Abstract
Objective: The aim of this study was to assess the feasibility, safety and therapeutic effects of ultrasound (US)-guided percutaneous microwave thermal ablation (PMTA) in situ for the treatment of symptomatic uterine fibroid.
Materials and methods: The microwave (MW) antenna was percutaneously inserted into the fibroid under US guidance to perform the ablation. The ultrasound images, any complications and side effects were assessed during and after ablation. The shrinkage rate of the fibroid was calculated after the treatment.
Results: Forty patients underwent the treatment. The baseline fibroid volume was 14.6 to 341.1 cm3 (mean 140.1 ± 87.4 cm3). When the MW therapy started, a heightened echo around the MW emission aperture of the antenna was observed and gradually propagated throughout the fibroid while the ablation continued. The mean ablation time was 490 s. Contrast enhanced MRI showed no enhancement in the fibroid post-ablation. The shrinkage rate of the fibroid was 61.8%, 78.7%, 73.2% and 93.1% at 3, 6, 9, and 12 months after ablation, respectively. Six patients felt pain in their lower abdomens or waists within 12 hours post-ablation, and the discomfort rapidly disappeared. Seven patients had a small amount of vaginal bloody secretions within one to two weeks after treatment, and six of these patients recovered from the bleeding without any therapy after one week. No patient developed complications and fever during or after the ablation.
Conclusions: PMTA for fibroid is feasible and safe, and it is an easy and fast procedure that is minimally invasive.
Introduction
Uterine fibroids are benign tumours that occur most commonly in women of childbearing age. Patients with symptoms often must be treated to alleviate those symptoms Citation[1]. Hysterectomy remains the reference standard in the treatment of uterine fibroid; however, it may cause severe trauma and undesirable comorbidities Citation[2]. Myomectomy is another conventional treatment with less invasion, but it is associated with a recurrence rate as high as 20% according to literature reports Citation[3]. In recent years, increasing attention has been paid to the study of minimally invasive or non-invasive therapies with uterine preservation such as uterine arterial embolisation (UAE) Citation[4], cryoablation Citation[5], radiofrequency (RF) Citation[6], microwave (MW) Citation[7] and high intensity focused ultrasound (HIFU) Citation[8]. According to reported literature, UAE may lead to exposure to X-ray radiation for both the patients and doctors and may promote the development of numerous complications Citation[4]. Cryoablation and RF ablation often require the aid of a laparoscopic procedure, which is invasive Citation[6]. HIFU is effective, but can be time consuming Citation[8]. Ultrasound (US)-guided percutaneous microwave thermal ablation (PMTA) is minimally invasive, has low time requirements, is easy to perform, and has been broadly used for the treatment of solid tumours in organs other than the uterus with favourable effects Citation[9–12]. The current study aims to explore the feasibility, safety and therapeutic effects of PMTA for the treatment of uterine fibroids.
Materials and methods
Forty patients with forty-three uterine fibroids were recruited into the study from April 2007 to July 2010. Patients were aged from 32–45 years (average: 41.3 ± 3.5 years). The inclusion criteria were as follows: all patients had been diagnosed with uterine fibroid by using ultrasonography and contrast enhanced MRI (ceMRI) in our hospital; they had one of the following symptoms of menorrhagia or metrorrhagia, pelvic pain, bulk pressure, or urinary frequency, with no history of the rapid enlargement of the fibroid in a short period of time (excluding the possibility of carcinomatous change of fibroid); the patients had either completed childbearing or no longer desired fertility. The patients sought treatment and rejected hysterectomy, myomectomy, HIFU and UAE. The exclusion criteria included patients who desired further pregnancy; the number of fibroids was more than three; the largest fibroid diameter was larger than 10 cm; the age of the patient was greater than 50 years; inability to exclude possible leiomyosarcoma, the patient had a pelvic infection, heart or brain disease or malignant tumours. The treatment procedures, the expected curative effect and the potential complications as well as the potential hazardous effect on fertility and adjacent organs were explained in detail to the patients. The applications for treatment and informed consent were signed by the patients themselves.
This therapy as a clinical trial has been approved by the medical ethics committee of the hospital.
Instruments
MW tumour coagulator: A KV2000 MW tumour coagulator (Kangyou Medical instruments Nanjing, China) with a frequency of 2450 MHz and capable of continuous and pulse MW emission modes was used. The needle antenna was 15 gauge in diameter and 20 mm in length. The distance from the aperture of the MW emission to the needle tip was 11 mm, and the emission aperture was 1 mm. For the antenna, an internal water cycle cooling system was used to lower the temperature of the needle shaft Citation[13].
Sonography system
A Siemens Sequoia 512 Computer Color ultrasonograph, with a puncture-guided device and low MI contrast-enhanced function was used. The frequency of the probe was 2.5 to 4.5 MHz.
Pre-ablation preparation
Before ablation, the patients received routine blood, urine and stool examinations along with a test measuring bleeding and clotting time and ECG and ceMRI. Examinations using 2D grey-scale and colour Doppler ultrasonography were performed to evaluate the site, size and blood supply of the fibroid. The mean diameter and volume of the fibroid were calculated according to the respective formulas (length + width + height)/3 and (4/3πγ3), where γ is the mean radius (mean diameter/2).
Contrast-enhanced ultrasonography (CEUS) was performed with 2.4 mL of SonoVue (Bracco, Milan, Italy) to evaluate the perfusion of the fibroid. The microbubble contrast agent was mixed with 5 mL of normal saline, and after vibration blending, 2.4 mL (5 mg/mL) was used for a quick bolus infusion into the median cubital vein followed immediately by a flush with 5 mL of normal saline.
A catheter was inserted and the bladder was filled for a half hour prior to the ablation in order to observe the location of the urinary bladder and its wall during the ablation. The capacity of the bladder was adjusted so that the anterior wall of the fibroid was as close as possible to the abdominal wall, which was helpful for making an accurate puncture.
MW ablation therapy procedures
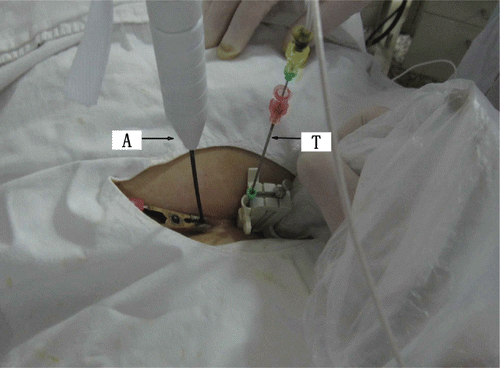
The ablation was performed under intravenous conscious sedation. The patients adopted a supine position. Under US guidance, a biopsy of the fibroid was performed via percutaneous puncture with an 18-gauge core needle for pathological diagnosis. Along the path of the biopsy, the MW antenna was then inserted into the centre of the fibroid with the tip of antenna located at 0.5 cm from the distal end of the tumour to avoid thermal damage to tissues outside the uterus. For temperature measurement in real-time during the ablation, one thermal couple was placed at a site of 0.5 cm inside the tumour adjacent to the urinary bladder if the fibroid was located at the anterior wall of the uterus, or adjacent to the rectum if the fibroid was located at the posterior wall (). The output energy of the MW was set at 50 W. Based on experience from our previous study Citation[14] using MW ablation in porcine musculature ex vivo in which we used one or two antennas with 50 W for 300 s or 600 s, coagulation zones can be induced covering 4.3 × 3.1 × 2.8 cm (one antenna, 300 s), 5.1 × 3.6 × 4.1 cm (one antenna, 600 s), 5.7 × 5.6 × 4.8 cm (two antennas, 300 s) or 6.7 × 5.9 × 5.3 cm (two antennas, 600 s). Therefore, in the current study, a single antenna was used for fibroids with mean diameters less than 5 cm and with lower perfusion; double antennas were used with an inter-antenna distance of 1 cm for fibroids with mean diameters larger than 5 cm or those less than 5 cm in mean diameter but with high perfusion. For large fibroids with mean diameters larger than 7 cm, the ablation was first performed using two antennas with 50 W for 300 s; then the antennas were withdrawn by 1 cm for a second ablation. For fibroids with non-spherical volumes, the margin of the thermal field was controlled at the shortest diameter, and the antenna was then withdrawn along the long axis or reinserted into the unablated zone for another ablation session. For patients with more than two fibroids, only the largest one was treated. During the ablation, variations in the echo from the fibroid were monitored by real-time ultrasonography. The MW therapy was stopped when the hyperecho (caused by microbubbles generated during MW emission and representing roughly the ablation zone Citation[15]) covered the whole nodule or when the measured temperature reached 60°C Citation[16]. The surveillance of the three-dimensional margin of the high echo was achieved with 2D imaging through continuous scanning in cross sections.
Post-ablation
The CEUS was performed after the MW therapy ended. If the CEUS showed an enhancement within the fibroid, re-ablation was required immediately. The patients were kept for 12 h under close observation for side effects and complications. If no abnormality was found during routine examination of their urine, the catheter was removed.
The second day after the ablation procedure, ceMRI was performed to evaluate the necrosis of the fibroid and the pelvic situation. The part of the fibroid that showed no enhancement in the ceMRI was considered to be necrotic tissue Citation[18]. The patients could be discharged from the hospital if no abnormality was found. They would then be provided with follow-up care for 12 months to assess potential complications and the shrinkage rate of the fibroid.
Statistical analysis
Statistical analysis was performed with Graphpad Prism for Windows version 4.0. The Wilcoxon matched-pairs signed rank test was used to compare the volumes and the percentage of volume reduction of the ablated fibroid. Statistical significance was set at p < 0.05.
Results
A total of 40 fibroids were ablated. Among them, 22 were intramural fibroids; 15 were subserous fibroids and three were submucous fibroids. The baseline mean diameter of the fibroids ranged from 3.7 to 9.0 cm, with an average of 6.4 ± 1.5 cm. The mean volume ranged from 14.6 to 341.1 cm3, with an average of 140.1 ± 87.4 cm3. The pre-ablation 2D grey-scale ultrasonography showed fibroids of intermediate echogenicity, with the internal echo being either homogeneous or inhomogeneous. The colour Doppler flow image (CDFI) showed an annular blood stream signal at the periphery of the fibroid and dotted or striped blood stream signals within the nodules. The ceMRI and CEUS showed enhancement within the fibroid.
When the MW therapy started, the hyperecho was seen around the MW emission aperture of the antenna, and it gradually propagated while the MW therapy continued (). After ablation, the CDFI found no blood stream signal in all fibroids (), and the CEUS showed no enhancement besides the circle enhancement in the periphery of the fibroid (). Twelve hours after the ablation, ceMRI showed no enhancement in the ablated zone, but circular or circle-like ‘black holes’ () that suggests tissue coagulated necrosis. The shrinkage rate of the fibroids with time after ablation is shown in .
Figure 2. (A) The ultrasound image of 2D grey-scale of uterus myoma at 10 s after MW therapy. Echo enhancement began around the antennas. (B) Ultrasound image of 2D grey-scale of uterus myoma at 70 s after MW therapy. The area of echogenicity is noted to be enlarged. (C) Ultrasound image of 2D grey-scale of uterus myoma at the end of ablation. The whole myoma is obscured due to echoes.
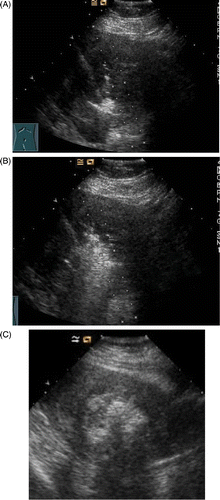
Figure 3. (A) CDFI of myoma before ablation shows the myoma with hyperperfusion. (B) CDFI of ablated myoma at the end of ablation. No residual colour Doppler is demonstrated.
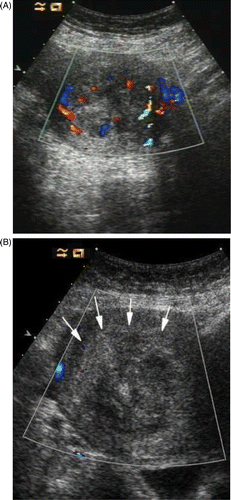
Figure 4. (A) Contrast enhanced ultrasound (ceUS) image of myoma before ablation. The fibroid was obviously enhanced. (B) CeUS of myoma 1 day following ablation showing no ultrasound contrast enhancement in the myoma.
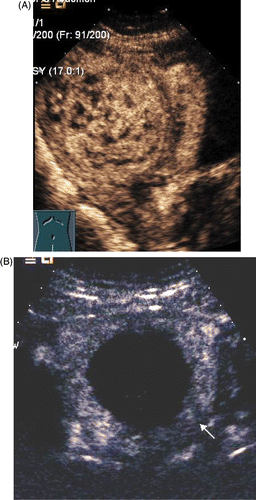
Figure 5. (A) CeMRI picture of myoma before ablation shows the fibroid was obviously enhanced. (B) Sagittal ceMRI of the pelvis following PMTA of a fibroid in the posterior wall of uterus. No enhancement is seen in the fibroid.
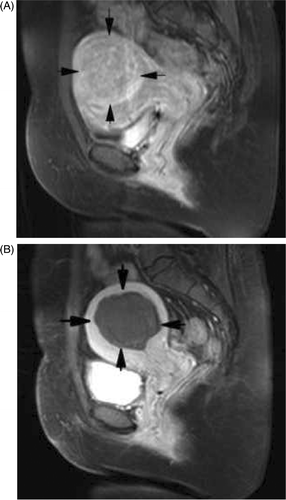
Table I. Fibroid volume and volume reduction at baseline, 3, 6, 9 and 12 months follow up.
The fibroid shrinkage rate was 100% in four patients who were noted to pass their targeted fibroid during subsequent menses. The MW therapy time ranged from 300 s to 930 s, with an average of 490 s. The response of patients to post-ablation therapy is shown in .
Table II. Patient symptoms after ablation.
Discussion
In recent years, thermal ablation in situ has been investigated as an alternative strategy to hysterectomy or myomectomy in the treatment of uterine fibroids with the uterus intact and demonstrated to be effective in shrinking uterine fibroids and improving symptoms Citation[4–8]. However, according to published reports, radiofrequency ablation and cryoablation are performed with the aid of laparoscopy Citation[5], Citation[6] by puncture through the cervical canal or Douglas pouch, which are all largely invasive Citation[7]. Moreover, radiofrequency ablation is only effective in fibroids with a volume of ≤75 cm3, and there is no significant improvement in the symptoms for fibroids with a volume of ≥75 cm3, with a high rate of reoperation Citation[6], Citation[17]. Although HIFU is non-invasive with definite curative effects, it can be time consuming, especially for large and hypervascular nodules Citation[8], Citation[18], Citation[19].
The potential advantages of MW technology include consistently higher intratumoural temperatures, larger tumour ablation volumes, faster ablation times, use of multiple applicators and less procedural pain. This technology is being widely used to treat solid tumors in organs other than the uterus, such as liver cancer, lung cancer, kidney cancer and adrenocortical carcinoma metastasis Citation[9–12], Citation[20], Citation[21]. Percutaneous puncture under ultrasound-guided with real time surveillance of the procedure overcomes the disadvantage of puncture through the cervical canal or Douglas pouch without real time imaging, and makes the puncture easier and more accurate.
Since uterine fibroids are benign, treatment is focused on symptom relief. Therefore, it is crucial to use minimal or non-invasive therapy which is safe and provides a good therapeutic result. The uterus is adjacent to the rectum and the bladder, so it is important to avoid thermal damage to the adjacent tissues during treatment procedures. According to our previous study, the edge of the ablation zone is the congestive zone that is 5 mm from the needle tip, with the necrosis zone located at the inner side of the congestive zone Citation[20]. In the current study, the edge of the ablation zone was designed to be located at the inner edge of the fibroid by keeping the tip of the needle 5 mm from the inner edge of the fibroid; this was done to avoid coagulated necrosis of the fibroid pseudo-capsule. Because most fibroids have a pseudo-capsule, as long as the thermal field is effectively controlled within the pseudo-capsule, a desired ablated volume can be formed, and undesirable thermal damage can be avoided. The needle MW antenna with water cooling system used in this study plays an important role in forming the desirable ablated configuration because of the lower temperature of the needle shaft Citation[21]. In addition, it is important to keep the extension of the hyperecho under surveillance in real time.
In this study, we have demonstrated that PMAT is feasible and safe for treatment of uterine fibroid. No complications were observed in any of the treated patients during or after the ablation and the fibroid shrinkage was significant following treatment. However, to assess its true clinical value, clinical effects and symptom improvements should be assessed in larger patient series with longer term follow-up.
Conclusions
The US-guidance PMTA in situ is feasible and safe for treatment of symptomatic uterine fibroids and it is an easy and fast procedure with minimal invasion. PMTA appears to be an optional therapy for patients with symptomatic uterine fibroids who prefer to keep their uterus intact. The long-term therapeutic effects need to be evaluated by long-term patient follow up and large sample collection.
Declaration of interest: This work was supported by the Chinese PLA General Hospital Miaopu Fund (grant no. 06MP57) and the first affiliated hospital of Chinese PLA General Hospital New Technique Innovation Fund (grant no. ZD200806). The research was guided by Baowei Dong. The authors alone are responsible for the content and writing of the paper.
References
- Levy BS. Modern management of uterine fibroids. Acta Obstet Gynecol Scand 2008; 87: 812–823
- Harding G, Coyne KS, Thompson CL, Spies JB. The responsiveness of the uterine fibroid symptom and health-related quality of life questionnaire (UFS-QOL). Health Qual Life Outcomes 2008; 6: 9
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyoma after myomectomy. Hum Reprod Update 2000; 6: 595–602
- Agdi M, Valenti D, Tulandi T. Intraabdominal adhesions after uterine artery embolization. Am J Obstet Gynecol 2008; 199: 482e1-3
- Exacoustos C, Zupi E, Marconi D, Romanini ME, Szabolcs B, Piredda A, Arduini D. Ultrasound-assisted laparoscopic cryomyolysis: Two- and three-dimensional findings before, during and after treatment. Ultrasound Obstet Gynecol 2005; 25: 393–400
- Bergamini V, Ghezzi F, Cromi A, Bellini G, Zanconato G, Scarperi S, Franchi M. Laparoscopic radiofrequency thermal ablation: A new approach to symptomatic uterine myomas. Am J Obstetr Gynecol 2005; 192: 768–773
- Kanaoka Y, Yoshida C, Fukuda T, Kajitani K, Ishiko O. Transcervical microwave myolysis for uterine myomas assisted by transvaginal ultrasonic guidance. J Obstet Gynaecol Res 2009; 35: 145–151
- Mikami K, Murakami T, Okada A, Osuga K, Tomoda K, Nakamura H. Magnetic resonance imaging-guided focused ultrasound ablation of uterine fibroids: Early clinical experience. Radiat Med 2008; 26: 198–205
- Liu FY, Yu XL, Liang P, Wang Y, Zhou P, Yu J. Comparison of percutaneous 915 MHz microwave ablation and 2450 MHz microwave ablation in large hepatocellular carcinoma. Int J Hyperthermia 2010; 26: 448–455
- Wang Y, Liang P, Yu X, Cheng Z, Yu J, Dong J. Ultrasound-guided percutaneous microwave ablation of adrenal metastasis: Preliminary results. Int J Hyperthermia 2009; 25: 455–461
- Wolf FJ, Grand DJ, Machan JT, Dipetrillo TA, Mayo-Smith WW, Dupuy DE. Microwave ablation of lung malignancies: Effectiveness, CT findings, and safety in 50 patients. Radiology 2008; 247: 871–879
- Liang P, Wang Y, Zhang D, Yu X, Gao Y, Ni X. Ultrasound guided percutaneous microwave ablation for small renal cancer: Initial experience. J Urol 2008; 180: 844–848
- Lu Y, Nan Q, Li L, Liu Y. Numerical study on thermal field of microwave ablation with water-cooled antenna. Int J Hyperthermia 2009; 25: 108–115
- Zhang J, Zhang BS, Feng L, Jiang X, Ren JT. Experimental study of microwave ablation for muscular tissues with water-cooling single needle antenna. Chinese J Med Ultrasound (Electronic Edition) 2009; 4: 22–25
- Dong BW, Liang P, Yu XL, Su L, Yu D, Cheng Z, Zhang J. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: Results in 234 patients. AJR Am J Roentgenol 2003; 180: 1547–1555
- Dong BW, Liang P, Yu XL, Zeng XQ, Wang PJ, Su L, Wang XD, Xin H, Li S. Sonographically guided microwave coagulation treatment of liver cancer: An experimental and clinical study. AJR Am J Roentgenol 1998; 171: 449–454
- Cho HH, Kim JH, Kim MR. Transvaginal radiofrequency thermal ablation: A day-care approach to symptomatic uterine myomas. Aust N Z J Obstet Gynaecol 2008; 48: 296–301
- Zhou XD, Ren XL, Zhang J, He GB, Zheng MJ, Tian X, Li L, Zhu T, Zhang M, Wang L, et al. Therapeutic response assessment of high intensity focused ultrasound therapy for uterine fibroid: Utility of contrast-enhanced ultrasonography. Eur J Radiol 2007; 62: 289–294
- Ren XL, Zhou XD, Yan RL, Liu D, Zhang J, He GB, Han ZH, Zheng MJ, Yu M. Sonographically guided extracorporeal ablation of uterine fibroids with high-intensity focused ultrasound: Midterm results. J Ultrasound Med 2009; 28: 100–103
- Zhou P, Liang P, Dong B, Yu X, Han Z, Xu Y. Phase I clinical study of combination therapy with microwave ablation and cellular immunotherapy in hepatocellular carcinoma. Cancer Biol Ther 2011; 11(5)450–456
- Lu Y, Nan Q, Du J, Li L, Qiao A, Liu Y. Experimental study on thermal field in the vicinity of arterial bifurcation in microwave ablation therapy. Int J Hyperthermia 2010; 26: 316–326