Abstract
Purpose: To report complications encountered after microwave ablation (MWA) of liver metastatic cholangiocarcinoma who had history of pancreatoduodenectomy or liver resection with bilioenteric anastomosis.
Materials and methods: Retrospective study of eight intrahepatic metastatic cholangiocarcinoma lesions treated with MWA (range from 1.5 to 3.6 cm, mean, 3.2 ± 1.1 cm) in five male patients from February 2008 to August 2009. All five patients underwent surgical resection of the primary neoplasm before MWA and bilioenteric anastomosis, four of them had intrahepatic biliary dilatation pneumatosis.
Results: All lesions were completely ablated according to results of contrast-enhanced ultrasound (CEUS) or contrast-enhanced CT/MRI 1 month after MWA. Liver abscess occurred in five ablation zones (5/8 62.5%) of four patients. Fistula encountered in all of the four cases including liver-pleural cavity fistula in two cases, liver-subcutaneous fistula in one case, both liver-pleural cavity and liver-subcutaneous fistula in one case. Two patients had right empyema, and one patient combined with melaena. Tumour cells were isolated from pus in one case. Three cases were satisfactorily cured with antibiotic administration, catheter drainage and supportive treatment; one case died 13 days after MWA.
Conclusion: There is a high incidence of abscess formation due to multiple risk factors when MWA was used for treatment of intrahepatic metastatic cholangiocarcinoma with bilioenteric anastomosis. Understanding the causes and grasping disposal methods will help to avoid or successfully cure this major complication.
Introduction
Image-guided microwave ablation (MWA) and radiofrequency ablation (RFA) as minimally invasive techniques have been widely used for the treatment of primary and metastatic liver cancer in the past decades Citation[1–3]. Abscess formation as a complication of MWA and RFA of intrahepatic malignancy occurred in approximately 0.3–1% according to different reports Citation[1–3]. These studies also highlighted that the two risk factors leading to septic complication were the presence of diabetes mellitus and/or the presence of air in the biliary tract. For different types of liver tumour, the incidence of liver abscess as a complication after intra-arterial chemotherapy to interhepatic cholangiocarcinoma (ICC) was obviously higher than that of hepatocellular carcinoma (HCC) and metastatic liver cancer (25%:7%:0.8%) Citation[4], According to one report about aggressive surgical resection for ICC with bilioenteric anastomosis, anastomosis with subsegmental bile ducts and vascular reconstruction, the incidence of pyogenic liver abscess was as high as 10–13.3% Citation[5]. In addition, pre-existing bilioenteric anastomosis as an independent risk factor for abscess formation was also repeatedly reported after RFA of intrahepatic metastatic cholangiocarcinoma Citation[6–8].
To our knowledge, no studies about hepatic abscess after MWA of intrahepatic metastatic cholangiocarcinoma with pre-existing bilioenteric anastomosis have been reported. Here we analysed five cases in which MWA was employed for liver metastatic cholangiocarcinoma after pancreatoduodenectomy or liver resection with bilioenteric anastomosis, as well as the incidence, causes and management of abscess formation as a major complication.
Materials and methods
Patients
This is a retrospective study. All treatments were performed at our institution with approval from the institutional ethics committee. Written informed consent was obtained from all patients at enrolment. Five male patients (range, 52–63 years, mean 57 ± 5.3 years) with eight intrahepatic metastases cholangiocarcinoma were treated with MWA from February 2008 to August 2009 and then followed up continuously until October 2010. All of them had a surgical resection before MWA, including pancreatoduodenectomy in three patients and resection of liver hilar mass with bilioenteric anastomosis in two patients. Four patients had cholangiectasis and intrahepatic bile duct pneumatosis before MWA treatment. The maximum diameter of the lesions ranged from 1.5 to 3.6 cm (mean, 3.2 ± 1.1 cm). The diagnosis of intrahepatic metastatic cholangiocarcinoma was confirmed in all patients with ultrasound (US)-guided needle biopsy. Of the five patients, one patient had a history of hepatitis B infection but no liver cirrhosis, the other four patients had no virus infection or cirrhosis.
As a general rule, patients who had uncontrollable ascites, obstructive jaundice or a marked tendency to bleed (prothrombin activity < 40%, prothrombin time > 25 s, platelet count < 50 cells × 109/L) were excluded from microwave ablation treatment.
Equipment
A KY2000 MW ablation system was applied (Kangyou Medical Instruments, Nanjing, People's Republic of China), which consists of a MW generator, two flexible coaxial cables and two cooled shaft antennae coated with polytetrafluoroethylene to prevent adhesion. The generator is capable of producing 1 to 100 W of power at 2450 MHz, which can drive up to two antennae simultaneously. The microwave system is also equipped with thermocouple needles which can monitor the temperature of its placement point in real time during ablation.
Techniques
All treatments were performed in our institution and were carried out under US guidance with the patients under unconscious intravenous anaesthesia (propofol, 6–12 mg/kg/h; ketamine, 1–2 mg/kg) in the operating room. All procedures were performed by two experienced doctors (L.P., Y.X.L.); both had more than 10 years’ experience in MW ablation of HCC. A detailed protocol including the placement of the antennae, power output setting, emission time and appropriate approach was worked out for each patient on an individual basis according to the experience we used for HCC Citation[9].
After MW ablation, the needle tracks were routinely cauterised to avoid tumour seeding and bleeding. Intravenously administered antibiotics were routinely used for three days after ablation treatment; this regimen was prolonged if the patient was suspected of having an infection.
Follow-up
CEUS or/and CECT/MR at days 3–5 after the treatment were used to evaluate the result of ablation. Then, CEUS or/and CECT/MR were repeated at 1 month after MWA, and at 3-month intervals within 1 year and then at 6-month intervals after MW ablation. In general, CEUS firstly was used, and the CECT/MR was further applied if the result of CEUS was not sure. The technique success rate was defined as ‘complete ablation was achieved according to CEUS or/and CECT/MR 3–5 days after ablation’, and technique effectiveness rate was defined as ‘complete ablation was achieved 1 month after ablation’.
Statistical analysis
Data analysis was done using SPSS 16.0(SPSS, Chicago, IL). Patients’ age and tumour size are expressed as mean ± SD, and the time of follow-up is expressed as mean.
Results
All of the eight lesions were completely ablated under the protocol. Both technique success rate and technique effectiveness rate are 100% (8/8). During the period of follow-up (range, 1–32 months, mean 14.4 months), liver abscess developed in five tumours (5/8 62.5%) of four patients (, , ). For the location of metastases – also the location of abscess, see . All of the four patients with abscess had cholangiectasis and intrahepatic bile duct pneumatosis before MWA treatment. Fistula occurred along the needle track after abscess formation including pleural cavity hepatic fistula in two cases (), liver-subcutaneous fistula in one case and both pleural cavity-hepatic and liver-subcutaneous fistula in one case (). For the three cases with pleural cavity-hepatic fistula, two had right pyothorax and pneumonia () who had symptoms of cough with occasionally purulent sputum. In addition, melaena was encountered in one case but was cured by continued medication haemostasis.
Figure 1. (a-c) US and MRI scans show abscess formation following microwave ablation (MWA) of intrahepatic cholangiocarcinoma with Cholangiojejunostomy. A 3.2-cm metastasis in a 52-year-old man was treated with MWA. (a) ultrasound scan shows the gas inside bile duct (thin arrows) arrived at ablation zone from liver hilar and hyperechoic ring of gas distribute around ablation zone 3 days after ablation (thick arrow). (b) MRI scan shows the pus developed from ablation zone to right thoracic cavity along needle track and forming a dumbbell-shaped abscess cavity (arrow). (c) Coronal MRI scan shows abscess in right thoracic cavity (thick arrow) coming from ablation zone involved soft tissue of chest wall (thin arrows) along coagulated needle track.
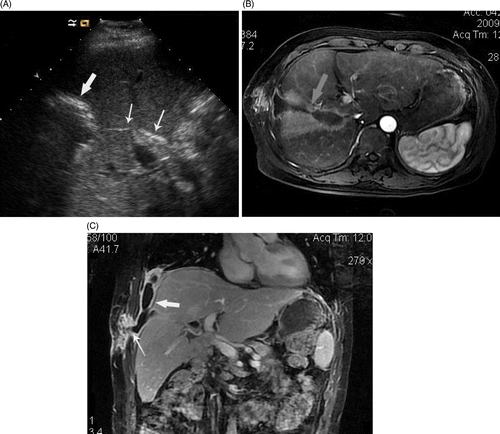
Figure 2. (a, b) MRI and CT scan showed abscess forming after ablation involved right thoracic cavity and inferior lobe of right lung. Four metastasis in a 56-year-old man were treated with MWA. (a) Coronal MRI scan shows two abscess forming 12 days after MWA (thin arrows) and superior abscess involved right thoracic cavity via needle track (thick arrow). (b) transverse CT scan shows pus in right thoracic cavity involved inferior lobe of right lung and induced consolidation of local lung (arrow) and limited inflammatory respond: the patient had symptom of cough and purulent sputum.
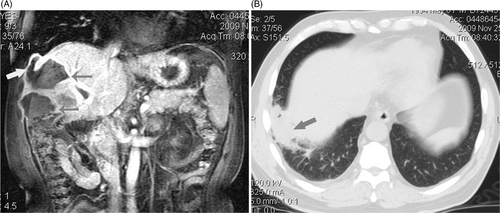
Figure 3. (a-c) transverse CT scan and digital subtraction angiography (DSA) shows abscess formation and a small account of bleeding in abscess cavity. A 53-year-old man had a symptom of bloody stool shortly after MWA of intrahepatic metastasis. (a) Transverse CT scan shows an abscess formation following MRA which had a large fluid- and air-filled cavity (arrow). (b) Hepatic arteriography hadn't found bleeding. (c) Coronal reconstruction of post-arteriography CT shows there was contrast agent deposition inside and around the ablation zone (arrow) in venous phase which help to deduce that there was a small number of active bleeding surrounding ablation zone.
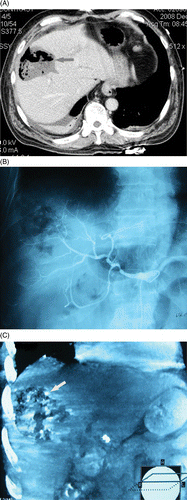
Table I Basic information of liver abscess and management after microwave ablation of liver cholangiocarcinoma metastases in four patients.
The four patients with liver abscess underwent immediate US and CECT/MR scan. Liver abscess was identified on CT or MR within 3–11 days after ablation. The CT findings of the abscesses that developed in the ablation zone were similar to those of other usual abscesses. All of the abscesses appeared as low-attenuation lesions (), and three (60%) of the five abscesses had enhancing rims with characteristic layering pattern in CECT or CEMR (). For all of the five abscesses, a substantial amount of air was found in CT which mostly appeared as bubbles. However, air–fluid levels were observed in two patients (). CECT and CEMR could demonstrate the fistula between liver and subcutaneous or pleural cavity which also had enhancing boundary (). For the patients with pleural cavity liver fistula, CT scan showed right pleural empyema and inflammation in lower lobe of the right lung () and right pleural empyema. The sonographic appearances of abscesses were highly echogenic region in the ablation zone (). Moreover, US could monitor gas inside the dilated bile duct entering into the abscess from the liver hilar region which showed linear hyperechogenicity (). Percutaneous arteriography was used for detection and treatment of possible biliary bleeding in patient with melaena, but got a negative result (). However, three-dimensional reconstruction of post-arteriography displayed that there was contrast agent deposition inside and around the ablation zone in the venous phase (), which helped to deduce that there was a small amount of active bleeding surrounding the ablation zone, gathered in the abscess cavity and draining to the bowel along the bile duct.
All of four patients with liver abscesses had a high fever, inappetence and increased leukocytes. Blood cultures were performed in all of the four patients, while sputum culture and pus culture from aspiration of abscess were performed in one patient respectively. The results of the above cultures demonstrated that all pathogens belonged to intestinal flora and biliary tract flora. Tumour cells were isolated from drainage of pus from abscess in one patient who had a negative result of imaging examination just after MWA, but a recurrence nodule was detected at the edge of ablation zone 6 months later in MR scan.
In general, amoxicillin sodium and/or metronidazole were used pre-operatively and post-operatively for prevention of infection. In the case of infection occurrence, antibiotics for Gram-negative bacteria were usually applied according to our experience. More aggressive antibiotics were applied under the guidance of the results of blood, sputum and pus culture, as well as the results of drug sensitive testing (). Percutaneous drainages were applied in two patients. For specific treatment in every patient see . All of the patients recovered from abscesses due to effective antibiotic and timely drainage except one patient who died from respiratory and cardiac failure 13 days after ablation.
Discussion
MWA has received increasing attention as a promising technique for treating focal malignancy in liver or other organs Citation[10–12], which could achieve a higher therapeutic efficacy through various methods Citation[13–15]. According to literature reports, MWA may cause a few complications including liver abscess when it is used for treatment of liver metastases Citation[1]. In many prior literatures, the incidence of abscess after RFA and MWA of malignant liver tumour ranges from 0.3% to 2% Citation[2], Citation[16–19]. For cholangiocarcinoma which had different biological characteristic from that of HCC,
Kim et al. found that patients with liver metastases of ICC after curative resection had a 7% incidence of abscess after RFA Citation[20]. However, for patients with pneumobilia, biliary abnormality and bilioenteric anastomosis, the incidence of abscess after RFA and laser-induced thermotherapy of intrahepatic metastases was significantly increased – often more than 20% Citation[2], Citation[18], Citation[21]. In the present study we exclusively focus on those patients with intrahepatic metastases of ICC after bilioenteric anastomosis. The frequency of liver abscess formation (62.5%, 5/8) after MWA was obviously higher than those of aforementioned studies.
The mechanism of abscess formation after RFA of liver tumour has been analysed in several studies Citation[16], Citation[18], Citation[20]. A few risk factors reported were correlated with the development of liver abscess, which included cholangitis, bilioenteric anastomosis, retained iodised oil in the position of ablation, an internally cooled electrode with greater power (200 W) and diabetes mellitus Citation[2], Citation[18], Citation[21]. As thermotherapy, although MWA has not been specially reported, it may share a similar mechanism for abscess formation in theory. In the present study, all of the four abscess cases had cholangiectasis and intrahepatic bile duct pneumatosis, which strongly supported the correlation between cholangiectasis – often accompanied by cholangitis – and abscess because the ablation can connect the biliary tract and ablation zone by thermal injury to the bile duct, and then the ablation zone could be contaminated by enteric bacteria Citation[18]. Another risk factor, bilioenteric anastomosis, which was related to retrograde enteric bacterial contamination of the biliary tract in 90% of patients with abscess after intervention procedure Citation[22], was encountered in all of the four cases with abscess in the present study. The process of air inside the bile duct entering the abscess from the liver hilar region was monitored by ultrasound, which further confirmed the pathological process of the above analysis. In addition, the fact that all of the pathogens cultured from blood, sputum and pus belong to intestinal flora and biliary tract flora could help to directly or indirectly define the etiology. For the other risk factors reported in prior studies, retained iodised oil and diabetes mellitus were not encountered in our enrolled patients. Microwave may be considered another possible risk factor for high incidence of abscess for its higher frequency and thermal efficiency compared to those of radiofrequency Citation[23]. The correlation between higher thermal efficiency and abscess formation has been reported in another study in which the high power radiofrequency (200 W) was used for ablation of HCC Citation[18]. In short, multiple risk factors including ICC, cholangiectasis and cholangitis, bilioenteric anastomosis and possible MWA simultaneously contributed to the high incidence of liver abscess after MWA in present study. Although the incidence is higher than those of prior studies in our knowledge Citation[1], Citation[2], Citation[20], the latter had not included all of the above risk factors simultaneously.
In the present study, fistula occurred in 100% (4/4) of patients with liver abscess after MWA. This high incidence of fistula has not been reported to our best knowledge. According to prior studies, the gas and pus in abscess often formed fistula along the coagulated needle track and possibly brought about other abscesses in other positions – for example, the pericardium and pleural cavity Citation[1], Citation[8]. In these conditions the inflammation became uncontrollable and even fatal Citation[8]. In our study group, two abscess patients had fistula combined with right empyema, and one of them died within 13 days after MWA. No doubt the fistula and empyema accelerated the progress and deterioration of disease, although they were not directly responsible for the perioperative death. For other patients with liver abscesses and fistula, percutaneous drainage and antibiotic could achieve successful therapy.
Local tumour progression may occur after MWA of interhepatic malignant tumour. However, in a prior study, local tumour progression was not found in patients with liver abscesses after RFA; the author attributed the absence of local tumour progression to excessive destruction of adjacent liver parenchyma surrounding the ablation zone, where the microscopic residual tumour could regrow Citation[18]. In our study tumour cells were isolated from pus in a patient with liver abscess shortly after MWA, and a recurrence nodule at the edge of the ablation zone emerged 6 months later. So, we suspect that a liver abscess at the position of the ablation zone could not ensure complete destruction of the possible residual tumour surrounding the ablation zone. However, the rate of local tumour progress in this study (12.5%, 1/8) is lower than that in another study (21.4%, 6/28) which applied RFA for recurrent intrahepatic cholangiocarcinoma after curative resection Citation[20].
One study reported that prophylactic antibiotic administration was effective in the prevention of infectious complications in interventional procedure Citation[22]. However, some reports found it failed to prevent the development of cholangitis or liver abscess after chemoembolization, PEI or RFA for patients with bilioenteric anastomosis Citation[2], Citation[24], Citation[25]. In the present study, although prophylactic antibiotic was applied in all of five patients, there was still a high incidence of abscess formation. So, a liver abscess as a most common complication should be anticipated when MWA is used for treatment of interhepatic metastatic cholangiocarcinoma after bilioenteric anastomosis. Further prospective studies are needed to determine the effectiveness of aggressive antibiotic prophylaxis, as well as the methods of timely diagnosis and effective management in the case of abscess formation to lower the morbidity and mortality.
There are several limitations in this study. Firstly, the number of subjects is relatively small, further study should include more patients to draw more confident conclusions. Secondly, cultures of pus aspirated from abscesses have not been performed in all patients, which hindered the exact etiology of abscesses. Thirdly, the mechanism of the formation of abscesses after MWA is speculative, which needs further experimental evidence.
Conclusion
In conclusion, due to collaborative interaction of multiple risk factors, there is a high incidence of abscess formation when MWA is used to treat liver metastatic cholangiocarcinoma with bilioenteric anastomosis. Fortunately, management via percutaneous drainage and antibiotic therapy achieves successful treatment efficacy for abscess. For interventional doctors, it is important to create mature and effective methods to prevent, diagnose and treat liver abscess after MWA.
Declaration of interest: This article was supported by grant from the National Scientific Foundation Committee of China (30825010). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Liang P, Wang Y, Yu X, Dong B. Malignant liver tumors: Treatment with percutaneous microwave ablation—complications among cohort of 1136 Patients. Radiology 2009; 251: 933–940
- Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: Complications encountered in a multicenter study. Radiology 2003; 226: 441–451
- Decadt B, Siriwardena AK. Radiofrequency ablation of liver tumors: Systematic review. Lancet Oncol 2004; 5: 550–560
- Inoue H, Hori A, Satake M, Kanetsuki I, Ueno K, Nishida H, Ikeda K, Kobayashi H, Nakajo M. Liver abscess formation after treatment of liver cancer by arterial injection using adriamycin/mitomycin C oil suspension (ADMOS). Nippon Igaku Hoshasen Gakkai Zasshi 1992; 2: 155–163
- Kubo S, Kinoshita H, Hirohashi K, Tanaka H, Tsukamoto T, Kanazawa A. Risk factors for and clinical findings of liver abscess after bilioenteric anastomosis. Hepatogastroenterology 1999; 46: 116–120
- Thomas KT, Bream PR, Jr, Berlin J, Meranze SG, Wright JK, Chari RS. Use of percutaneous drainage to treat hepatic abscess after radiofrequency ablation of metastatic pancreatic adenocarcinoma. Am Surg 2004; 6: 496–499
- Zuber-Jerger I, Blum HE. Chologenic abscess after radiofrequency ablation of a cholangiocellular carcinoma. Dtsch Med Wochenschr 2003; 44: 2300–2302
- Thiemann M, Benhidjeb T, Anders S, Gebauer B, Strik MW. Hepato-pericardial fistula following radiofrequency ablation (RFA) for liver metastases: A case report and review of the literature. Langenbecks Arch Surg 2008; 393: 1013–1016
- Zhou P, Liang P, Yu X, Wang Y, Dong B. Percutaneous microwave ablation of liver cancer adjacent to the gastrointestinal tract. J Gastrointest Surg 2009; 13: 318–324
- Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology 2007; 72: 124–131
- Wang Y, Liang P, Yu X, Cheng Z, Yu J, Dong J. Ultrasound-guided percutaneous microwave ablation of adrenal metastases: Preliminary results. Int J Hyperthermia 2009; 25: 455–461
- Liang P, Wang Y, Zhang D, Yu X, Gao Y, Ni X. Ultrasound guided percutaneous microwave ablation for small renal cancer: Initial experience. J Urology 2008; 180: 844–848
- Shen WF, Zhong W, Xu F, Kan T, Geng L, Xie F, Sui CJ, Yang JM. Clinicopathological and prognostic analysis of 429 patients with intrahepatic cholangiocarcinoma. World J Gastroenterol 2009; 15: 5976–5982
- Mayo-Smith WW, Dupuy DE. Adrenal neoplasms: CT-guided radiofrequency ablation – Preliminary results. Radiology 2004; 231: 225–230
- Dong B, Liang P, Yu X, Su L, Yu D, Cheng Z, Zhang J. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: Results in 234 patients. Am J Roentgenol 2003; 180: 1547–1555
- Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, Lee WJ, Lim HK, Nam GJ, Han SS, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: Spectrum of imaging findings. RadioGraphics 2003; 23: 123–134
- de Baère T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, Gamal El Din M, Letoublon C, Elias D. Adverse events during radiofrequency treatment of 582 hepatic tumors. Am J Roentgenol 2003; 181: 695–700
- Choi D, Lim HK, Kim MJ, Kim SJ, Kim SH, Lee WJ, Lim JH, Paik SW, Yoo BC, Choi MS, et al. Liver abscess after percutaneous radiofrequency ablation for hepatocellular carcinomas: Frequency and risk factors. Am J Roentgenol 2004; 184: 1860–1867
- Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: A prospective review of a 5-year experience. Ann Surg Oncol 2010; 17: 171–178
- Kim JH, Won HJ, Shin YM, Kim PN, Lee SG, Hwang S. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur J Radiol 2010; 19: 1–5
- Vogl TJ, Straub R, Eichler K, Woitaschek D, Mack MG. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: Experience with complications in 899 patients (2520 lesions). Radiology 2002; 225: 367–377
- Spies JB, Rosen RJ, Lebowitz AS. Antibiotic prophylaxis in vascular and interventional radiology: A rational approach. Radiology 1988; 166: 381–387
- Dupuy DE. Science to practice: Microwave ablation compared with radiofrequency ablation in lung tissue – Is microwave not just for popcorn anymore?. Radiology 2009; 3: 617–618
- Shibata T, Yamamoto Y, Yamamoto N, Maetani Y, Shibata T, Ikai I, Terajima H, Hatano E, Kubo T, Itoh K, et al. Cholangitis and liver abscess after percutaneous ablation therapy for liver tumors: Incidence and risk factors. J Vasc Interv Radiol 2003; 14: 1535–1542
- Kim W, Clark TW, Baum RA, Soulen MC. Risk factors for liver abscess formation after hepatic chemoembolization. J Vasc Interv Radiol 2001; 12: 965–968
