with conventional radiotherapy (RT) combined with intracavity hyperthermia versus conventional RT alone.
Methods and materials: Previously untreated NPC patients were assigned randomly into the conventional RT group and the hyperthermia group. In addition to curative RT, hyperthermia group patients received intracavity hyperthermia before or after RT; T90 was 42.5°–43°C for 50 min twice a week for 7 weeks.
Results: From August 2001 to July 2006, 180 eligible patients with NPC were enrolled in this study. The complete response (CR) rate in the two arms (RT plus hyperthermia versus conventional RT) was 95.6% and 81.1%, respectively (p = 0.003, χ2 test). CR rates for T2 and T3 patients in the hyperthermia group were 97.1% and 96.9%, respectively, while in the conventional RT group they were 79.5% and 76.7%, respectively. The difference between the two groups was statistically significant (p = 0.03 and p = 0.024, respectively). The 5-year local control rate was 91.1% and 78.9% for the two arms, respectively (p = 0.022). Oral mucous toxicity in both arms was comparable. The 5-year PFS and 5-year OS rate for the hyperthermia arm vs. the conventional arm were 72.7% versus 63.1% (p = 0.039) and 78.2% versus 70.3% (p = 0.14), respectively.
Conclusions: Conventional RT treatment followed by intracavity hyperthermia was well tolerated by the NPC patients. The addition of hyperthermia improved the local tumour control, and our results indicated a positive impact on PFS of NPC patients.
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common malignant tumours in China, and radiotherapy is the treatment strategy of choice. As novel imaging technologies became available and radiotherapy techniques advanced, the prognosis of patients with NPC significantly improved. Long-term survival rates have been reported at 50–82.4% Citation[1–3]. Although a high percentage of patients have been cured by radiation therapy (RT), more than 30% of patients with NPC still die from local failure, especially in locally advanced cases. For example, Chan et al. reported that 28% of the patients in their study experienced local recurrence at a median follow up of 2.7 years Citation[4]. Innovative strategies to overcome this dilemma include dose escalation or the addition of after-loading radiotherapy. Nevertheless, these approaches can cause some serious acute and/or late normal tissue reactions.
With its unique anti-tumour mechanism and great tolerance by patients, local hyperthermia has become another useful option for supplemental therapy. Hyperthermia enhances the local-regional efficacy of radiotherapy Citation[5–8]. A phase II clinical study previously conducted at our institute suggested that radiotherapy combined with hyperthermia could promote local tumour control without increasing the ambient normal tissue injury (data not published). Based on our experience with this treatment technique, we conducted a phase III clinical study that began in 2001. One hundred and eighty patients with NPC were recruited into the study and the median follow-up time was 58 months. The purpose of this paper is to describe the procedure of nasopharyngeal cavity hyperthermia and the treatment protocol and to report the clinical outcome in terms of local tumour control, overall survival, and normal tissue toxicity.
Materials and methods
Patients with NPC who met the following criteria were enrolled in this study: (1) newly diagnosed and histopathologically proven NPC without any other primary cancers; (2) performance status (PS) between 0 and 2 according to the criteria proposed by the Eastern Cooperative Oncology Group Citation[9]; (3) aged between 18 and 70 years old; (4) blood test: WBC count 4 × 109/L or higher and platelet count 100 × 1012/L or higher; (5) no abnormality observed in the liver, kidneys, heart, and lungs; (6) no metastasis found in the skeletal system; and (7) informed consent. All patients enrolled in this study were randomly divided into a conventional group and a hyperthermia group. Before treatment, the range/extension of the primary lesion was evaluated by pharyngeal fibrotic endoscopy and magnetic resonance imaging (MRI), and cervical lymph nodes metastasis was evaluated by CT or MRI.
All patients received conventional radiotherapy. A total dose of 70 Gy was prescribed to the primary tumour and the upper neck in 2 Gy fractions. The RT was delivered daily, five times a week. Fields were reduced to exclude the spinal cord at the midplane at 44 Gy. However, the entire neck was irradiated to a total dose of at least 50 Gy (even in cases of N0 stage) at the anatomical levels of lymph nodes, usually 20–40 mm below the skin surface. Clinically positive neck nodes received a minimum dose of 60 Gy in 30 fractions delivered over the course of 6 weeks. The anterior lower neck field was treated at 2 Gy per fraction once a day up to a total dose of at least 50 Gy over 5 weeks. If the tumour in the nasopharyngeal cavity did not show complete recession by the time the entire radiotherapy course was completed, the patient would receive high dose-rate (HDR) brachytherapy with a dose prescription of 15–20 Gy in 2–3 fractions.
Cisplatin and 5-FU were administered every 3 weeks for 2–4 courses during and post RT in all stage T3 and T4 patients. One course of chemotherapy consisted of cisplatin (100 mg/m2, day 1) given over 30 min with pre-hydration and diuretics followed by 5-FU (1 g/m2/24 h for 4 days, days 1–4) by continuous infusion over 96 h during RT.
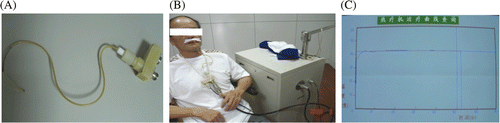
In the hyperthermia group, local external microwave hyperthermia was delivered using a commercially available device (WE2102-A Microwave Hyperthermia System, 915 MHz Yuan De Biomedical Engineering, Beijing). This procedure used microwave radiation as the target to produce heat. The electrodes were placed in the nasopharyngeal cavity from either nostril (). Thermometry was performed by inserting one thermocouple directly on the surface of the nasopharyngeal tumour. Hyperthermia sessions took place once a week within 30 min before or after RT for a total of seven times, or 2 h after cisplatin finished. Temperatures were monitored and recorded every 10 s. T90 (temperature covering 90% of target) was maintained within 42.5°–43°C.
Figure 1. (A) Instrument used for hyperthermia and temperature monitor; (B) patient being treated with hyperthermia; (C) temperature curve during hyperthermia therapy.
The response of primary lesions was evaluated based on the results of pharyngeal fibrotic endoscopy and MRI scans performed 1 month after the completion of the treatment according to the WHO criteria Citation[10]. Results were recorded as CR (complete recession), PR (partial recession), SD (stable disease), and PD (progressed disease).
The toxicity of the acute oral mucosa membrane was evaluated every week according to the RTOG-Acute Radiation Morbidity Scoring Criterion Citation[11]: grade 0, no change over baseline; grade 1, mild pain not requiring analgesic; grade 2, patch mucositis, which may produce an inflammatory serosanguinitis discharge/may experience moderate pain requiring analgesia; grade 3, confluent fibrinous mucositis/may include severe pain requiring a narcotic; grade 4, ulceration, haemorrhage, or necrosis.
Patients were followed up every 3 months for the first 2 years, every 6 months in the third year and fourth year, and yearly thereafter. The following assessments were performed at follow-up visits: (1) physical examination; (2) nasopharyngeal fibrotic endoscopy; and (3) CT or MRI.
Statistical analyses were performed using SPSS software for windows (version 13.0, Chicago, IL). Survival rates were calculated using the Kaplan-Meier method. The log-rank test was used in univariate analyses to determine potential prognostic factors. The survival time was measured starting from the day RT was initiated. The chi-square test was used to compare the correlation variables between the hyperthermia group and the conventional group. Variables analysed in this study included gender, histology, parapharyngeal space involvement, overall stage, T stage, N stage, CR, and mucous toxicity. The t test was used to compare the mean age and the mean dose. A p value < 0.05 was considered statistically significant and all tests were two-sided tests.
The study was ethically based on the Declaration of Helsinki and the principles of good clinical practice. The study protocol was approved by the human studies and ethics committee of the cancer hospital in Zhejiang Province.
Results
Data analysis
From August 2001 to July 2006, 180 patients with NPC were enrolled in the study and randomly assigned to one of the two study arms (conventional RT group versus hyperthermia group); there were 90 patients in each arm. Patient characteristics are summarised in . The two treatment arms were well balanced. All enrolled patients completed the scheduled course of treatment in 7 weeks.
Table I. Patients’ characteristics.
CR time and dose
Out of the 90 patients in each group, 73 patients (81.1%) and 86 patients (95.6%) were evaluated as CR in the conventional treatment group and the hyperthermia group, respectively (p = 0.003). summarises the completed response to the treatment according to stage. For patients with paranasopharyngeal space involvement, the CR rate was 95% (57/60) in the hyperthermia group and 83.9% (52/62) in the conventional RT group (p = 0.046). The time required to show a complete response corresponded a mean RT dose of 52.4 Gy (range 32–64 Gy) in the hyperthermia group and 55.9 Gy (range 40–70 Gy) in the conventional group. In T1 patients, the mean dose decreased from 50 Gy for the conventional group to 42 Gy for the hyperthermia group.
Table II. Complete response (CR) rate among different tumour stages. Numbers in parentheses are patient numbers.
Clinical outcome
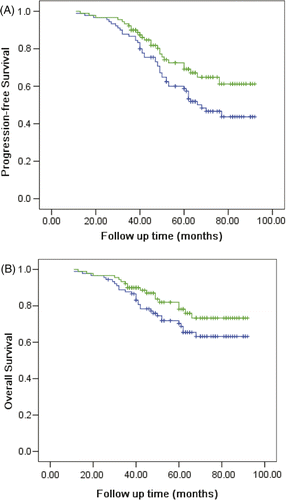
With a median follow-up of 58 months (range 11–92 months), the 5-year local control rate was 91.1% and 78.9% for the two arms (hyperthermia versus conventional groups), respectively (p = 0.022). The 5-year PFS (progression free survival) values were significantly different for the two arms (69.1% versus 58.7% respectively, p = 0.042). The 5-year overall survival rates differed for the two arms (78.2% for hyperthermia treatment arm and 70.3% for the conventional treatment arm), but the difference was not statistically significance (p = 0.14). shows the survival curves.
Toxicity
The T90 in the hyperthermia group was controlled within the range of 42.5°–43°C, which was higher than the human body temperature. In this condition, the most common toxicities were local mucositis, erythema, and blisters. In our clinical trial, acute oral mucous toxicity in both arms was comparable (). No lethal toxicity occurred during this clinical trial. And the late adverse response was manageable in hyperthermia group ().
Table III. Acute mucosa toxicity in the two groups.
Table IV. Late adverse reaction in the two groups.
Discussion
Hyperthermia plays an important role in tumour treatment because of its unique anti-tumour features and great patient tolerance. RT in combination with hyperthermia has been suggested as a method to improve the efficiency of tumour control. Cells are sensitive to radiotherapy in G2/M phase and resistant in S phase, but cells in S phase are sensitive to hyperthermia. Moreover, hypoxic cells are resistant to radiotherapy but sensitive to hyperthermia. Thus, radiation and hyperthermia have a synergistic effect on tumour cell killing. Most clinical trials of hyperthermia therapy combined with RT have shown encouraging results for tumour control. Zhu et al. summarised the treatment results of 70 patients with NPC Citation[12] and found that the tumour remission and CR + PR rates were 65.7% and 31.4% for thermotherapy plus RT and 95.1% and 65.7% for RT alone. Svetisky studied 54 patients with local recurrent laryngeal carcinoma who after RT were given RT and chemotherapy in combination with or without hyperthermia Citation[13]. Eight patients (33%) in the RT + chemotherapy + hyperthermia group survived for over 3 years, whereas no patients in the control group survived. Recently, Nagraj reported the trimodality of treatment with paclitaxel, cisplatin, hyperthermia and radiation was feasible and effective in head and neck cancer Citation[14]. Our study also suggested that RT with hyperthermia could significantly improve the local tumour control rate. In terms of the stratification analysis by T stage, RT in combination with hyperthermia could reduce the local residue of the tumour in patients with stage T2–T3 disease. All of the T1 tumours in both groups showed complete regression, and a 100% CR rate was achieved. However, hyperthermia can accelerate tumour regression and reduce the required radio-therapeutic dose. According to the data presented here, hyperthermia did not improve the local tumour control in T4 patients. Based on the analysis of this dataset, we found that all of the residual focuses located in the base or ceiling of the skull were beyond the scope of hyperthermia, but tumours within the range of hyperthermia demonstrated complete regression.
The paranasopharyngeal space was easily invaded by NPC tumours because of its loose tissue structure. According to CT or MRI scans, the paranasopharyngeal space is involved in over 60% of patients of NPC Citation[15]. Teo et al.'s studies indicated that the involvement of the bulky parapharyngeal space was one of the most significant prognostic factors that indicated distant metastasis and poor survival Citation[16–18]. Wu et al. reported that intracavity microwave hyperthermia plus radiation could improve the local tumour control in the parapharyngeal space Citation[19]. In their study, the CR rate of the primary tumour at the end of treatment was 95.9% (47/49) for the combined group and 83.7% (41/49) for the control group. However, some researchers doubt whether the effective range of hyperthermia could reach the parapharyngeal space. An anaesthetised beagle dog was used in our study to evaluate the effective scope of hyperthermia. We put an instrument that produces heat into the dog's nose and placed seven thermometers in different locations around the nasopharyngeal cavity to measure the temperature distribution. A spindle-like distribution of 30–35 mm in transdiameter was found when T90 was 42.5°–43°C in our experiment. Thus, the T90 coverage could include the parapharyngeal space, and better results of intracavity microwave hyperthermia plus radiation were confirmed in our study. The local tumour control in the parapharyngeal space was increased from 83.9% for irradiation alone to 95% for hyperthermia plus radiation.
In general, hyperthermia prevented the tumour cells from repairing the radiation damage and increased the injury to nearby normal tissue as well. Wu et al. reported that the grade 4 injury rate of the oral membrane was significantly higher in the combined group than that in the control group Citation[19]. However, our study showed that the morbidity of radiation was similar between the two arms. This has already been demonstrated in other prospective trials reported by Rau et al. and van der Zee et al. Citation[6], Citation[7]. Regional hyperthermia did not increase radiation-induced toxicity, which had also been shown in the treatment of recurrent or locally advanced prostate cancer Citation[20]. This difference might be due to the different blood supply between tumour and normal tissue. In normal tissue, with the vessel dilated and the branch open during hyperthermia, the blood supply increases rapidly. The increased blood supply could take away the excessive heat, limit the level of hyperthermia, and alleviate the harm to the normal oral membrane. At the same time, it could accelerate the proliferation and reparation of oral membrane cells. In contrast, in the tumour, new vessels are disorganised and form a blood sinus, which leads to poor heat tolerance. On the other hand, bleeding, hyperaemia, congestion, and occlusion could often be found after hyperthermia. In these circumstances the blood supply was decreased and a higher temperature in the tumour was achieved. It aggravated the harm to tumour cells and inhibited their reparation.
As novel imaging technologies became available for clinical application and RT techniques improved, the prognosis of NPC also improved. In 1989 the 5-year OS was 47% Citation[21]. In Lee's analysis of 5037 patients in Hong Kong, the 5-year local, regional, and distant failure-free rates were 66%, 67%, and 62%, respectively Citation[22]. Recently reported studies have showed better results Citation[23–24]. In 2007, Yi et al. reported the 5-year OS, local control, and PFS to be 76.1%, 81.7%, and 58.4%, respectively Citation[23]. In their study, the patients treated with hyperthermia had a higher overall survival rate and PFS rate than the patients without hyperthermia, and the corresponding 5-year OS and PFS rates of patients treated with or without hyperthermia were 78.2% and 69.1% versus 70.3% and 58.7%. From these data, one could conclude that hyperthermia combined with RT could improve the 5-year OS and PFS. Although our results did not show a significant difference in OS between the two groups, local hyperthermia appeared to enhance the effectiveness of therapy, as suggested in various trials of other tumour entities. Subgroup analysis showed hyperthermia produced better local control in T2/T3 patients, but there was still no significant overall survival advantage. This might be due to the small number of patients in our study. A large series of randomised trials is needed to verify the positive influence of hyperthermia + RT treatment in T2/T3 patients.
Compared with conventional radiotherapy, intensity modulated radiation therapy (IMRT) permits the delivery of high dose to the target tumour, and results in better local control and adequate sparing of the surrounding normal tissue. Radiation Therapy Oncology Group (RTOG) trial 0225 showed that IMRT in treating NPC achieved a 2-year OS rate of 80.2%, and the incidence of acute and late toxicities was reduced Citation[25]. The results of our study indicated that the addition of hyperthermia to conventional therapy could achieve a similar outcome as well, and avoid the effects of low-dose hypersensitivity in IMRT.
In summary, local hyperthermia is a very feasible and well-tolerated procedure for treating tumours. Combined with conventional treatment, it might improve the effectiveness of local radiotherapy, promote long term survival, and have acceptable toxicity.
Acknowledgement
This work was sponsored by Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Au JS, Law CK, Foo W, Lau WH. In-depth evaluation of the AJCC/UICC 1997 staging system of nasopharyngeal carcinoma: Prognostic homogeneity and proposed refinements. Int J Radiat Oncol Biol Phys 2003; 56: 413–426
- Liu XQ, Luo W, Liu MZ, Ye L, Sun Y, Xia YF. Treatment results and prognostic analysis of 1093 primary nasopharyngeal carcinoma: The experience of a single institution of Guangzhou in the beginning of the 21st century. Chin-Ger J Clin Oncol 2008; 7: 187–195
- Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT, Zee BC, Law SC, Teo PM, Tung SY, et al. Treatment results for nasopharyngeal carcinoma in the modern era: The Hong Kong experience. Int J Radiat Oncol Biol Phys 2005; 61: 1107–1116
- Chan AT, Teo PM, Ngan RK, Leung TW, Lau WH, Zee B, Leung SF, Cheung FY, Yeo W, Yiu HH, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: Progression-free survival analysis of a phase III randomized trial. J Clin Oncol 2002; 20: 2038–2044
- Overgaard J, Gonzalez-Gonzalez D, Hulshof MC, Arcangeli G, Dahl O, Mella O, Bentzen SM. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Lancet 1995; 345: 540–543
- Rau B, Wust P, Tilly W, Gellermann J, Harder C, Riess H, Budach V, Felix R, Schlag PM. Preoperative radio-chemotherapy in locally advanced recurrent rectal cancer: Regional radiofrequency hyperthermia correlates with clinical parameters. Int J Radiat Oncol Biol Phys 2000; 48: 381–391
- Van der Zee J, González Gonzáles D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumors: A prospective, randomised, multicentre trial. Lancet 2000; 355: 1119–1125
- Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD, González González D, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int J Radiat Oncol Biol Phys 1996; 35: 731–744
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–655
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–214
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995; 31: 1341–1346
- Zhu L, Chen ZJ, Wang W, Li RY, Wang P. Intracavitary thermotherapy combined with radiotherapy for nasopharyngeal carcinoma. Chin J Clini Oncol 2005; 10: 585–587
- Svetisky PV. Effect of microwave and ionizing radiation in patients with recurrent laryngeal carcinoma. J Laryngol Otol 1990; 104: 704–705
- Huilgol NG, Gupta D, Dixit R. Chemoradiation with hyperthermia in the treatment of head and neck cancer. Int J Hyperthermia 2010; 26: 21–25
- Chen ZB, Ren QR, Li CQ. Comparison of the long-term results between two radiotherapeutic protocols for nasopharyngeal carcinoma patients with involvement of the parapharyngeal space. Chin J Radiat Oncol 1999; 8: 69–72
- Teo P, Lee WY, Yu P. The prognostic significance of parapharyngeal tumor involvement in nasopharyngeal carcinoma. Radiother Oncol 1996; 39: 209–221
- Yeh SA, Tang Y, Lui CC, Huang YJ, Huang EY. Treatment outcomes and late complications of 849 patients with nasopharyngeal carcinoma treated with radiotherapy alone. Int J Radiat Oncol Biol Phys 2005; 62: 672–679
- Sham JS, Choy D. Prognostic value of paranasopharyngeal extension of nasopharyngeal carcinoma on local control and short-term survival. Head Neck 1991; 13: 298–310
- Wu JB, Chen CQ. A controlled clinical study of intracavitary microwave hyperthermia combined with lrradiation for nasopharyngeal carcinoma. J QILU Oncol 1996; 3: 200–203
- Tilly W, Gellermann J, Graf R, Hildebrandt B, Weissbach L, Budach V, Felix R, Wust P. Regional hyperthermia in conjunction with definitive radiotherapy against recurrent or locally advanced prostate cancer T3 pN0 M0. Strahlenther Onkol 2005; 181: 35–41
- Zhang EP, Lian PG, Cai KL, Chen YF, Cai MD, Zheng XF, Guang XX. Radiation therapy of nasopharyngeal cancer: Prognostic factors based on a 10-year follow-up of 1302 patients. Int J Radiat Oncol Biol Phys 1989; 16: 301–305
- Lee AW, Poon YF, Foo W, Law SC, Cheung FK, Chan DK, Tung SY, Thaw M, Ho JH. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: Overall survival and patterns of failure. Int J Radiat Oncol Biol Phys 1992; 23: 261–270
- YI JL, Gao L, Huang XD, Li SY, Luo JW, Cai WM, Xiao JP, Xu GZ. Nasopharyngeal carcinoma treated by radical radiotherapy alone: Ten year experience of a single institution. Int J Radiat Oncol Biol Phys 2006; 65: 161–168
- Gao YS, Hu CS, Ying HM, Zhu GP, Kong L, He XY, Xu TT, Wang XS, Yuan J, Wu SQ, et al. Treatment results of nasopharyngeal carcinoma: A retrospective analysis of 1837 cases in a single institute. Chin J Radiat Oncol 2008; 17: 335–339
- Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, Bosch W, Morrison WH, Quivey J, Thorstad W, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: Radiation therapy oncology group phase II trial 0225. J Clin Oncol 2009; 27: 3684–3690