Abstract
Purpose: To prospectively evaluate safety and effectiveness of intratumoural injection of superantigen staphylococcal enterotoxin C (SEC) for hepatocellular carcinoma (HCC) after percutaneous microwave ablation (PMWA).
Methods: HCC patients (260) with tumours of 60 mm or less (average 29.2 ± 11.1 mm) that achieved technique effectiveness evidenced on imagings within one week of PMWA were enrolled. The SEC group consisted of 45 patients with 2000 U SEC injected into the marginal area of ablated tumour under ultrasound guidance on day 24, 28 and month 2, 5, and 7 after PMWA. The control group consisted of 215 patients receiving PMWA alone.
Results: The overall survival rates for 1, 3, and 5 years were 93.3%, 72.9%, 60.8% in the SEC group and 94%, 66%, 44.4% in the control group. The overall survival time had significant difference (p = 0.032). Treatment method was prognostic significance for overall survival on univariate (p = 0.045) and multivariate (p = 0.049) analyses. The disease-free survival time of the SEC group was longer than the control group (p = 0.195). The disease free survival rates at 1 and 3 years were 62% versus 56.6%, 37% versus 9.4% in the SEC and control subgroup of larger (D > 30 mm) tumours, and disease free survival time had significant difference (p = 0.032). Treatment method was a prognostic significance factor for disease free survival of larger tumours on univariate (p = 0.03) and multivariate (p = 0.023) analyses but was not for small tumours. No serious adverse event related to SEC injection happened.
Conclusions: Local superantigen injection at ablated HCC early after PMWA is safe, and achieves longer overall survival as well as disease free survival of larger tumours.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignant tumour in the world, causing more than 500,000 deaths every year Citation[1]. Its incidence has been increasing worldwide due to the spread of hepatitis B and C virus infection. Treatment methods include surgical resection, liver transplantation, percutaneous ablation and transarterial chemoembolisation Citation[2]. Although orthotopic liver transplantation offers the chance for therapeutic success Citation[3] its performance is limited by a shortage of donor organs. Percutaneous ablation therapy has been accepted widely as an effective, less invasive treatment for small HCC. The 5-year survival rate was reported between 41% ∼ 55% Citation[4–6]. Although the overall survival rates were not different between surgical resection and percutaneous ablation, surgical resection may provide less cumulative recurrence rate Citation[7]. The cumulative disease-free survival rates of thermal ablation were 54.6% ∼ 74.3%, 27.3% ∼ 52.4% and 20% ∼ 29.7% for 1, 2 and 3 years Citation[8], Citation[9]. The 1 and 3 years cumulative intrahepatic distant recurrence after ablation was reported to be 10.4% ∼ 21.7% and 52.5% ∼ 70.6% Citation[8], Citation[10]. Local tumour progression rate after ablation was 12.8% ∼ 23% Citation[8], Citation[11]. Systemic chemotherapy with different agents is marginally effective against HCC but severely toxic and not associated with increased survival Citation[12]. Searching for new and effective methods to prolong the overall survival and disease free survival time requires further advances.
Although the accurate reason for pathogenesis and recurrence of HCC remains to be fully elucidated, the result of much research showed that antiviral immunity for hepatitis and antitumour immunity are intimately associated with cellular immunity Citation[13], Citation[14]. T lymphocytes are potent cellular effectors of the immune system and possess a memory that responds to rechallenge by the same antigen Citation[15], Citation[16].
Coley's toxins, bacterial products used to stimulate inflammatory responses in cancer patients reported in 1893 Citation[17], were one of the first immunological manipulators to treat cancer. Since then, several toxins or proteins produced by various microorganisms such as bacteria, mycoplasma or virus, which were dominated as superantigen Citation[18] had been proven to stimulate anti-tumour reaction by reactive T lymphocytes Citation[19–21] and to be safe in clinical use for digestive tract tumours at lower dosage by intravenous injection Citation[22] since the 1990s. Superantigens are the most powerful T-cell activation known today, and are promising as an immunological manipulator in tumour treatment. In June 1999, superantigen staphylococcal enterotoxin C (SEC), brand named Gaojusheng (Shengyang Xiehe Bio-pharmaceutical, Shengyang, China) was permitted for clinical use with intramuscular injection by the State Drug Administration of China.
In this study we designed a prospective non-randomised controlled trial to test the hypothesis that SEC injected at the site of ablated tumour, may increase overall survival rate and disease free survival rates of HCC patients after percutaneous microwave ablation (PMWA). Here we report results of this prospective non-randomised controlled phase II clinical trial in HCC patients with combined therapy of PMWA and SEC injection at the ablated tumour site in comparison with those of PMWA alone, to observe the effect of SEC in promoting long-term survival and preventing tumour recurrence.
Patients and methods
Study design
This trial was approved by our institutional human research review committee. From December 1999 to April 2006, all patients who had pathologically proven HCC and hepatitis B-related cirrhosis and received PMWA with curative intention at the authors‘ institution were considered to be enrolled in our study. If technique effectiveness was evidenced at contrast-enhanced imaging within 1 week of PMWA, every eligible patient was explained the purpose of PMWA and SEC treatment and was assigned to the SEC or control group according to their willingness.
Patients
In total 260 patients were enrolled in our study and were divided into SEC and control groups according to their preference. Inclusion criteria for PMWA were unresectable cancer; tumour accessible via a percutaneous approach; single nodular HCC tumours of 60 mm or smaller; three or fewer multiple nodular hepatic tumours with a maximum dimension of 40 mm or less in each nodule; absence of portal vein thrombosis or extra-hepatic metastases; prothrombin time of less than 25 s, prothrombin activity higher than 40%, and platelet count higher than 40 cells × 109/L. Inclusion criteria for SEC treatment were no history of allergy, willing to receive SEC therapy after PMWA and no severe major complications of ablation requiring hospitalisation one month after PMWA. The SEC group included 45 patients with 66 tumours. Another 215 patients with 294 tumours willing to receive PMWA alone were included in the control group. Written informed consent was obtained from all patients. Histological diagnosis was obtained by US-guided percutaneous biopsy using an 18-gauge needle in all patients. In patients with multiple nodules, at least one biopsy was performed.
Subgroups were divided according to the maximum diameter of the tumour. Small tumours, diameter of 30 mm or less, were subgroup 1. Intermediate and large tumours, diameter of more than 30 mm, were subgroup 2. The baseline data of the two groups and the subgroups are listed in . There was no statistical difference between the two groups and subgroups in gender, age, constituents of the number of tumour, size of tumour, Child-Pugh classification or condition of preablation treatment.
Table I. Patient characteristics and tumour profile.
Preablation imaging work-up
Pretreatment examination included ultrasound (US), contrast-enhanced computed tomography (CT) and/or contrast-enhanced magnetic resonance imaging (MRI), and tumour marker assay in all subjects. US was performed using a Sequoia US system (Acuson, Mountain View, CA) with 3.5–5.0 MHz curved-array multi-frequency transducers. CT studies were carried out with spiral CT (Tomoscan SR 7000; Philips Medical Systems, Best, the Netherlands) or multi-detector row CT (Lightspeed 16; GE Medical Systems, Milwaukee, WI) and contrast medium (iopromide, Ultravist 300; Schering, Berlin, Germany). MRI studies were carried out with a 1.5-T unit (Signa Echo-Speed, GE Medical Systems), and contrast medium (Magnevist, Schering; 0.1 mmol/kg body weight).
Microwave ablation technique and equipment
All treatments were performed at the authors’ institution and were carried out under US guidance in the interventional room. General anaesthesia was achieved by using a combination of two anaesthetics, propofol (Diprivan; Zeneca Pharmaceuticals, Wilmington, DE) and ketamine (Shuanghe Pharmaceuticals, Beijing, China). Two types of microwave equipment were applied in the study. From 1999 to 2004, a UMC-I microwave applicator (PLA General Hospital and Institute 207 of the Aerospace Industry Company, Beijing) with a frequency of 2450 MHz delivering a maximum power of 80 W through 16-gauge needle antennae was applied Citation[4], Citation[6], Citation[23]. From 2005 to 2006, a KY-2000 microwave applicator (Kangyou Medical, Nanjing, China) with a frequency of 2450 MHz delivering a maximum power of 100 W through two 15-gauge internally cooled antennae was applied Citation[24], Citation[25]. With US guidance, a single antenna or multiple antennae were used, depending on index tumour size Citation[4], Citation[6], Citation[23]. Percutaneous placement of the antenna was applied by any one of the three experienced radiologists (P.L., B.D., X.Y.) for all tumours. A detailed protocol was worked out for each patient on an individual basis before treatment Citation[4], Citation[6], Citation[23]. In general, for tumours less than 17 mm in diameter, a single antenna was used; for tumours 17 mm or larger, multiple antennae were applied synchronously or consecutively. An output setting of 60 W for 300 s was routinely used during ablations. A thermal monitoring system was used during treatment. With US guidance, one to three thermal couples were placed at different sites 5 mm outside the tumour to monitor temperature throughout the procedure. If the measured temperature at 5 mm outside the tumour did not reach 60°C by the end of 300 s and did not remain at 54°C for at least 3 min, an increase in application time was required depending on the temperature monitored dynamically (emission time range 180–1620 s).
SEC injection protocol
For patients in the SEC groups, 500 U, 1000 U and 2000 U SEC were injected intramuscularly on day 21, 22 and 23 after PMWA to observe the possibility of allergy and other severe adverse effects. If severe adverse effects did not happen, intratumoural SEC injection therapy was started. The margin of the ablation zone as well as the border between the ablated zone and surrounding liver parenchyma were obscure and hard to distinguish on day 24 after PMWA. We planned to inject SEC into the peripheral zone of the ablation zone as this would minimise diffusion of liquid outside the coagulated tissue because of lack of blood flow as well as lymphoid circulation. To reduce damage, only two 21G PTC needles were used to inject SEC. SEC was injected into the posterior peripheral area of ablated tumour through two 21G PTC needles under US guidance, then into the anterior peripheral area of ablated tumour after withdrawing the needles with local anaesthesia of lidocaine. The injection dates of SEC were arranged on days 24 and 28, and 2, 5 and 7 months after PMWA. Diffusion of SEC was observed on sonography, although not covering all the periphery of ablated zone. All patients completed the treatment protocol except one in the SEC group who suffered a sudden death 5 months after PMWA unrelated to the SEC injection. Eight patients in the SEC group had intrahepatic recurrence including 3 patients with local tumour progression within 7 months (1 in month 2, 5 in month 5, 2 in month 7) after PMWA Citation[26]. However, they continued to finish 5 doses of SEC injection. Thirty-four patients received 5 doses of local SEC injection. Eleven patients who had no recurrence at 1 year follow-up received an additional 1–9 (median 2) local SEC injections at the site of ablation with 6 months interval based on their own willingness.
Follow-up
The follow-up protocol was performed according to previously published recommendations Citation[4], Citation[6], Citation[23]. Therapeutic effectiveness was assessed on the basis of the result of contrast-enhanced imaging and serum tumour marker levels. Contrast-enhanced CT or MRI was repeated at 1-month and at 3-month intervals within 1 year after PMWA and then at 6-month intervals after treatment. If any intrahepatic new foci or local tumour progression was noted, PMWA was performed as soon as it was suitable for PMWA. The appearance over follow-up of foci of untreated disease in tumours that were previously considered to be completely ablated was defined as local tumour progression Citation[26]. The follow-up period was calculated starting from the beginning of PMWA for all patients. Condition of patients was discovered through phone calls follow-up till December 2008. Survival time was calculated from the date of PMWA to the date of death or the end of the follow-up period. Disease free survival time was calculated from the date of PMWA to the date of intrahepatic new tumour, local tumour progression or distant malignancy confirmed by contrast-enhanced imaging, or to the end of the follow-up period if no such sign was found. In the control group 21 patients were lost to follow-up, and disease free survival or survival time was calculated from the date of PMWA to the date of last follow-up if intrahepatic new foci, local tumour progression or distant malignancy did not happen before the date of last follow-up. The follow-up period of the lost patients ranged from 36 months to 79 months (54.2 ± 14.3 months).
Statistical analysis
Data analysis was conducted using Chinese High Intellectualised Statistical Software (CHISS, Beijing Yiyuantang Science and Technology Company, Beijing, China) for windows. Overall survival and disease free survival rates were calculated by the Kaplan-Meier method and analysed by the log-rank test. Univariate and multivariate analyses were based on the Cox proportional hazards regression model. The data were expressed as means ± standard deviation (SD). For comparison of patient characteristics and tumour profile between two groups, independent-samples t-test, Wilcoxon rank sum test, and chi-square test were carried out as appropriate. Two-tailed p < 0.05 was judged to be significant.
Results
Overall survival rate and prognostic factors
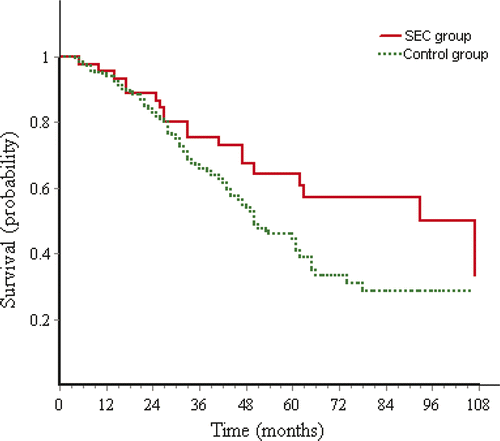
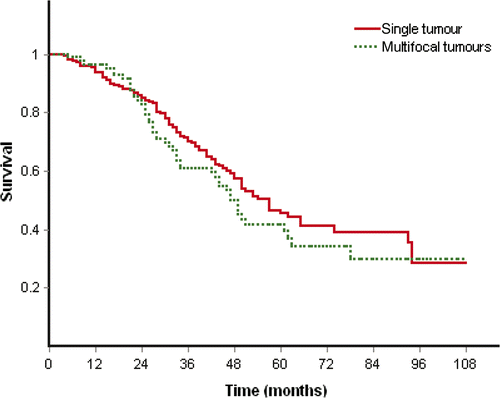
The overall survival rates at 1, 2, 3, 4 and 5 years were 93.3%, 86.7%, 72.9%, 64.4% and 60.8% in the SEC group, and 94%, 82.9%, 66%, 54.1% and 44.4% in the control group (), and overall survival time had significant difference (p = 0.032) between two groups. The observation period was more than 1 year for 43 patients (95.5%) and 204 patients (94.9%), 3 years for 32 patients (71.1%) and 140 patients (65.1%), 5 years for 19 patients (42.2%) and 60 patients (27.9%) in the SEC group and control group respectively. The median survival time was 49 months (range 5 to 107 months; 2.5 to 97.5 percentiles, 10.4 to 107 months) in the SEC group and 40 months (range 4 to 106 months, 2.5 to 97.5 percentiles 7.4 to 91.8 months) in the control group. The mean survival time was 74.4 ± 4.8 months and 58.8 ± 1.2 months in the SEC and the control group respectively. Treatment method (p = 0.045, p = 0.049), size of tumour (p = 0.02, p = 0.008) and Child-Pugh classification (p = 0.0, p = 0.012) showed prognostic significance for overall survival on univariate and multivariate analyses. Single tumour or multifocal tumours was not prognostic significance for overall survival on univariate (p = 0.25) and multivariate analyses (p = 0.856). The overall survival had no significant difference between patients of single tumour and multifocal tumours (p = 0.257, ).
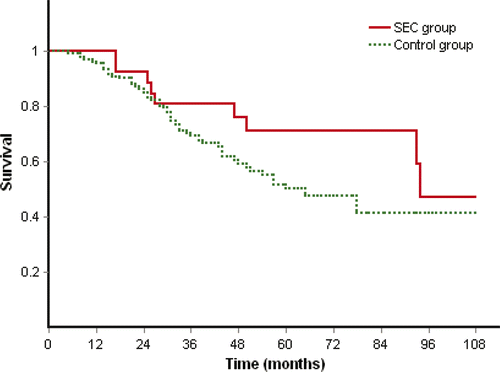
Subgroup analysis by Kaplan-Meier method and log-rank test showed that overall survival had no significant difference between SEC and control group in subgroup 1 of small tumours (p = 0.153, ) and in subgroup 2 of larger tumours (p = 0.132). But the mean survival time of the SEC group was clearly longer than the control group in both subgroup 1 (94.1 ± 8.9 months versus 65.6 ± 1.9 months) and subgroup 2 (76.8 ± 8.2 months versus 48.6 ± 2 months).
Disease-free survival rate and prognostic factors
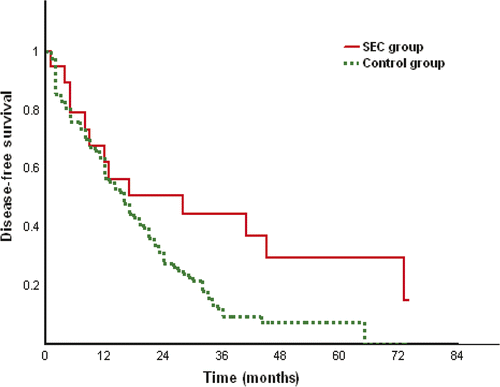
The median disease free survival time was 17 months (range 1 to 95 months; 2.5 to 97.5 percentiles 2.2 to 82.1 months) in the SEC group and 17 months (range 1 to 98 months, 2.5 to 97.5 percentiles 1 to 64.3 months) in the control group. The mean disease free survival time was 30.8 ± 3.4 months and 25.2 ± 0.7 months in the SEC and the control group respectively. The disease free survival rates at 1, 2, 3, 4 and 5 years were 64%, 43.2%, 31.2%, 16.7% and 16.7% in the SEC group, and 59.1%, 36.7%, 21.2%, 14.5% and 7.6% in the control group which had no significant difference (p = 0.195) between the two groups. Local tumour progression was found in four tumours (6.06%) in the SEC group and 31 tumours (10.54%) in the control group. The rate of local tumour progression had no significant difference (p = 0.267) between the two groups.
The result of subgroup analysis showed that the disease free survival rates at 1, 2, 3, 4 years were 62%, 44.4%, 37% and 14.8% in the SEC group and 56.6%, 27.7%, 9.4% and 7.5% in the control group of subgroup 2 of larger tumours (), and disease free survival time had significant difference (p = 0.032) between two groups. Treatment method was the only factor showing prognostic significance for disease free survival on univariate (p = 0.030) and multivariate (p = 0.023) analyses. The mean disease free survival time was 42.4 ± 7.1 months and 19.6 ± 1.3 months in the SEC and the control group of subgroup 2 respectively. However, the disease free survival time had no significant difference (p = 0.984) between the two groups in subgroup 1 of small tumours.
Adverse event
Local injection SEC was associated with minimal systemic toxicity. No haematological abnormalities were observed. The most frequent adverse event related to treatment included fever, general malaise and muscular soreness. Fever usually started from 6–8 h after intratumoural injection of SEC and decreased to normal in 3 days without medication. The maximum temperature range from 37.5–40.2°C (38.5° ± 1.2°C) The symptom of malaise and muscular soreness also diminished when body temperature decreased to normal. Two patients did not enrol in the SEC group because of grade II allergy with local flushing and indurations after the first intramuscular injection of SEC. No serious hypersensitive reaction happened.
Major complications related to ablation included pleural effusion requiring thoracentesis in three patients and bile duct injury in one patient of the control group and pleural effusion requiring thoracentesis in two patients and skin burn requiring resection in one patient of SEC group Citation[27].
Discussion
Microwave ablation is one of the minimally invasive thermal ablation techniques which obtain tumour destruction without significant damage to normal liver parenchyma by focal hyperthermia. Tissue injury caused by focal hyperthermia occurs in two distinct phases - direct and indirect phase Citation[28]. Direct heat injury is predominantly determined by the total energy applied to the tumour, tumour biology, and tumour microenvironment Citation[29]. Indirect injury after focal hyperthermia application, which may involve a balance of several factors including micro-vascular damage, ischaemia-reperfusion injury, induction of apoptosis, Chuffer cell activation, altered cytokine expression, and modulation of the immune response, produces a progression in tissue damage. Human liver tumours are generally of low immunogenic potential Citation[30]. Hyperthermia may modify tumour antigens to produce a host immune response Citation[31]. Local immune response may be one mechanism involved in progressive tissue injury after focal hyperthermia application and potentially influence patterns of tumour recurrences Citation[32–34]. It is highly likely that tumour destruction by focal hyperthermia increases expression of cancer antigens and stimulates the immune response. A large amount of activated T lymphocytes at the marginal area of ablation region are helpful to stimulate local antitumour immunity. Meanwhile, the recurrence of HCC mostly happens in liver and local tumour progression is also a notable problem after ablation Citation[8–11]. The attempt to change the immune microenvironment after ablation with massive cytokines such as IL-1, IL-2, IL-6, TNF and IFN-γ which was released by activated T lymphocytes after stimulation by superantigen Citation[35–37] may influence tumour recurrence and long-term survival. For those two reasons, we aimed to inject superantigen SEC into the marginal region of ablated area. Our result demonstrated that overall survival was significantly improved in the patients of the SEC group and disease free survival was also significantly improved in the subgroup of larger tumours (D > 30 mm) with combined therapy of PMWA and local injection of SEC, compared with those of the control group with PMWA alone. Combined therapy was the prognostic factor of overall survival rates as well as of disease free survival rates for the subgroup of larger tumours as shown by univariate and multivariate analyses. This result demonstrated that local SEC injection at the site of the ablated tumour may have a positive contribution to immunity modulation. Although disease free survival and local tumour progression were statistically lacking significant difference between SEC and control group, however disease free survival had statistically significant difference between SEC and control subgroup of larger tumour. This impliesthat combining immunotherapy with ablation may be of more value for larger tumours, which may be due to the fact that smaller tumours were adequately treated with percutaneous microwave ablation alone, but greater failure was expected for the population of larger tumours.
The result of our previous research showed the number of immunocytes, including T lymphocytes, natural killer cells and macrophages, significantly increased both at the marginal region of the ablated tumour and the adjacent liver tissue, and peaked on day 17 after PMWA, then mildly decreased but maintained at high level on day 30 after PMWA Citation[38], Citation[39]. Based on the results of our previous research, we aimed to start local injection of SEC into the marginal region of ablated tumour on day 24 after PMWA to achieve further stimulation of local immunocytes infiltration at the marginal region of the ablating area. Local injection of SEC would hold superantigen at the local site for longer and maintain persistent stimulating capability. The immune responses stimulated by SEC could change from tumouricidal activity to dosage-related immunosuppression Citation[40], Citation[41]. Meanwhile, considering the follow-up interval, we planned the local injection of SEC as a low dose at 3–6 months interval after initial three injections.
Systemic exposure to superantigens can be associated with serious toxicity Citation[42]. Local injection of superantigens may be able to effectively activate tumour infiltrating immune effector cells, while avoiding the adverse effects associated with systemic exposure. The result of our clinical observation of local SEC injection after PMWA showed no serious adverse effects. Grade II allergy was observed after the first intramuscular SEC injection in two patients who were excluded from the SEC group. Other adverse effects such as fever, general malaise and muscular soreness were well tolerated, which confirmed the safety of local injection of superantigen SEC. However, we should still pay close attention to the possibility of allergy.
The rate of local tumour progression showed no statistical difference between the two groups and although the rate of local tumour progression was lower in the SEC group compared with that in the control group. The overall survival rate showed no significant difference between the SEC and control subgroups of small tumours and larger tumours, although mean survival times were longer in the SEC subgroups compared with those in the control subgroups. This may be due to the small sample, especially in the SEC group.
This study had some limitations. First, this study was a phase II clinical trial in which all data were obtained from a single centre which had much experience with PMWA procedures. A multicentre study with a larger number of patients and longer follow up is required. Second, this study was a non-randomised comparison trial. A randomised controlled clinical study is needed to further confirm the effect of local injection of superantigen for HCC after ablation. Third, the SEC injection with two needles at four sites of the peripheral area of ablated tumour could not obtain a globular cuff of ablation zone which may weaken the immunostimulating effect.
In conclusion, local superantigen SEC injection at ablated tumour site in patients with HCC early after PMWA is safe and can achieve longer overall survival as well as disease free survival for larger tumours. With the advance of biotherapy, the combination application of new methods such as immunity modulation may result in better overall survival and disease free survival for HCC patients after ablation.
Declaration of interest: Supported by grants from the Natural Science Foundation of Beijing (30672016). The authors alone are responsible for the content and writing of the paper.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108
- Bruix J, Hessheimer AJ, Forner A, Vilana R, Llovet JM. New aspects of diagnosis and therapy of hepatocellular carcinoma. Oncogene 2006; 25: 3848–3856
- Bruix J, Fuster J, Llovet JM. Liver transplantation for hepatocellular carcinoma: Foucault pendulum versus evidence-based decision. Liver Transpl 2003; 9: 700–702
- Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, Xiao Q. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005; 235: 299–307
- Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: Long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005; 234: 961–967
- Dong BW, Liang P, Yu X, Su L, Yu D, Cheng Z, Zhang J. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: Results in 234 patients. AJR 2003; 180: 1547–1555
- Hasegawa K, Makuuchi M, Takayama T, Kokudo N, Arii S, Okazaki M, Okita K, Omata M, Kudo M, Kojiro M, et al. Surgical resection versus percutaneous ablation for hepatocellular carcinoma: A preliminary report of the Japanese nationwide survey. J Hepatol 2008; 49: 589–594
- Zytoon AA, Ishii H, Murakami K, El-Kholy MR, Furuse J, El-Dorry A, El-Malah A. Recurrence-free survival after radiofrequency ablation of hepatocellular carcinoma. A registry report of the impact of risk factors on outcome. Jpn J Clin Oncol 2007; 37: 658–672
- Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: Is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment?. Radiology 2009; 252: 905–13
- Okuwaki Y, Nakazawa T, Shibuya A, Ono K, Hidaka H, Watanabe M, Kokubu S, Saigenji K. Intrahepatic distant recurrence after radiofrequency ablation for a single small hepatocellular carcinoma: Risk factors and patterns. J Gastroenterol 2008; 43: 71–78
- Lam VW, Ng KK, Chok KS, Cheung TT, Yuen J, Tung H, Tso WK, Fan ST, Poon RT. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg 2008; 207: 20–29
- Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005; 42: 1208–1236
- Milich DR, Jones J, Hughes J, Maruyama T. Hepatitis B virus infection, the immune response and hepatocellular carcinoma. Ciba Found Symp 1994; 187: 113–129
- Cerny A, Chisari FV. Immunological aspects of HCV infection. Intervirology 1994; 37: 119–125
- Takagi S, Chen K, Schwarz R, Iwatsuki S, Herberman RB, Whiteside TL. Functional and phenotypic analysis of tumour infiltrating lymphocytes isolated from human primary and metastatic liver tumours and cultured in recombinant interleukin-2. Cancer 1989; 63: 102–111
- Chiriva-Internati M, Grizzi F, Wachtel MS, et al. Biological treatment for liver tumor and new potential biomarkers. Dig Dis Sci 2008; 53: 836–843
- Coley WB. The treatment of malignant tumours by repeated inoculations of erysipelas with a report of 10 original cases. Am J Med Sci 1893; 105: 487–511
- White J, Andrew H, Ann M, Iwatsuki S, Herberman RB, Whiteside TL. The Vβ-specific superantigen staphloccoal enterotoxin: Stimulation of nature T cells and colonal deletion in neonatal mice. Cell 1989; 56: 27–35
- Hedlund G, Dohlsten M, Petersson C, Kalland T. Superantigen-based tumor therapy: In vivo activation of cytotoxic T cells. Cancer Immunol Immunother 1993; 36: 89–93
- Lamphear JG, Bohach GA, Rich RR. Structural dichotomy of staphylococcal enterotoxin C superantigens leading to MHC class II-independent activation of T lymphocytes. J Immunol 1998; 160: 2107–2114
- Inoue M, Plautz GE, Shu S. Treatment of intracranial tumors by systemic transfer of superantigen-activated tumor-draining lymph node T cells. Cancer Research 1996; 56: 4702–4708
- Nielsen SE, Zeuthen J, Lund B, Persson B, Alenfall J, Hansen HH. Phase I study of single, escalating doses of a superantigen-antibody fusion protein (PNU-214565) in patients with advanced colorectal or pancreatic carcinoma. J Immunother 2000; 23: 146–153
- Dong BW, Liang P, Yu XL, Zeng XQ, Wang PJ, Su L, Wang XD, Xin H, Li S. Sonographically guided microwave coagulation treatment of liver cancer: An experimental and clinical study. Am J Roentgenol 1998; 171: 449–454
- Wang Y, Sun Y, Feng L, Gao Y, Ni X, Liang P. Internally cooled antenna for microwave ablation: Results in ex vivo and in vivo porcine livers. EJR 2008; 67: 357–361
- Liu FY, Yu XL, Liang P, Wang Y, Zhou P, Yu J. Comparison of percutaneous 915 MHz microwave ablation and 2450 MHz microwave ablation in large hepatocellular carcinoma. Int J Hyperthermia 2010; 26: 448–455
- Goldberg SN, Charboneau JW, Dodd GD, III, Dupuy DE, Gervais DA, Gillams AR, Kane RA, Lee FT, Jr, Livraghi T, McGahan JP, et al. Image-guided tumor ablation: Proposal for standardization of terms and reporting criteria. Radiology 2003; 228: 335–345
- Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: Treatment with percutaneous microwave ablation - complications among cohort of 1136 patients. Radiology 2009; 251: 933–940
- Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of Focal Heat Destruction of Liver Tumors. Journal of Surgical Research 2005; 127: 208–223
- Nikfarjam M, Malcontenti-Wilson C, Christophi C. Focal hyperthermia produces progressive tumor necrosis independent of the initial thermal effects. J Gastrointest Surg 2005; 9: 410–417
- Mihich E. Biological response modifiers: Their potential and limitations in cancer therapeutics. Cancer Invest 1985; 3: 71–83
- Isbert C, Ritz JP, Roggan A, Schuppan D, Rühl M, Buhr HJ, Germer CT. Enhancement of the immune response to residual intrahepatic tumor tissue by laser induced thermotherapy (LITT) compared to hepatic resection. Lasers Surg Med 2004; 35: 284–292
- Dickson JA, Shah SA. Hyperthermia: The immune response and tumor metastasis. Natl Cancer Inst Monogr 1982; 61: 183–192
- Mondovì B, Santoro AS, Strom R, Faiola R, Fanelli AR. Increased immunogenicity of Ehrlich ascites cells after heat treatment. Cancer 1972; 30: 885–888
- Wu F, Zhou L, Chen WR. Host antitumour immune responses to HIFU ablation. Int J Hyperthermia 2007; 23: 165–171
- Dohlsten M, Hedlund G, Akerblom E, Lando PA, Kalland T. Monoclonal antibody-targeted superantigens: A different class of anti-tumor agents. Proc Natl Acad Sci USA 1991; 88: 9287–9291
- Dohlsten M, Sundstedt A, Bjorklund M, Hedlund G, Kalland T. Superantigen-induced cytokines suppress growth of human colon-carcinoma cells. Int J Cancer 1993; 54: 482–488
- Litton MJ, Dohlsten M, Lando PA, Kalland T, Ohlsson L, Andersson J, Andersson U. Antibody-targeted superantigen therapy induces tumor-infiltrating lymphocytes, excessive cytokine production, and apoptosis in human colon carcinoma. Eur J Immunol 1996; 26: 1–9
- Dong B, Zhang J, Liang P, Yu XL, Su L, Yu DJ, Ji XL, Yu G. Sequential pathological and immunologic analysis of percutaneous microwave coagulation therapy of hepatocellular carcinoma. Int J Hyperthermia 2003; 19: 119–133
- Zhang J, Dong B, Liang P, Yu XL, Su L, Yu DJ, Ji XL, Yu G. Significance of changes in local immunity in patients with hepatocellular carcinoma after percutaneous microwave coagulation therapy. Chin Med J (Engl) 2002; 115: 1367–1371
- Jie K, Jiang H, Sun L, Wang HR, Zheng YL, Li Y, Jiang YQ. The pilot study of anti-tumor effects versus immunosuppression of staphylococcal enterotoxin C. Cancer Biol Therapy 2007; 6: 1584–1591
- Seo KS, Lee SU, Park YH, Davis WC, Fox LK, Bohach GA. Long-term staphylococcal enterotoxin C1 exposure induces soluble factor-mediated immunosuppression by bovine CD4+ and CD8+ T cells. Infect Immun 2007; 75: 260–269
- McCormack JE, Callahan JE, Kappler J, Marrack PC. Profound deletion of mature T cells in vivo by chronic exposure to exogenous superantigen. J Immunol 1993; 150: 3785–3792