Abstract
Purpose: Partial splenectomy is technically more complicated than total splenectomy due to difficulty in haemostasis, but it can preserve splenic function after operation. We evaluated the feasibility and safety of partial splenectomy performed by an electromagnetic thermal surgery system in a porcine model.
Methods: Our system was comprised of an alternating electromagnetic field generator, an extensible coil applicator, comb-needle arrays, and a temperature feedback control component. Ten Lanyu pigs were anaesthetised to conduct partial splenectomy. Two rows of comb-like stainless-steel needle arrays were inserted into the tissue at 15 cm from the distal tip of the spleen. The temperature of the tissues around the needle arrays was raised to 150°C for 3 min and the spleen was transected directly between the needle arrays and then sent for histological examination. Two weeks later, the animals underwent a second celiotomy to remove the remaining spleen for histological examination.
Results: The average duration of the partial splenectomy was 10 min as timed from insertion of the needle arrays to the transection of the spleen. There was no blood loss during the procedure. The cut surface of the spleen was well coagulated without any oozing sites. During the re-exploration, no intra-abdominal blood was found. There were dense adhesions between the spleen and the surrounding organs. Histological examination of the cut surface of the excised portion of the spleen showed coagulative necrosis with clot formation in the blood vessels.
Conclusions: Partial splenectomy using our electromagnetic thermal system can achieve effective haemostasis and is safe and easy to perform.
Introduction
Total splenectomy carries certain severe health problems even when it is performed in adults Citation[1–2]. Among these problems, overwhelming infection is of greatest concern Citation[3]. Partial splenectomy is an alternative developed to preserve splenic function while removing benign tumours of the spleen, such as hamartomas, inflammatory pseudotumours, lipomas, haemangiomas, and splenic cysts Citation[4]. Partial splenectomy can also be used to treat splenic injuries restricted to one pole of the spleen, and haematological problems related to hypersplenism such as thrombocytopenia and haemolytic anaemia. Importantly, partial splenectomy can restore the normal functions impaired by the aforementioned haematological problems Citation[5–7].
The conventional technique of partial splenectomy consists of dividing the segmental branches of the splenic artery and vein to the pole being resected. After the pole becomes ischaemic, the parenchyma is then divided at the boundary of the ischaemia. However, significant blood loss can occur, occasionally resulting in the need for a total splenectomy. To reduce blood loss during splenic resection, techniques other than ligation and resection, such as the application of radiofrequency or microwave energy to partial splenectomy, have been utilised Citation[8–9].
Radiofrequency (RF) and microwaves generate heat by applying energy through electrodes to the adjacent splenic parenchyma. The heat causes coagulative necrosis and vessel shrinkage in the spleen resection margins and seals off the blood vessels, which allows division of the parenchyma without significant bleeding. Heat can also be generated by magnetic particles or fluids Citation[10–12]. By using stainless-steel needles and extensile coils to generate heat we have successfully used electromagnetic power to resect liver in rats, rabbits and pigs Citation[13–15]. The purpose of this study was to evaluate the feasibility and safety of this electromagnetic thermal system for splenic resection in a porcine model.
Materials and methods
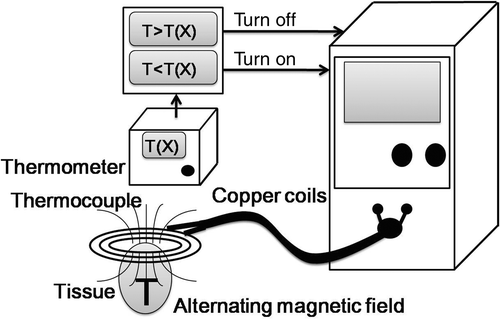
Our system comprises an alternating magnetic field (AMF) generator, coils with an extensor, needle arrays and a temperature feedback control component.
Alternating magnetic field generator
The physical properties and specifications of the AMF generator developed in our laboratory have been previously reported Citation[16]. Briefly, the machine generates a high frequency alternating magnetic field with an input of 220V-55A-60Hz, an output frequency of 62.1 kHz, and a power of 2.2 kW at the centre of the induction coils, which are 7.5 cm in diameter (). To facilitate its operation, the coil was extended by using cables to connect it to the machine. The entire device functions as a high-frequency magnetic generator. The magnetic flux density at 1 cm from the centre of the coils was 2 teslas (measured with a 7030 Gauss/Tesla meter, F.W. Bell (Milwaukie, OR, USA)).
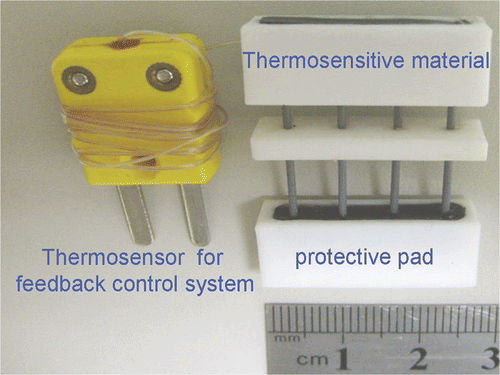
Comb-needle array
To facilitate the needle puncture over the planned resection line, we designed a needle array consisting of 26-gauge, 3-cm long stainless-steel needles with a linear layout and capped with a thermo-responsive material (). The needles were arranged 0.5 cm apart from each other. The thermo-responsive block was made of Thermolite (New Prismatic, Taipei, Taiwan) that changes colour from black to white when its temperature reaches 70°C. In addition, a temperature sensor was positioned inside one of the needles to provide for temperature control. Since all needles were placed in the same alternating electromagnetic field, the temperature at the top of each needle was almost identical, as confirmed in our preliminary experiments. Accurately measuring tissue temperature is beneficial to the safety of the animals, and it also ensures an effective temperature for coagulation where the local circulation is higher than expected. Measurements were made once every second. The length of the needle array can be varied to satisfy the length of resection and the depth of the tissue. To create a 1-cm wide transection line, two rows of needle arrays 0.5 cm apart were used to puncture the planned resection line when using AMF for partial splenectomy.
Figure 2. Comb-needle array used in this study. Note that the conductive coil and plug provide feedback to the control system to obtain the correct needle temperature. There is a temperature sensor embedded in one of the needles, which is connected with the plug. The needles are separated by 0.5 cm and can be of various lengths. The needle array has a protective pad over the sharp end. The pad will be applied after tissue penetration and before heating.
Animals
The protocol of the animal experiments was approved by the animal research committee of National Cheng Kung University Medical College and is in accordance with the guidelines set forth by the Agriculture Council of Taiwan on Animal Care.
Ten male Lanyu pigs weighing between 20 and 33 kg (mean 24.5 kg) were fasted overnight with free access to water. The animals were premedicated with intramuscular injection of 0.02 mg/kg atropine (Sintong, Taoyuan, Taiwan), 0.1 mL/kg xylazine (Bayer, Leverkusen, Germany), and 10 mg/kg Zoletil 50 (Virbac, Carros, France). Following endotracheal intubation, the animals were anaesthetised using 8 mL/kg i.v. thiamylal sodium and 200 mL/kg/min 1–3% isoflurane (Halocarbon, River Edge, NJ, USA) throughout the operation. Blood pressure, heart rate and oxygen saturation were continuously monitored and recorded every 15 min throughout the surgical procedure. All animals were closely observed by a veterinarian during the operation.
Surgical procedure
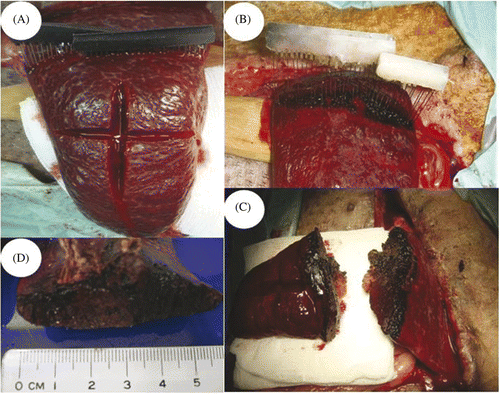
The abdomen was shaved and disinfected with povidone-iodine solution. An upper midline incision was made and the abdomen was carefully explored to eviscerate the medial portion of the spleen. Two needle arrays were inserted into and through the splenic tissue, and minimal bleeding was noted from the puncture holes (). The line of splenic resection was chosen arbitrarily at 15 cm from the tip of the spleen to standardise the surgical approach. According to our previous experiments on rats and rabbits, the temperature and the time needed to achieve coagulative necrosis was 150°C for 3 min. The temperature of the tissue was confirmed both by observing the change of colour of the Thermolite from black to white as well as the measurement from the built-in temperature feedback control component of the system (). After thermocoagulation, the needle arrays were removed and the spleen was separated along the planned resection line by scalpel (). The hilar vessels to the distal portion of the spleen were divided to complete the partial splenectomy. The cut surface was examined to ensure adequate coagulation ().
Figure 3. Procedures of the partial splenectomy using the electromagnetic thermal surgery system. (A) The needle arrays were inserted 15 cm from the tip of the spleen. (B) After heating, the Thermolite attached to the needle arrays changed colour from black to white when the temperature was above 70°C. (C-D) The cut surfaces of the spleen showed effective coagulation without signs of oozing.
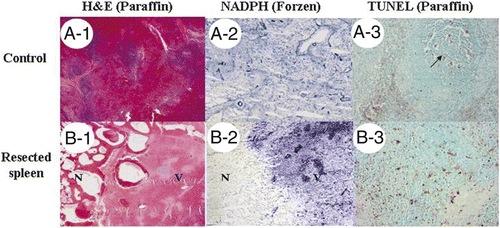
The midline abdominal incision was then closed in layers. The animals were allowed to wake up and to resume a regular diet. The removed portion of the spleen was sent for histological examination ().
Figure 4. Histological examination of surgical margins. (A) Control splenic tissue (A1) was positive for NADPH-diaphorase activity (A2) and showed apoptosis only within the white pulp under TUNEL assay (A3). (B) Immediately resected spleen (B1) showed loss of NADPH (B2) in necrotic area (N) compared to viable splenic tissue (V). Increase of apoptosis in necrotic area was also noted (B3). (A1, A2, B-1, and B2: magnified 10×; A3 and B3: 100×).
Two weeks after the first operation the animals underwent a second abdominal exploration through the previous midline incision. The ligaments between the spleen and the surrounding organs were divided. The adhesions between the cut surface of the spleen and omentum or adjacent organs were carefully preserved and removed en bloc with the spleen for histological examination ().
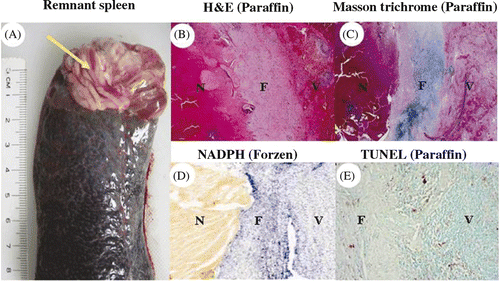
Figure 5. Histological examinations of remnant spleen. (A) The remnant spleen was removed 14 days after the procedure; arrow points to the dense adhesions. Histological examination showed a fibrous band (F) between viable splenic tissue (V) and necrotic tissue (N) (B: HE stain; C: Masson's trichrome stain; D: NADPH stain, for viability from frozen section; E: TUNEL assay from paraffin block; B-D: 10×, E: 100×).
Histological examinations
To examine the immediate effect of electromagnetic thermoablation, one part of the transected spleen was immediately fixed in 10% formalin, sectioned, and stained with haematoxylin-eosin; the other part was frozen in an optimal cutting temperature compound by using liquid nitrogen to assess tissue viability with nicotinamide-adenine dinucleotide phosphate (NADPH)-diaphorase staining. In brief, the frozen samples were sectioned, mounted on glass slides, and washed with water. The samples were then allowed to react for 20 min at room temperature with NADPH-diaphorase reaction solution (10 mL of 10 mmol/L PBS, pH 7.4, containing 10 mg NADPH, and 5 mg nitroblue tetrazolium).
The remaining spleen removed on the day of sacrifice was fixed in 10% formalin, sectioned, and stained with haematoxylin-eosin and Masson's trichrome to examine the repair processes of the remnant of spleen.
Results
The average total operating time was 30 min. The average time needed to separate the distal part of spleen from the proximal with our system was 10 min (including the electromagnetic thermocoagulation and transection). All ten animals survived the surgical procedures. The recorded blood pressure, heart rate, and oxygen saturation during the procedure were within normal ranges. No animals had any post-operative complications.
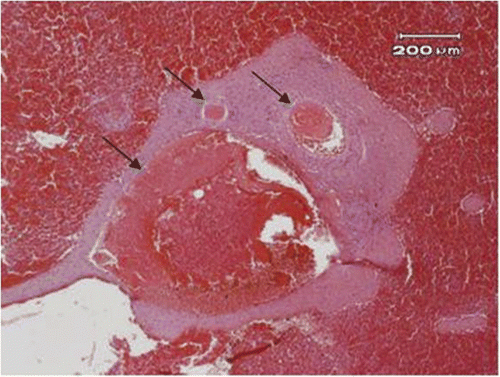
The cut surfaces of the spleens did not have signs of active oozing even before the division of the blood vessels (). Histological examination of the cut surface from the removed portion of the spleen showed coagulative necrosis () and increased apoptosis (), compared with a control spleen from a non-treated pig (). The blood vessels were coagulated with blood clot formation in the lumen (). The maximal diameter of the blood vessels coagulated by electromagnetism was estimated as 3 mm.
Figure 6. The blood vessels (arrow) near the resected margin of the spleen showed thrombus formation (40×).
During the second exploration there was no evidence of bleeding from the previous operation, and no abscess formation in the left upper abdomen in any of the ten pigs studied. Dense adhesions were found in all animals; eight between the cut surface of the spleen and the omentum, one with the stomach, and one with the small bowel. The dense adhesions were mainly composed of fibrotic tissue ().
Tissue examination of the remnant spleen showed a healing process by fibrous band formation between necrotic tissue and viable tissue (). Apoptosis in the remnant tissue was comparable to the control tissue levels ().
Discussion
Our study demonstrated that the electromagnetic thermal system is able to achieve coagulation of splenic tissues, allowing the subsequent bloodless division of the spleen. By heating the tissue with our electromagnetic thermal system the blood vessels were completely sealed off by thrombus and no prior ligation of segmental splenic vessels was required. During the second exploration 14 days later, dense adhesions were observed between the splenic cut surface and surrounding tissues and organs, including omentum, stomach, and small intestines, and no delayed bleeding occurred during this observation period. The formation of dense adhesions between the cut surface of the spleen and the surrounding tissues and organs is likely due to local inflammation as a result of the surgical trauma as well as the loss of the visceral serosa covering the spleen. The adhesions should not be affected by the method of splenic resection, whether it is our thermal surgery system or simply with a scalpel.
The short- and long-term septic complications after total splenectomy have changed the conventional surgical technique from total splenectomy to partial splenectomy when managing patients with spleen trauma or splenic tumours Citation[17–18]. However, despite segmental ligation, bleeding from the splenic parenchymal surface occurs and is difficult to control. Many innovative techniques to reduce bleeding from the cut splenic surfaces have been proposed, including linear staples Citation[19], CO2 laser Citation[20], argon beam coagulator Citation[21], and RF-based thermal surgery Citation[22].
RF delivers a high frequency current to vibrate the electrons in the tissue and generate heat. By applying 6 RF electrodes at the same time, Haghighi and his colleagues created a 1 × 6 × 6-cm coagulative block in 12 min in ovine spleen Citation[23]. Such a technique successfully transects the spleen with minimal blood loss, and no mobilisation of the spleen or ligation of segmental vessels was required. The success of applying RF for a partial splenectomy proves that heat can effectively coagulate the vessels and allow bloodless transection of the spleen. However, an elegant study by Mertyna et al. showed that the effect of distance and baseline temperature for radiofrequency ablation were quite variable. The thermal doses to achieve coagulation were not constant and were difficult to predict Citation[24].
Previous technologies in the 1980s using alternating electromagnetic fields and metal implants generated enough heat for hyperthermia but were never developed into thermal surgery. At that time, no one proposed opening the abdomen to perform electromagnetic thermocoagulation as we have done in studies using rats, rabbits and pigs Citation[13–15]. By opening the abdomen and using an extensile coil, we have successfully resected livers and spleens in small animals. However, the blood vessels in small animals were not large enough to generate the ‘heat-sink’ effect, and one might doubt its coagulative efficiency when applied to large animals and humans. This is no longer a concern, since the current study has demonstrated that our electromagnetic thermal system can coagulate blood vessels up to 3 mm and transect the porcine spleen without prior ligation of blood vessels. Additional minor modifications of the apparatus, including energy output, needle design, computer software, and the treatment protocol can potentially coagulate vessels with diameters larger than 3 mm.
Our success in using electromagnetism should also be attributed to the design of the comb-needle array, which allowed the simultaneous insertion of multiple in-line needles. This design has facilitated the insertion of needles and accelerated the heating procedure, which will be especially important when applying our system to patients with splenic trauma. Our comb-needle arrays are designed with a distance of 0.5 cm between each needle. As explained in our previous report Citation[14], a needle array consisting of nine needles raised the temperature to 93° ± 2°C in 3 min and resulted in 0.5 cm-wide coagulation. Each needle was fixed to a Thermolite block, which can help to ensure the completeness of the heating procedure. The Thermolite block is soft and can be easily cut by scissors to fit varying lengths of transection lines.
Our electromagnetic thermal system may be the most promising one to be applied to surgery among the techniques that use heat to resect parenchymal tissues. Unlike RF-based thermal surgery, our system is based on ferromagnetism that uses stainless-steel needles (containing iron) to generate heat when placed in a high-frequency alternating magnetic field. Since the magnetic force can penetrate the body without conducting wires, there is no electrical current flowing through the body and no grounding pads are needed on the skin. The effective treatment length of RFA electrodes and microwave probes is usually within 3 cm, whereas our technology uses inductive heat and therefore the therapeutic length is easy to adjust according to treatment demands. Besides these safety and efficiency aspects, another compelling advantage is the relative cheapness of our disposable comb-needle arrays over RF electrodes or microwave probes.
There are, however, a few limitations to our study. The partial splenectomy was performed on a normal spleen and the resection line was chosen beforehand. Partial splenectomy is possible for most patients receiving elective surgery, but is quite unlikely in traumatic patients whose ruptured spleens have irregular edges. The other important limitation is that no metallic materials can be placed in the magnetic field. The presence of stainless-steel instruments in the operative field may burn contacted tissues since they will be heated. Many clips or staples are available in plastic materials and should be used with our thermal system, along with non-metallic surgical tools or retractors. Although patients with metallic implants such as artificial joints or a pacemaker might appear to be contraindicated, we believe that safe practices can be attained by rearranging the orientation of the magnetic field to keep at least 10 cm between coils and implants. Finally, open surgery is still needed to apply the extensile coils and needle arrays; our current technology is not yet suitable for laparoscopic surgery.
Conclusion
Our system is a powerful tool to generate tissue coagulation and has the potential to be used for partial splenectomy. The present system is simple and effective as it brings a minimal amount of equipment into the body, thereby reducing the need for sterilising a lot of cable. Our system should simplify surgical techniques and make surgery safer for patients with various kinds of splenic problems that require splenectomy. It is worth pursuing clinical trials to prove its usefulness in humans.
Acknowledgements
We thank veterinarian Chih-Ting Liu for her assistance in the anaesthesia and perioperative care of the animals, also Chih-Hao Huang and Sheng-Chieh Huang, Tung-Jen Lee, Ming-Hwa Chen, Yi-Chun Lai, and Szu-Yin Chen for their assistance in the study.
Declaration of interest: This study was supported partly by a grant from the National Science Council of Taiwan, NSC 97-2627-B-006-007, and NSC 98-3114-Y-076-009, and partly by a grant from the Industry Technology Research Institute, ITRI 2009-08569, 98-EC-17-D-02110953. Roberto Zuchini, a physician and PhD student, was supported by an international scholarship from National Cheng Kung University. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cadili A, de Gara C. Complications of splenectomy. Am J Med 2008; 121: 371–375
- Waghorn DJ. Overwhelming infection in asplenic patients: Current best practice preventive measures are not being followed. J Clin Pathol 2001; 54: 214–218
- Schwartz PE, Sterioff S, Mucha P, Melton LJ, III, Offord KP. Postsplenectomy sepsis and mortality in adults. JAMA 1982; 248: 2279–2283
- Coon WW. Surgical aspects of splenic disease and lymphoma. Curr Probl Surg 1998; 35: 543–646
- Millikan JS, Moore EE, Moore GE, Stevens RE. Alternatives to splenectomy in adults after trauma. Repair, partial resection, and reimplantation of splenic tissue. Am J Surg 1982; 144: 711–716
- Rice HE, Oldham KT, Hillery CA, Skinner MA, O’Hara SM, Ware RE. Clinical and hematologic benefits of partial splenectomy for congenital hemolytic anemias in children. Ann Surg 2003; 237: 281–288
- N'Kontchou G, Seror O, Bourcier V, Mohand D, Ajavon Y, Castera L, Grando-Lemaire V, Ganne-Carrie N, Sellier N, Trinchet JC, Beaugrand M. Partial splenic embolization in patients with cirrhosis: Efficacy, tolerance and long-term outcome in 32 patients. Eur J Gastroenterol Hepatol 2005; 17: 179–184
- Pikoulis E, Felekouras E, Papaconstantinou I, Kontos M, Prassas E, Griniatsos I, Bacoyiannis C, Pappa P, Papalois A, Tsigris C, et al. A novel spleen-preserving laparoscopic technique using radiofrequency ablation in a porcine model. Surg Endosc 2005; 19: 1329–1332
- Gao Y, Wang Y, Duan Y, Li C, Sun Y, Zhang D, Lu T, Liang P. 915MHz microwave ablation with high output power in in vivo porcine spleens. Eur J Radiol 2010; 75: 87–90
- Salloum M, Ma R, Zhu L. Enhancement in treatment planning for magnetic nanoparticle hyperthermia: Optimisation of the heat absorption pattern. Int J Hyperthermia 2009; 25: 309–321
- Jordan A, Wust P, Fahling H, John W, Hinz A, Felix R. Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int J Hyperthermia 2009; 25: 499–511
- Jordan A. Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int J Hyperthermia 2009; 25: 512–516
- Zuchini R, Huang CH, Tsai HW, Huang SC, Lin CP, Chen CY, Lee GB, Lin XZ. Electromagnetic thermoablation to treat thrombocytopenia in cirrhotic and hyperesplenic rats. J Gastroenterol Hepatol 2010; 25: 1578–1586
- Chen CY, Zuchini R, Tsai HW, Huang CH, Huang SC, Lee GB, Lin XZ. Electromagnetic thermal surgery system for liver resection – An animal study. Int J Hyperthermia 2010; 26: 604–609
- Shan YS, Zuchini R, Tsai HW, Lin PW, Lee GB, Lin XZ. Bloodless liver resection using needle arrays under alternating electromagnetic fields. Surg Innov 2010; 17: 95–100
- Tseng HY, Lee GB, Lee CY, Shih YH, Lin XZ. Localised heating of tumours utilising injectable magnetic nanoparticles for hyperthermia cancer therapy. IET Nanobiotechnol 2009; 3: 46–54
- Ziemski JM, Rudowski WJ, Jaskowiak W, Rusiniak L, Scharf R. Evaluation of early postsplenectomy complications. Surg Gynecol Obstet 1987; 165: 507–514
- Holdsworth RJ, Irving AD, Cuschieri A. Postsplenectomy sepsis and its mortality rate: Actual versus perceived risks. Br J Surg 1991; 78: 1031–1038
- Uranus S, Kronberger L, Kraft-Kine J. Partial splenic resection using the TA-stapler. Am J Surg 1994; 168: 49–53
- Reynolds M, LoCicero J, III, Young S, Michaelis LL. Partial splenectomy with the CO2 laser: An alternative technique. J Surg Res 1986; 41: 580–586
- Go PM, Goodman GR, Bruhn EW, Hunter JG. The argon beam coagulator provides rapid hemostasis of experimental hepatic and splenic hemorrhage in anticoagulated dogs. J Trauma 1991; 31: 1294–1300
- Zacharoulis D, Poultsidis A, Katsogridakis E, Kalala F, Nakou M, Chatzitheofilou C. Radiofrequency-assisted partial splenectomy: Histopathological and immunological assessment of the splenic remnant in a porcine model. Surg Endosc 2008; 22: 1309–1316
- Haghighi KS, Steinke K, Hazratwala K, Kam PC, Daniel S, Morris DL. Controlled study of in-line ovine spleen transection assisted by radiofrequency ablation. J Trauma 2005; 58: 841–844
- Mertyna P, Dewhirst MW, Halpern E, Goldberg W, Goldberg SN. Radiofrequency ablation: The effect of distance and baseline temperature on thermal dose required for coagulation. Int J Hyperthermia 2008; 24: 550–559