Abstract
Purpose: Terminally differentiated neurones in the central nervous system need to be protected from stress. We ask here whether differentiation of progenitor cells to neurones is accompanied by up-regulation of Hsp72, with acquisition of enhanced thermotolerance.
Materials and methods: Human neuroblastoma SH-SY5Y cells were propagated in an undifferentiated form and subsequently differentiated into neurone-like cells. Thermotolerance tests were carried out by exposure of cells to various temperatures, monitoring nuclear morphology as index of cell death. Abundance of Hsp72 was measured in cell lysates by western immunoblotting.
Results: The differentiation of SH-SY5Y cells was accompanied by increased expression of Hsp72. Further, in both cell states, exposure to mild hyperthermic stress (43°C for 30 min) increased Hsp72 expression. After differentiation, SH-SY5Y cells were more resistant to hyperthermic stress compared to their undifferentiated state, correlating with levels of Hsp72. Stable exogenous expression of Hsp72 in SH-SY5Y cells (transfected line 5YHSP72.1, containing mildly elevated levels of Hsp72), led to enhanced resistance to hyperthermic stress. Hsp72 was found to be inducible in undifferentiated 5YHSP72.1 cells; such heat-treated cells displayed enhanced thermotolerance. Treatment of cells with KNK437, a suppressor of Hsp72 induction, resulted in acute thermosensitisation of all cell types tested here.
Conclusions: Hsp72 has a major role in the enhanced hyperthermic resistance acquired during neuronal differentiation of SH-SY5Y cells. These findings model the requirement in intact organisms for highly differentiated neurones to be specially protected against thermal stress.
Introduction
Neurones in the central nervous system are constantly challenged by many kinds of stresses such as hyperthermia, accumulation of abnormal proteins and toxic stress Citation[1]. Terminally differentiated neurones, which no longer divide, have a high priority for preservation in the central nervous system, unlike their neuronal progenitor cells. Disturbances to cellular defence mechanisms can lead to a variety of acute and chronic conditions. Hyperthermia resulting from fever or heat stroke can seriously disturb the central nervous system, leading to convulsion and coma. Hyperthermia is associated with the degradation and aggregation of intracellular proteins, which eventually leads to cytotoxicity Citation[2].
Heat shock proteins (HSPs) are molecular chaperones that act as a cellular defence system against cells exposed to elevated temperatures and other insults. They function by minimising the accumulation of misfolded or damaged proteins Citation[3], Citation[4]. Moreover, HSPs facilitate the correct folding of partially misfolded proteins to preserve cell viability. These molecular chaperones may play a significant role in some neurodegenerative diseases, which represent protein disaggregation disorders, such that misfolded proteins accumulate and aggregate to eventually form inclusions within the body of neurones Citation[5].
Hsp72 has been found in the areas of the brain under stress and may be expressed to protect neurones from challenging stresses such as hyperthermia and neurodegenerative diseases Citation[6–9]. Protection from hyperthermic stress has been observed to correlate with induction of Hsp72 in other tissues. For example, patients exposed to 39°C air temperature, but without experiencing symptoms of heat stroke, were found to have elevated blood serum Hsp72 levels. In contrast, symptomatic heat stroke patients were found to have low serum Hsp72 levels Citation[10].
When cultured mammalian cells, and also intact mice, are exposed to a pre-treatment of mild non-lethal hyperthermic stress (thermal preconditioning) they can acquire resistance to a subsequent more severe stress that would otherwise be lethal Citation[11–15]. Development of such thermotolerance is often accompanied by the induction of Hsp72 under mild hyperthermic stress Citation[16], Citation[17].
Thermally induced death of cells is sometimes manifested as apoptotic death Citation[18], Citation[19]. Recent studies have suggested that Hsp72 promotes the survival of cells through direct or indirect intervention with components of the apoptotic machinery Citation[20–22]. An apoptotic cell undergoes characteristic morphological changes as well as alterations of the nuclear chromatin including fragmentation of DNA. How Hsp72 is able to inhibit the onset of apoptosis and thus promote cellular survival is currently under debate Citation[23].
This study investigates the role of Hsp72 in the protection of neuronal cells from hyperthermic stress. Human neuroblastoma SH-SY5Y cells were used in this study, since they provide a well established model of neural differentiation Citation[24]. These cells can be propagated in an undifferentiated form and can be differentiated into neurone-like cells with the use of retinoic acid (RA) and brain derived neurotrophic factor (BDNF) Citation[24]. In the undifferentiated form, SH-SY5Y cells can easily be transfected with plasmid vectors (unlike primary neuronal cells from intact animals) and subsequently they can be differentiated into neurone-like cells that no longer proliferate.
These features enabled us to test the relationship between Hsp72 and the cellular response of neurone-like cells to hyperthermic stress, particularly to evaluate whether differentiation confers an altered response to thermal stress. We found that after differentiation SH-SY5Y cells were more resistant to severe hyperthermic stress than their undifferentiated counterparts. Moreover, upon differentiation, neurone-like SH-SY5Y cells expressed and induced higher levels of Hsp72. Undifferentiated SH-SY5Y cells stably expressing exogenous Hsp72 displayed a level of resistance to severe hyperthermic stress equivalent to that of neurone-like SH-SY5Y cells that were not transfected. Our results indicate that there is a strong relationship between the resistance to thermal stress in SH-SY5Y cells and the level of expression of Hsp72 within neuronal cells.
Materials and methods
Cell culture
Human neuroblastoma SH-SY5Y cells (ATCC catalogue number CRL-2266) were cultured in DMEM medium (Gibco, Carlsbad, CA) supplemented with 1 mM L-glutamate, 10 mM HEPES and 10% heat inactivated foetal calf serum (Parkville, VIC). All cells were cultured in a humidified incubator at 37°C with 5% CO2. Human neuroblastoma SH-SY5Y stable cell lines over-expressing Hsp72 (denoted 5YHSP72.1) were generated by transfecting pCI-neo Hsp72 Citation[21] using Lipofectamine Plus (Invitrogen, Carlsbad, CA) reagent. Stably transfectant clones were selected and maintained in medium containing G418 (500 µg/mL).
To differentiate SH-SY5Y and 5YHSP72.1 cells, they were seeded at a density of 7.5 × 103 cells/cm2 and treated with 10 µM of all-trans retinoic acid (Sigma-Aldrich, St Louis, MO) in DMEM. After 5 days, RA was removed and 5 ng/mL of BDNF (Sigma-Aldrich) was added to the cells prior to incubation in DMEM without serum for a further 2 days. This differentiation protocol, whereby neurone-like cells are sustained by BDNF while non-neuronal cells die in the absence of other serum factors resulted in 97% of the culture population being differentiated based on presence of neurites longer than twice the length of its cell body, the neuronal state confirmed by immunostaining with anti-MAP-2 antibodies (data not shown). In our hands, use of RA alone with SH-SY5Y cells enabled no more than 90% of cells to express these differentiation characteristics.
For heat shock treatments, cells were immersed in a water bath (Memmert, Schwabach, Germany) at the temperatures indicated below (±0.1°C) for 30 min and then allowed to recover at 37°C for the indicated times. Control cells were maintained at 37°C throughout.
Heat shock protein inhibitor, KNK437
KNK437 (N-formyl-3,4-methylenedioxy-benzylidene-γ-butyrolactam, EMD Biosciences, San Diego, CA) was dissolved in DMSO to a final concentration of 50 mM. KNK437 was added to cells at the concentrations indicated, but keeping the input of DMSO constant (equivalent to 0.1% of total volume) equivalent to the total volume and cultures returned to the incubator at 37°C for 1 h before hyperthermic stress or thermal preconditioning treatments.
Cell death assay
Nuclear morphology was analysed using propidium iodide and fluorescence microscopy. Cells were collected and resuspended in 200 µL of phosphate-buffered saline containing 10 mg/mL propidium iodide, 0.1% Triton X-100, and 0.1% sodium acetate. Cells were incubated at room temperature under darkened conditions for 10 min before being examined for nuclear morphology using an Olympus IX71 inverted fluorescence microscope. Cells considered undergoing cell death displayed either fragmented or condensed nuclei, readily distinguishable from the normal rounded diffusely stained nuclei. The mean percentage of cell death in each population, from three independent experiments, was determined.
Western immunoblotting and densitometry analysis
Cells were collected and resuspended in cell lysis buffer (1% Triton X-100, 4 mM EGTA, 15 mM MgSO4, 25 mM glycylglycine) and incubated on ice for 5 min. Cells were centrifuged at 10 000 g for 5 min and the cell debris discarded. Protein concentration in the cell lysates was determined using a protein assay kit (BCA protein assay, Pierce, Rockford, IL). Cell lysates containing equivalent amounts of protein were then separated on a 12% SDS-PAGE gel and resolved proteins transferred to PVDF membrane. This membrane was probed with antibodies specific for Hsp72 (SPA 810, Stressgen Biotechnologies, Ann Arbor, MI) and actin (Neomarkers, Fremont, CA). Secondary antibodies conjugated to Alexa 488 or Alexa 568 (Invitrogen) enabled visualisation of bands, and imaging of the bands on the PVDF membrane was carried out using a Typhoon Trio (GE Healthcare, Chalfont St Giles, UK), excitation with Argon laser at 488 nm and HeNe laser at 568 nm, as required. Band density values were analysed using the ImageQuant TL densitometry program (GE Healthcare). In some cases, as specified, bands were alternatively detected by use of secondary antibodies conjugated to horseradish peroxidise (GE Healthcare). Here, the PDVF membrane was then treated with enhanced chemiluminescence (ECL) substrate (Bio-Rad Laboratories, Hercules, CA) for 5 min. Detection was performed by exposing the PDVF membrane to hyperfilm (GE Healthcare), which was then scanned and band density values were analysed using ImageQuant as above.
Statistical tests
Comparison of Hsp72 levels in cell culture, and thermal killing outcomes at particular temperatures was carried out using student's t-test (P < 0.05 was considered significant). Results were obtained from at least three independent experiments on different batches of cells on different days.
Results
Neurone-like SH-SY5Y cells show increased resistance to severe hyperthermic stress relative to that of undifferentiated SH-SY5Y cells
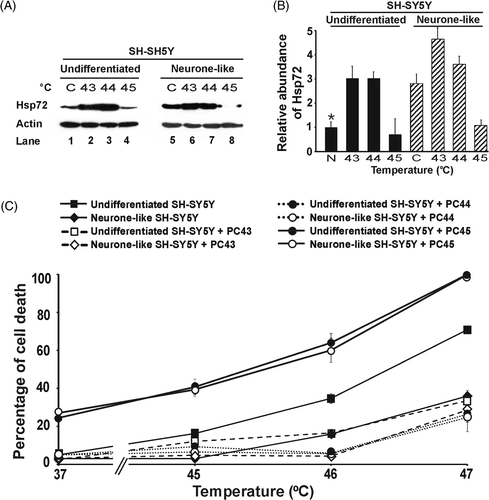
The resistance to hyperthermic stress at different temperatures was compared in undifferentiated and neurone-like SH-SY5Y cells, by measuring the extent of heat-induced cell death. Cells were exposed to different temperatures for 30 min and allowed to recover at 37°C for 18 h before harvesting. Cells were permeabilised, stained with propidium iodide and the percentage of cells displaying fragmented or condensed nuclei determined using fluorescence microscopy as described previously Citation[20] (). Neurone-like SH-SY5Y cells were found to be significantly more resistant to hyperthermic stress between 45°C and 47°C compared to their undifferentiated counterparts (P < 0.001 at 46°C). Thus, at 46°C, differentiated cells showed only half the extent of death of the undifferentiated cells, about 35% of which were killed.
Figure 1. Hyperthermic sensitivity in undifferentiated and neurone-like SH-SY5Y cells. Cells were exposed to the indicated temperatures for 30 min. After 18 h recovery at 37°C, the percentage of cells undergoing cell death was determined. Where indicated, cells were thermally preconditioned (PC) by heating at 43°C for 30 min followed by recovery at 37°C for 8 h before subsequent severe hyperthermic stress at the temperatures shown. All results are from three independent experiments. Error bars indicate standard deviation.
It has been shown in several other studies that with increasing heat stress cells switch from an apoptotic death (fragmented nuclei) to a necrotic death (condensed nuclei) Citation[25]. In this study it was observed that at the highest temperatures applied (47°C in and up to 48°C in later experiments below) the abundance of nuclei with condensed, rather than fragmented, morphology increased. This suggests necrosis may be occurring at these elevated temperatures in this study.
Applying the same procedure to five single-cell colonies isolated from the original population of SH-SY5Y cells, a similar differential in resistance to hyperthermic stress between neurone-like cells and their undifferentiated precursors was observed in each case (data not shown).
Neurone-like SH-SY5Y cells display elevated Hsp72 expression and induction levels relative to those of undifferentiated SH-SY5Y cells
To test for a possible relationship between Hsp72 levels and the hyperthermic response of SH-SY5Y cells in undifferentiated and neurone-like states, the expression of Hsp72 in SH-SY5Y cells before and after differentiation was examined. Differentiation was accompanied by elevated levels of Hsp72 (3.6- fold, P < 0.001) (, Lane 3, and ). To test for induction of Hsp72, a mild hyperthermic stress of 43°C for 30 min was applied, followed by 8 h recovery at 37°C. Under these conditions, in which no loss of cell viability was observed (data not shown), SH-SY5Y cells displayed up-regulated Hsp72 levels (, Lane 1). The expression of Hsp72 was increased 4-fold in the undifferentiated cells and 10-fold in the differentiated cells (P < 0.001, ). Thus, neurone-like SH-SY5Y cells express higher basal levels of Hsp72 and have greater capacity to up-regulate Hsp72 upon mild hyperthermic stress, compared to their undifferentiated counterparts. The basal levels of Hsp72 before and after differentiation of SH-SY5Y cells correlated well with the acquisition of thermotolerance following differentiation ().
Figure 2. Hsp72 expression levels of SH-SY5Y and 5YHSP72.1 cells. (A) Undifferentiated and neurone-like SH-SY5Y cells were heated at 43°C for 30 min and allowed to recover for 8 h at 37°C before harvesting. Cell lysates were obtained from before and after differentiation with RA and BDNF, with or without mild hyperthermic stress at 43°C for 30 min (PC). Lysates were subjected to western immunoblotting with anti-Hsp72 and actin antibodies. In this case secondary antibodies conjugated to Alexa fluorochromes were used. (B) Levels of Hsp72 induction were quantified by densitometric analysis of images in . (C) Undifferentiated and neurone-like 5YHSP72.1 cells were heated at 43°C for 30 min and allowed to recover for 8 h at 37°C. Lysates were subjected to western immunoblotting as in . These were run on the same gel as in panel A, hence the continuity in lane numbering. (D) Levels of Hsp72 induction were quantified by densitometric analysis of images in . *Fold induction values from all densitometric analyses here were normalised to the levels of Hsp72 in undifferentiated SH-SY5Y cells (, Lane 1). All results are from three independent experiments. Error bars indicate standard deviation.
Thermal preconditioning of SH-SY5Y cells protects against severe hyperthermic stress, with greater protection manifested by neurone-like SH-SY5Y cells
In light of the inducibility of Hsp72 before and after differentiation, we determined whether the mild hyperthermic treatment effected reduced susceptibility to a subsequent more severe hyperthermic stress. Indeed, we found that such prior thermal preconditioning protects undifferentiated SH-SY5Y and neurone-like SH-SY5Y cells from a subsequent severe hyperthermic stress at 46°C and 47°C compared to cells not exposed to thermal preconditioning (, broken lines, P < 0.001, for both the respective comparisons involving undifferentiated or neurone-like cells, at 46°C). Thus, undifferentiated preconditioned cells show about half the extent of cell death (about 16%) compared to untreated cells (about 36%). The preconditioned neurone-like cells show negligible death at 46°C, in contrast to the killing of untreated neurone-like cells (about 16%). Therefore, thermal preconditioning is associated with greater hyperthermic resistance in both differentiated and non-differentiated cells. The level of hyperthermic resistance correlates with expression of Hsp72, consistent with the idea that Hsp72 is involved in the resistance to hyperthermic stress. Further, Hsp72 is a candidate for being a key factor in the thermal preconditioning that leads to thermotolerance of SH-SY5Y cells.
SH-SY5Y cells over-expressing Hsp72 show enhanced resistance to hyperthermic stress
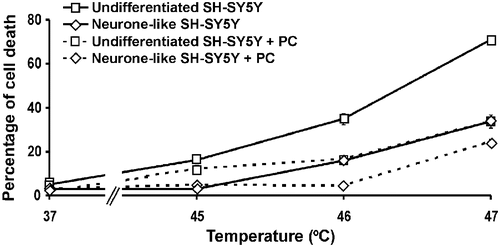
To test directly the proposition that Hsp72 is involved in the protection against severe hyperthermic stress, SH-SY5Y cells over-expressing Hsp72 (5YHSP72.1) were generated. Whereas some stably transfected cells displayed grossly elevated levels of Hsp72 (data not shown), 5YHSP72.1 cells were found to express enhanced levels of Hsp72 (, Lane 5) comparable to the Hsp72 level seen in non-transfected undifferentiated SH-SY5Y cells following induction of thermotolerance (, Lane 2). These mildly elevated levels are thus at the upper end of the physiological range. Hsp72 levels were further increased in 5YHSP72.1 cells following induction of thermotolerance by exposure to 43°C (, Lane 6). 5YHSP72.1 cells could be differentiated into neurone-like 5YHSP72.1 cells using the RA/BDNF procedure at the same efficiency as the non-transfected cells (data not shown). However, the differentiated 5YHSP72.1 cells showed no further increase in Hsp72 (, Lane 7) compared to their undifferentiated precursors (, Lane 5). Mild hyperthermic treatment of the neurone-like transfected cells failed to induce Hsp72 to a significant extent (, Lane 8).
Undifferentiated and neurone-like 5YHSP72.1 cells were heat stressed at different temperatures for 30 min, without thermal preconditioning (). Undifferentiated 5YHSP72.1 cells were significantly more resistant to severe hyperthermic stress than undifferentiated SH-SY5Y cells (P < 0.001 at 46°C). Undifferentiated 5YHSP72.1 cells and neurone-like SH-SY5Y cells which express similar levels of Hsp72 () showed equivalent tolerance to hyperthermic stress (). Significantly, neurone-like 5YHSP72.1 cells were more susceptible to hyperthermic stress (about 25% cell death at 46°C) compared to both undifferentiated 5YHSP72.1 cells (about 5% cell death at 46°C, P < 0.001) and neurone-like SH-SY5Y cells (about 12% cell death at 46°C, P < 0.001). This increase in thermal sensitivity correlated with the lack of thermo-inducibility of Hsp72 in neurone-like 5YHSP72.1 cells ( and ), whereby Hsp72 levels do not rise after mild hyperthermic treatment unlike the situation with differentiated non-transfected cells ( and ). However, such neurone-like 5YHSP72.1 cells were still more resistant to severe hyperthermic stress than undifferentiated SH-SY5Y cells. This is probably due to the elevated levels of Hsp72 in the transfected neurone-like cells compared to the very low levels in undifferentiated non-transfected SH-SY5Y cells.
Figure 3. Hyperthermic resistance of undifferentiated but not neurone-like 5YHSP72.1 cells. (A) Cells were exposed to the indicated temperatures for 30 min and after 18 h recovery at 37°C, the percentage of cells undergoing cell death was determined. Results from untransformed SH-SY5Y cells are included for comparison. (B) Cells were thermally preconditioned (PC) at 43°C for 20 min and allowed to recover for 8 h at 37°C before subsequent heating at the temperatures shown. Results for non-preconditioned 5YHSP72.1 are included for comparison. All results are from three independent experiments. Error bars indicate standard deviation.
Finally, we tested 5YHSP72.1 cells for levels of expression of other HSP proteins other than Hsp72 relative to non-transfected cells. In pair-wise comparisons of undifferentiated SH-SY5Y and 5YHSP72.1 cells, level of expression of Hsp90, Hsp73 (constitutive), Hsp60 or Hsp27 were very similar in both cell types (data not shown). Hence, the 5YHSP72.1 cell line over-expresses Hsp72 but has relatively small changes in other HSP members.
Features of thermal preconditioning of undifferentiated and neurone-like 5YHSP72.1 cells correlate with cellular levels of Hsp72
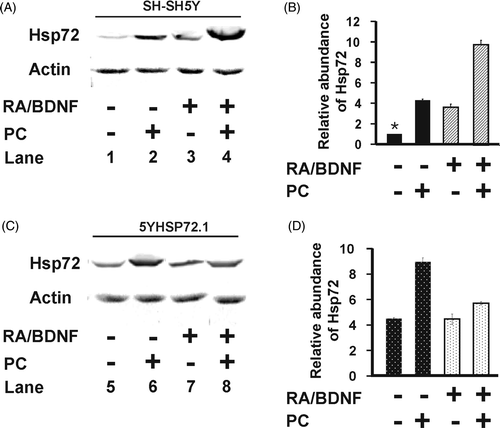
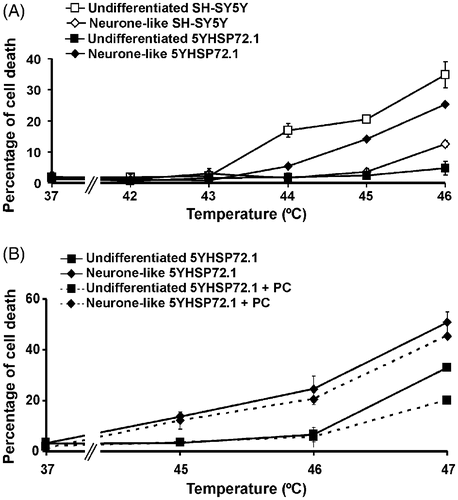
Since undifferentiated 5YHSP72.1 cells were able to express increased levels of Hsp72 following mild hyperthermic treatment, we predicted that thermal preconditioning of undifferentiated 5YHSP72.1 cells should increase hyperthermic resistance. On the other hand, in light of the lack of further elevation of Hsp72 levels in neurone-like 5YHSP72.1 cells and their poor ability to increase Hsp72 following mild hyperthermic stress, we anticipated a much weaker thermoprotective effect of the preconditioning dose. Accordingly, we investigated the degree of protection against severe hyperthermic stress following thermal preconditioning of undifferentiated and neurone-like 5YHSP72.1 cells. As predicted, induction of thermotolerance in undifferentiated 5YHSP72.1 cells increased their hyperthermic resistance at 47°C (P < 0.001, ). A small hyperthermic protection was found in neurone-like 5YHSP72.1 following induction of thermotolerance, but the difference was not significant (P > 0.05, at 47°C). Overall, thermal preconditioning achieved a greater protective effect in undifferentiated 5YHSP72.1 cells compared to neurone-like 5YHSP72.1 cells, as predicted on the basis of their relative Hsp72 levels.
In order to check in more detail this apparent inability to further increase Hsp72 synthesis in the differentiated 5YHSP72.1 cells on thermal stimulation, we examined Hsp72 levels after 30 min exposure of these cells to 44°C and 45°C, higher than the 43°C used above. The results indicated clearly that at 44°C, while there is further induction of Hsp72 synthesis in undifferentiated cells, in neurone-like 5YHSP72.1 cells the Hsp72 levels were greatly reduced ( and ). This is indicative of extensive degradation of Hsp72, rather than its induction. Interestingly, the undifferentiated 5YHSP72.1 cells did show loss of Hsp72 after treatment at 45°C. Such degradation of Hsp72 at 45°C (but not 44°C) also occurred in untransformed SH-SY5Y cells (Supplementary ).
Figure 4. Correlation of Hsp72 expression levels and thermotolerance in both undifferentiated and neurone-like 5YHSP72.1 cells. (A) Undifferentiated and neurone-like 5YHSP72.1 cells were heated at the temperatures (°C) indicated for 30 min and allowed to recover for 8 h at 37°C before harvesting. Control cells (denoted C) were maintained at 37°C throughout. Lysates were subjected to western immunoblotting with anti-Hsp72 and actin antibodies. In this case secondary antibodies conjugated to horseradish peroxidise were used. (B) Levels of Hsp72 induction were quantified by densitometric analysis of the image in . *Fold induction values were normalised to the levels of Hsp72 in undifferentiated SH-SY5Y cells (denoted N) (see Supplementary ). Note that the values for fold induction are relatively smaller here than those shown for corresponding cells in because of systematic differences in the quantification of bands using ECL techniques as opposed to fluorometric techniques. (C) Cells were exposed to the indicated temperatures for 30 min and after 18 h recovery at 37°C, the percentage of cells undergoing cell death was determined. Where indicated, cells were thermally preconditioned (PC) at 43°C (PC43), 44°C (PC44) or 45°C (PC45) for 30 min before subsequent heating at the temperatures shown. All results are from three independent experiments. Error bars indicate standard deviation.
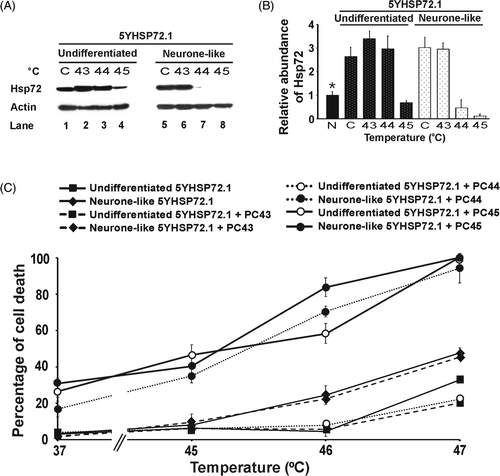
The biological correlates of these 30 min pretreatment conditions at different temperatures are shown in the quantified death indices of cells subsequently exposed for 30 min to elevated temperatures up to 47°C (). Significantly, when 5YHSP72.1 cells expressed increased Hsp72 levels following the pretreatment, they showed enhanced survival at higher temperatures. On the other hand, those with greatly reduced levels of Hsp72 showed exacerbated thermosensitivity, such that at secondary treatment at 47°C close to 100% death of 5YHSP72.1 cells was observed. Specifically, neurone-like 5YHSP72.1 cells preconditioned at 44°C showed enhanced cell death on secondary treatment at elevated temperatures, consistent with their grossly reduced Hsp72 levels (unlike the other cells tested which were more robust after preconditioning at 44°C). Corresponding data for untransformed SH-SY5Y cells (Supplementary ) shows similar relationships between Hsp72 levels and thermoresistance, except that the untransformed cells do not degrade Hsp72 until a threshold of 45°C. These findings, taken together, explain why the transformed 5YHSP72.1 cells after differentiation are (unexpectedly) less thermotolerant than most other cells studied here.
KNK437 inhibits the induction of Hsp72 by mild hyperthermic stress in undifferentiated and neurone-like SH-SY5Y cells
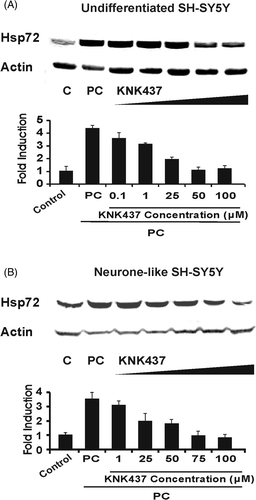
KNK437 is a benzylidene lactam compound that inhibits the induction of heat shock proteins including Hsp72 and abrogates the acquisition of thermotolerance after mild hyperthermic stress in various human cancer cell lines Citation[26], Citation[27]. Thus KNK437 potentially provides a pharmacological means of suppressing Hsp72 induction in SH-SY5Y cells (notwithstanding its lack of specificity for this particular heat shock protein). Therefore, we investigated whether KNK437 inhibits the heat-induced up-regulation of Hsp72 in SH-SY5Y and 5YHSP72.1 cells (both undifferentiated and neurone-like). With increasing KNK437 concentrations, there was a progressively greater inhibition of Hsp72 induction in SH-SY5Y cells. At 50 µM, KNK437 completely inhibited the up-regulation of Hsp72 in undifferentiated SH-SY5Y cells (). In neurone-like SH-SY5Y cells, a higher concentration of KNK437 (75 µM) was required to inhibit the up-regulation of Hsp72 (). The same KNK437 concentrations were found for complete suppression of Hsp72 induction in 5YHSP72.1 cells, namely 50 µM and 75 µM for undifferentiated and neurone-like cells, respectively (data not shown).
Figure 5. Inhibition of heat-induced expression of Hsp72 by KNK437. Cells were treated with various concentrations of KNK437 1 h before heating at 43°C for 30 min followed by 8 h recovery at 37°C (thermal preconditioning, PC) before harvesting. Control cells (denoted C) were maintained at 37°C throughout. Lysates were subjected to western analysis with anti-Hsp72 and actin antibodies. Levels of induction of Hsp72 were quantified by densitometric analysis of images. (A) Undifferentiated SH-SY5Y cells. (B) Neurone-like SH-SY5Y cells. In both cases, fold induction was normalised to the level of Hsp72 in the respective control. Note that for all concentrations of KNK437 applied, the input of DMSO was held constant; vehicle DMSO alone at 0.1% as used here had no measurable effect on Hsp72 levels. All results are from three independent experiments. Error bars indicate standard deviation.
KNK437 sensitises SH-SY5Y and 5YHSP72.1 cells to hyperthermic stress and blocks thermotolerance
Having shown that KNK437 inhibits Hsp72 up-regulation in SH-SY5Y cells, we examined the response to hyperthermia of SH-SY5Y and 5YHSP72.1 cells in the presence of 50 µM and 75 µM KNK437, respectively. KNK437 itself did not cause significant cell death 37°C (); however, it sensitised SH-SY5Y cells to hyperthermic stress (). In the presence of KNK437, neurone-like cells were more heat sensitive than undifferentiated precursors. Thus at 44°C there is about 80% cell death for neurone-like cells compared to approximately 20% for undifferentiated cells, while at 46°C, cells in both states show more than 80% killing in the presence of KNK437 (). As with SH-SY5Y cells, KNK437 markedly increased the sensitivity of 5YHSP72.1 cells to hyperthermic stress ().
Figure 6. KNK437 sensitises SH-SY5Y and 5YHSP72.1 cells to thermal stress. Cells (either undifferentiated or neurone-like) were treated with or without KNK437 1 h before being heated at different temperatures for 30 min. Concentrations of KNK437 were 50 µM and 75 µM for undifferentiated and neurone-like cells, respectively. After the hyperthermic stress, cells were allowed to recover at 37°C for 18 h before harvesting and the percentage of cells displaying apoptotic nuclei was determined. Where indicated (PC), cells were treated with or without KNK437 1 h before being thermally preconditioned at 43°C for 30 min. After such thermal preconditioning (PC), cells were allowed to recover at 37°C for 8 h and then a secondary insult of a severe heat stress at the indicated temperature was carried out for 30 min. After the secondary heat stress, cells were allowed to recover at 37°C for 18 h before harvesting. (A) SH-SY5Y cells with and without KNK437. (B) 5YHSP72.1 cells with and without KNK437. (C) Thermally preconditioned SH-SY5Y cells with and without KNK437. (D) Thermally preconditioned 5YHSP72.1 cells with and without KNK437. Control cells treated with equivalent concentrations of KNK437 (without heat) or the vehicle DMSO alone, did not show measurable changes in cell viability relative to controls. All results are from three independent experiments. Error bars indicate standard deviation. Note the different temperature range adopted for the studies reported in each of the two rows.
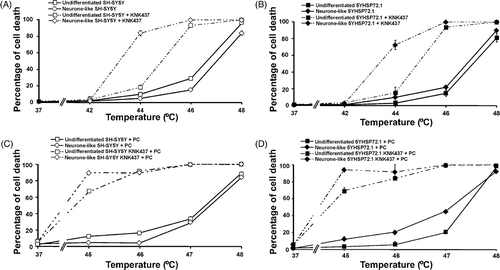
Following exposure of cells to mild hyperthermic stress (thermal preconditioning), KNK437 abrogates the acquisition of thermotolerance in SH-SY5Y () and in 5YHSP72.1 cells () irrespective of their state of differentiation. Thus, in spite of elevated levels of Hsp72 in 5YHSP72.1 cells, the presence of KNK437 generated a similar extent of hyperthermic sensitivity to that shown by non-transfected cells, regardless of the state of differentiation. Overall, KNK437 increases basal hyperthermic sensitivity of the SH-SY5Y cells as well as inhibiting thermotolerance. Nonetheless, the results are consistent with a role for Hsp72 in the hyperthermic resistance of SH-SY5Y cells, as KNK437 clearly abrogates the enhanced thermotolerance that is acquired either by differentiation into neurone-like cells or by expression of exogenous Hsp72.
Discussion
Neurones are vulnerable to stresses that can lead to a decline in viability. For terminally differentiated neurones, the factors that ensure the long term survival of these irreplaceable cells are critical for normal brain function. Increases in core body temperature (hyperthermia) can occur in a number of conditions such as heatstroke, leading to dysfunction of the central nervous system. Consequences include intracranial hypertension, cerebral hypoperfusion and cerebral ischaemia Citation[2], Citation[28]. HSPs are stimulated by heat, and their expression, in particular that of Hsp72, has been correlated with the acquisition of thermotolerance Citation[16], Citation[17]. Elevated levels of Hsp72 may lead to better patient outcome during heatstroke by attenuation of central nervous system damage. Therefore, it is important to understand the contribution of the stress chaperone Hsp72 to neuroprotection.
The degree of resistance of neuroblastoma cells to hyperthermic stress correlates with levels of Hsp72
The protective effects of thermal preconditioning have been well documented in many cell types since the first observation three decades ago Citation[15], Citation[29–31]. In general, the degree of thermotolerance manifested by a given cell type is a function of both the basal level of heat shock proteins and the extent to which levels of such proteins rise during exposure to heat. Our data show that the degree of resistance to hyperthermic stress correlates with Hsp72 levels in SH-SY5Y cells, both before and after differentiation. The increase in basal levels of Hsp72 following differentiation of SH-SY5Y into neurone-like cells correlates with their enhanced ability to survive after severe hyperthermic stress. The induction of thermotolerance in undifferentiated SH-SY5Y cells leads to an equivalent extent of resistance to severe hyperthermic stress as shown by the neurone-like SH-SY5Y cells. Thermotolerant SH-SY5Y cells express similar levels of Hsp72 to those in the neurone-like cells. Following induction of thermotolerance, neurone-like SH-SY5Y cells express even more Hsp72 and show greater thermal resistance.
The increased expression and induction of Hsp72 that we observed in neurone-like SH-SY5Y cells is contrary to some published reports. Other studies using different cells types and differentiation agents have shown differentiated cells to be less efficient inducers of Hsp72 following mild hyperthermic stress, compared to their precursor cells Citation[32–36]. In studies using human cell lines, human retinal pigment ARPE-19 and D-407 neuroepithelial cells, a time-dependent increase of basal Hsp70 expression was seen after differentiation with fenretinide Citation[37], Citation[38]. ARPE-19 and D-407 cells also showed neuronal-like characteristics upon differentiation with fenretinide, although the authors did not test the induction of Hsp72 upon hyperthermic stress. In another study, a decrease of Hsp72 induction in differentiated SH-SY5Y cells, compared to undifferentiated SH-SY5Y cells, was observed upon treatment with the drug celastrol that induces a range of HSPs Citation[36]. However, such Hsp72 decrement was found to be due to higher concentrations of the drug, which resulted in cell death. It is not possible to generalise the reasons for the varying basal expression and extents of inducibility of Hsp72 in various neurone-like cells, which encompass different cell lines and species, as well as the use of a range of differentiating agents.
Thermal preconditioning up-regulates other stress proteins such as Hsp27, Hsp90 and Hsp104 Citation[15], Citation[39]. To address the possibility that these other HSPs are responsible for the protective effect against severe hyperthermic stress, we over-expressed Hsp72 in the SH-SY5Y cells, generating the stably transfected cell line 5YHSP72.1. In this transfected cell line, no evidence for up-regulation of HSPs other than Hsp72 was obtained. We show clearly that in undifferentiated cells, expression of exogenous Hsp72 confers a significant degree of protection from severe hyperthermic stress, to a level similar to that seen in the neurone-like SH-SY5Y cells. Once again, the extent of protection correlates with the level of Hsp72. We attribute enhanced thermotolerance to severe hyperthermic stress in neurone-like SH-SY5Y and undifferentiated 5YHSP72.1 cells to the common feature that they both have increased basal levels of Hsp72. Further, since each was shown to exhibit a strong up-regulation of Hsp72 upon mild hyperthermic stress, we infer that this is an important factor in enabling the cells to achieve resistance to even higher temperatures. Thermal preconditioning of 43°C and 44°C provided greater resistance to severe hyperthermic stress in undifferentiated 5YHSP72.1 cells compared to neurone-like 5YHSP72.1 cells.
It is interesting to note that 5YHSP72.1 cells neither increase Hsp72 levels after their differentiation nor do they express more endogenous Hsp72 after preconditioning at 43°C (). Furthermore, not only are neurone-like 5YHSP72.1 cells unable to switch on induction of Hsp72 at other preconditioning temperatures (44°C and 45°C), but they seem to actively degrade their Hsp72 content. This loss of Hsp72 from the neurone-like 5YHSP72.1 cells is reflected not only in failure of these cells to show enhanced thermotolerance after standard preconditioning, but in their manifestation of enhanced killing when pretreated at mildly elevated temperatures above 43°C. In addition, this indicates that differentiation-dependent factors in neurone-like SH-SY5Y cells are not involved in their increased protection against severe hyperthermic stress; rather it is the Hsp72 levels that determine thermotolerance.
Evidently, the neurone-like 5YHSP72.1 cells are more thermosensitive than most other cells studied here, contrary to expectation. We showed this is due to at least two factors, lack of thermal induction of Hsp72, and active degradation of this protein. The basis is not yet clear for the impaired thermal induction of Hsp72 in the stably transformed 5YHSP72.1 cells after differentiation to the neurone-like state. Neither is it immediately obvious why Hsp72 is degraded at elevated temperatures in these cells (compared to undifferentiated 5YHSP72.1 cells or untransformed SH-SY5Y cells). Whether these are separate or related phenomena in relation to the molecular biology of Hsp72 remains to be clarified.
The effects of KNK437 on thermotolerance in neuronal cells
KNK437 is reported to inhibit thermotolerance by blocking the up-regulation of Hsp72 Citation[26], Citation[27], Citation[40]. We investigated whether KNK437 would abrogate the thermotolerance observed in SH-SY5Y and 5YHSP72.1 cells. KNK437 inhibited the up-regulation of Hsp72 and increased the hyperthermic sensitivity of SH-SY5Y cells. Further, the neurone-like SH-SY5Y cells, which display resistance to hyperthermic stress, became highly sensitive to such stress in the presence of KNK437. This is consistent with the proposal that Hsp72 is involved in the increased resistance seen in neurone-like SH-SY5Y cells and that the protection against thermal stress can be lost if Hsp72 is down-regulated.
Interestingly, the treatment of KNK437 in thermally preconditioned SH-SY5Y or 5YHSP72.1 cells subjected to a secondary insult of hyperthermic stress resulted in an enhanced extent of killing, compared to that in cells not thermally preconditioned. In general, KNK437 caused an excessive amount of cell death irrespective of whether Hsp72 was explicitly up-regulated (up-regulation conditions tested here include: differentiation, thermal preconditioning or vector-driven over-expression of Hsp72). In this context, it seems that the ability of cells to synthesise heat shock protein after thermal stress may be essential for resistance. KNK437 prevents acquisition of hyperthermic resistance in cells exposed to a preconditioning heat treatment, but KNK437 treatment also leads to loss of hyperthermic resistance in cells already with high levels of Hsp72.
Another aspect is that KNK437 may be inherently toxic to cells and this toxicity is enhanced in cells exposed to hyperthermia. Thus, KNK437 may have additional targets, other than Hsp72, which are involved in conferring thermotolerance (for example, other HSP molecules). This drug has been reported to also inhibit expression of Hsp105 and Hsp40 Citation[27]. In spite of these possible off-target effects, the data we obtained using KNK437 are consistent with Hsp72 being an important factor in neuronal hyperthermic resistance. In this light, data using KNK437 should be interpreted cautiously, as we have done here.
Conclusions
Hsp72 has been shown here in a cellular model to be a key factor in the acquisition of thermal resistance of neural cells following differentiation. In a broader biological context, it is attractive to consider the notion that during neuronal differentiation in the central nervous system, additional survival mechanisms are acquired to ensure the long-term survival of these irreplaceable cells. It is conceivable that neuronal precursor cells have less Hsp72 but retain proliferative capacity and hence are ‘disposable’. On the other hand, fully differentiated neurones need greater protection from prevailing stresses.
Acknowledgements
We thank Rohan Steel for useful discussions.
Declaration of interest: This study was supported by grants from the National Health and Medical Research Council of Australia (NHMRC, Program Grant on Brain Injury and Repair) and the Australian Research Council (ARC Centre of Excellence) to Phillip Nagley, and the National Institute of Health/National Cancer Institute (CA81421) to Robin L. Anderson. Lesley Cheng was supported by a PhD training scholarship from Neurosciences Victoria.
References
- Ohtsuka K, Suzuki T. Roles of molecular chaperones in the nervous system. Brain Res Bull 2000; 53: 141–146
- Yan YE, Zhao YQ, Wang H, Fan M. Pathophysiological factors underlying heatstroke. Med Hypotheses 2006; 67: 609–617
- Feldman DE, Frydman J. Protein folding in vivo: The importance of molecular chaperones. Curr Opin Struct Biol 2000; 10: 26–33
- Frydman J, Nimmesgern E, Ohtsuka K, Hartl FU. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature 1994; 370: 111–117
- Muchowski PJ, Wacker JL. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci 2005; 6: 11–22
- Chen S, Brown IR. Neuronal expression of constitutive heat shock proteins: Implications for neurodegenerative diseases. Cell Stress Chaperones 2007; 12: 51–58
- Nishimura RN, Dwyer BE. Evidence for different mechanisms of induction of Hsp70i: A comparison of cultured rat cortical neurons with astrocytes. Brain Res Mol Brain Res 1996; 36: 227–239
- Satoh J, Kim SU. Hsp72 induction by heat stress in human neurons and glial cells in culture. Brain Res 1994; 653: 243–250
- Tytell M, Barbe MF, Brown IR. Stress (heat shock) protein accumulation in the central nervous system. Its relationship to cell stress and damage. Adv Neurol 1993; 59: 293–303
- Wang ZZ, Wang CL, Wu TC, Pan HN, Wang SK, Jiang JD. Autoantibody response to heat shock protein 70 in patients with heatstroke. Am J Med 2001; 111: 654–657
- Subjeck JR, Sciandra JJ, Chao CF, Johnson RJ. Heat shock proteins and biological response to hyperthermia. Br J Cancer Suppl 1982; 5: 127–131
- Subjeck JR, Sciandra JJ, Johnson RJ. Heat shock proteins and thermotolerance; a comparison of induction kinetics. Br J Radiol 1982; 55: 579–584
- Li GC, Meyer JL, Mak JY, Hahn GM. Heat-induced protection of mice against thermal death. Cancer Res 1983; 43: 5758–5760
- Amin V, Cumming DV, Coffin RS, Latchman DS. The degree of protection provided to neuronal cells by a pre-conditioning stress correlates with the amount of heat shock protein 70 it induces and not with the similarity of the subsequent stress. Neurosci Lett 1995; 200: 85–88
- Bettaieb A, Averill-Bates DA. Thermotolerance induced at a mild temperature of 40°C protects cells against heat shock-induced apoptosis. J Cell Physiol 2005; 205: 47–57
- Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci USA 1982; 79: 3218–3222
- Mizzen LA, Welch WJ. Characterization of the thermotolerant cell. I. Effects on protein synthesis activity and the regulation of heat-shock protein 70 expression. J Cell Biol 1988; 106: 1105–1116
- Mosser DD, Martin LH. Induced thermotolerance to apoptosis in a human T lymphocyte cell line. J Cell Physiol 1992; 151: 561–570
- Poe BS, O'Neill KL. Inhibition of protein synthesis sensitizes thermotolerant cells to heat shock induced apoptosis. Apoptosis 1997; 2: 510–517
- Buzzard KA, Giaccia AJ, Killender M, Anderson RL. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J Biol Chem 1998; 273: 17147–17153
- Clemons NJ, Buzzard K, Steel R, Anderson RL. Hsp72 inhibits Fas-mediated apoptosis upstream of the mitochondria in type II cells. J Biol Chem 2005; 280: 9005–9012
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2000; 2: 469–475
- Steel R, Doherty JP, Buzzard K, Clemons N, Hawkins CJ, Anderson RL. Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1. J Biol Chem 2004; 279: 51490–51499
- Encinas M, Iglesias M, Liu Y, Wang H, Muhaisen A, Cena V, Gallego C, Comella JX. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem 2000; 75: 991–1003
- Harmon BV, Corder AM, Collins RJ, Gobé GC, Allen J, Allan DJ, Kerr JF. Cell death induced in a murine mastocytoma by 42–47°C heating in vitro: Evidence that the form of death changes from apoptosis to necrosis above a critical heat load. Int J Radiat Biol 1990; 58: 845–858
- Yokota S, Kitahara M, Nagata K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res 2000; 60: 2942–2948
- Ohnishi K, Takahashi A, Yokota S, Ohnishi T. Effects of a heat shock protein inhibitor KNK437 on heat sensitivity and heat tolerance in human squamous cell carcinoma cell lines differing in p53 status. Int J Radiat Biol 2004; 80: 607–614
- Sucholeiki R. Heatstroke. Semin Neurol 2005; 25: 307–314
- Spiro IJ, Sapareto SA, Raaphorst GP, Dewey WC. The effect of chronic and acute heat conditioning on the development of thermal tolerance. Int J Radiat Oncol Biol Phys 1982; 8: 53–58
- Kampinga HH. Thermotolerance in mammalian cells. Protein denaturation and aggregation, and stress proteins. J Cell Sci 1993; 104: 11–17
- Gerner EW, Schneider MJ. Induced thermal resistance in HeLa cells. Nature 1975; 256: 500–502
- Dwyer DS, Liu Y, Miao S, Bradley RJ. Neuronal differentiation in PC12 cells is accompanied by diminished inducibility of Hsp70 and Hsp60 in response to heat and ethanol. Neurochem Res 1996; 21: 659–666
- Hatayama T, Takahashi H, Yamagishi N. Reduced induction of HSP70 in PC12 cells during neuronal differentiation. J Biochem 1997; 122: 904–910
- Yang J, Oza J, Bridges K, Chen KY, Liu AY. Neural differentiation and the attenuated heat shock response. Brain Res 2008; 1203: 39–50
- Oza J, Yang J, Chen KY, Liu AY. Changes in the regulation of heat shock gene expression in neuronal cell differentiation. Cell Stress Chaperones 2008; 13: 73–84
- Chow AM, Brown IR. Induction of heat shock proteins in differentiated human and rodent neurons by celastrol. Cell Stress Chaperones 2007; 12: 237–244
- Chen S, Fariss RN, Kutty RK, Nelson R, Wiggert B. Fenretinide-induced neuronal differentiation of ARPE-19 human retinal pigment epithelial cells is associated with the differential expression of Hsp70, 14-3-3, pax-6, tubulin beta-III, NSE, and bag-1 proteins. Mol Vis 2006; 12: 1355–1363
- Chen S, Samuel W, Fariss RN, Duncan T, Kutty RK, Wiggert B. Differentiation of human retinal pigment epithelial cells into neuronal phenotype by N-(4-hydroxyphenyl)retinamide. J Neurochem 2003; 84: 972–981
- Parsell DA, Taulien J, Lindquist S. The role of heat-shock proteins in thermotolerance. Philos Trans R Soc Lond B Biol Sci 1993; 339: 279–285; Discussion 285-276
- Koishi M, Yokota S, Mae T, Nishimura Y, Kanamori S, Horii N, Shibuya K, Sasai K, Hiraoka M. The effects of KNK437, a novel inhibitor of heat shock protein synthesis, on the acquisition of thermotolerance in a murine transplantable tumor in vivo. Clin Cancer Res 2001; 7: 215–219
Supplementary Figure S1. Correlation of Hsp72 expression levels and thermotolerance of SH-SY5Y cells upon various pretreatment conditions. (A) Undifferentiated and neurone-like SH-SY5Y cells were heated at the temperatures (°C) indicated for 30 min and allowed to recover for 8 h at 37°C before harvesting. Control cells (denoted C) were maintained at 37°C throughout. Lysates were subjected to western immunoblotting with anti-Hsp72 and actin antibodies. In this case secondary antibodies conjugated to horseradish peroxidise were used. (B) Levels of Hsp72 induction were quantified by densitometric analysis of the image in . *Fold induction values were normalised to the levels of Hsp72 in undifferentiated SH-SY5Y cells (denoted N); this reference value was likewise used for normalisation of data in (N). Note that the values for fold induction are relatively smaller here than those shown for corresponding cells in because of systematic differences in the quantification of bands using ECL techniques as opposed to fluorometric techniques. (C) Cells were exposed to the indicated temperatures for 30 min and after 18 h recovery at 37°C, the percentage of cells undergoing cell death was determined. Where indicated, cells were thermally preconditioned (PC) at 43°C (PC43), 44°C (PC44) or 45°C (PC45) for 30 min before subsequent heating at the temperatures shown. All results are from three independent experiments. Error bars indicate standard deviation.