Abstract
Purpose: To investigate the feasibility, safety and efficacy of ultrasound guided percutaneous microwave (MW) ablation for small splenic metastasis.
Materials and methods: A total of four patients with five pathologically proven splenic metastases (from ovarian, pulmonary, gastric adenocarcinoma and hepatocellular carcinoma, respectively) 1.3 to 2.9 cm in diameter were treated with microwave ablation. A cooled-shaft needle antenna was percutaneously inserted into the tumour under ultrasound guidance. One thermocouple was placed about 0.5 cm away from the tumour to monitor temperature in real time during ablation. Microwaves were emitted at 60 W for 600 s routinely and prolonged as necessary to attain temperatures sufficient to ensure tumour killing. Treatment efficacy was assessed by contrast-enhanced imaging at 1, 3 and 6 months, and every 6 months thereafter.
Results: All tumours were completely ablated at a single session and no complications occurred. No local tumour progression was observed at a mean follow up of 22 ± 17.1 months (range 4 to 43 months). The ablation zone was well defined on contrast-enhanced imaging and it gradually shrank with time. One new metastatic lesion was detected in the spleen at 11 months after the ablation and was successfully treated by another MW ablation. The post-ablation survival was mean 22 months. No other complications were observed except for fever and abdominal pain.
Conclusions: Ultrasound-guided percutaneous MW ablation appears to be a safe and effective minimally invasive technique for management of small splenic metastasis in selected patients.
Introduction
The spleen is an important component of the body's defence against infection and is the tenth most frequent site of secondary tumour, with a low incidence of carcinomatous metastasis in 2% to 8% of autopsy cases examined Citation[1]. The most frequent solid tumours metastasising to the spleen are reported to be melanoma and carcinomas followed by lung, breast, and ovarian carcinomas Citation[1], Citation[2]. The optimal management of splenic tumours has shifted during the last decades from traditional splenectomy to conservative treatment. The shift toward spleen preservation is justified on the basis of the importance of the spleen in immunological function. The literature suggests that 25% to 50% of the spleen needs to be preserved to ensure sufficient immunological function in patients Citation[3–5]. Thermal ablation including radiofrequency (RF) and microwave (MW) ablation has recently been successfully adopted for minimally invasive treatment of some primary and metastatic tumours in solid organs Citation[6–8]. However, until now only two cases of colorectal and renal splenic metastasis treated by RF energy were reported Citation[9], Citation[10] and no data of experimental or clinical study regarding the safety and efficacy of MW ablation for splenic metastasis is available.
Recently, cooled-shaft antenna development in MW ablation technology has enabled larger tumour volumes to be treated and has led to its wide clinical application in oncology Citation[11–13]. We report our initial experience of ultrasound (US)-guided percutaneous MW ablation of splenic metastasis in four cases to preliminarily observe the feasibility and safety of MW ablation technique in this very vascular organ.
Subjects and methods
The study was carried out at our institution with approval from the institutional ethics committee. Written informed consents for participation in this study were obtained from all the patients. All ablations were performed as an inpatient procedure.
Patients
Case 1
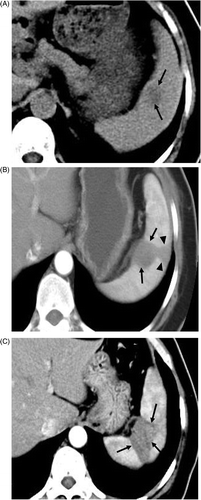
A 55-year-old woman underwent a radical hysterosalpingo-oophorectomy because of right ovarian adenocarcinoma in May 2002. In August 2006 an abdominal ultrasound scan showed two hypoechoic tumours 1.4 × 1.1 cm and 1.3 × 1.1 cm, respectively located at the right lobe of the liver and the site adjacent to the splenic hilum, both with regular borders, and colour signals were obtained at the peripheral margin of the tumours. A computed tomography (CT) scan showed a solitary tumour with low attenuation near the splenic hilum (). The 18F-2-fluoro-2-deoxyglucose (18F-FDG) positron emission tomography (PET) scan showed hypermetabolic spots at the splenic hilum and the right lobe of the liver consistent with metastasis. The liver lesion was ablated with MW technique 1 week before treating splenic metastasis. Another lesion 2.3 × 1.8 cm was identified in the upper pole of the spleen by contrast-enhanced ultrasound (CEUS) scan 11 months later, which was treated by another MW ablation procedure under the establishment of artificial pleural effusion to make the lesion in the upper pole of the spleen visible distinctly under ultrasound scan and to make the insertion feasible and safe by avoiding the pulmonary complications. No other metastasis was detected by imaging examinations.
Figure 1. Transverse contrast-enhanced CT scans in a 55-year-old woman with ovarian carcinoma metastasis. (A) Preablation scan showed one hypoattentuating neoplasm (arrows) near the hilum. (B) Scan obtained 9 months after the first ablation shows hypoattenuating ablation zone (arrows) without enhancement. The needle track was cauterized as the probe was removed, resulting in the subcapsular triangle-shaped thermal lesion (arrowheads). (C) Scan obtained 32 months after the second ablation shows hypoattenuating ablation zone (arrows) without enhancement corresponding to treated region.
Case 2
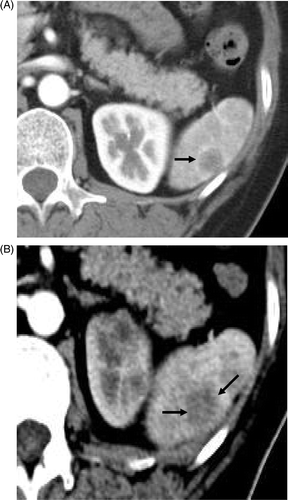
A 56-year-old woman was admitted to our hospital because of poorly differentiated lung adenocarcinoma 5.9 × 4.2 cm in November 2007. The PET scan showed a left lobe lung mass and a splenic lesion that were both hypermetabolic. The CT scan showed a solitary 1.6 × 1.4 cm lesion with low attenuation and peripheral enhancement at the lower pole of the spleen (). The metastasis was isolated to the spleen. The lung lesion underwent six courses of chemotherapy and the patient refused any other treatment method for the lung. The splenic lesion was treated by one MW ablation procedure.
Figure 2. Transverse contrast-enhanced CT scans in the 56-year-old woman with lung adenocarcinoma metastasis. (A) Preablation scan showed one solitary neoplasm (arrow) with low attenuation and peripheral enhancement at the lower pole of the spleen. (B) On an arterial phase CT scan obtained 28 months after treatment no enhancement is seen in the enlarged coagulation zone (arrows), suggesting the absence of residual tumour.
Case 3
A 56-year-old male patient with moderately differentiated gastric adenocarcinoma underwent six courses of chemotherapy in June 2007. His tumour was not considered operable at his local hospital. In February 2008 he was admitted to our hospital because of four hepatic metastases with the largest size of 2.9 cm, which were treated by five microwave ablation procedures. Five months later a CT scan of the abdomen revealed a solitary 2.5 × 2.3 cm lesion with low attenuation at the lower pole of the spleen, which was treated by MW ablation.
Case 4
A 32-year-old male patient was diagnosed with hepatocellular carcinoma and underwent resection in January 2007. In November 2008 he was admitted to our hospital because of two hepatic lesions with the largest size of 4.9 cm and a solitary 2.9 × 2.7 cm metastasis at the upper pole of the spleen, which were all detected by contrast-enhanced magnetic resonance imaging (MRI) and CEUS scan with high enhancement in arterial phase. The liver lesions and splenic lesion were all treated by microwave ablation.
Technique of procedure
Microwave system
The microwave unit (KY-2000, Kangyou Medical, Shanghai, China) consists of a microwave generator, a flexible coaxial cable, a controlled temperature circulating water bath, and a needle antenna. The generator is capable of producing 100 W of power at 2450 MHz. The needle antenna has a diameter of 1.9 mm with an 18-mm shaft which is easily visualised with ultrasound. The shaft has a coating of polytetrafluoroethylene to prevent tissue adhesion. A narrow radiating segment of 3mm is embedded on the shaft, 11 mm away from the tip. Inside the shaft there are dual channels through which distilled water under room temperature is pumped by a peristaltic pump, continuously cooling the shaft proximal to the radiating segment. The flow rate of the distilled water was constant at 40 ml/min for all ablations, which can effectively prevent overheating of the shaft. The MW machine is also equipped with a thermocouple system (Kangyou Medical) which can measure temperature in real time during ablation. The metallic shaft of the thermocouple needle is insulated with epoxy resin to avoid any interference from the metal in the electromagnetic field.
Microwave ablation procedures
Patients were placed in the supine oblique position in the interventional US suite. Colour Doppler and grey-scale US scans were performed to choose the safest lateral intercostal approach to the splenic tumour. This path did not traverse major hilar vasculature or pleura. After local anaesthesia with 1% lidocaine, US-guided biopsy was performed first by an automatic biopsy gun with an 18-gauge cutting needle to obtain 2–3 specimens. Then the antenna was percutaneously inserted into the tumour and placed at the designated place under US guidance. For tumours less than 1.5 cm, one antenna was inserted, for tumours measuring 1.5 cm or greater, two antennae were inserted with an inter-antenna distance of no more than 1.8 cm, which were used simultaneously during MW ablation to obtain a larger ablation zone. A 20-gauge thermocouple was inserted about 0.5–1.0 cm away from the tumour for real-time temperature monitoring during MW ablation. A power output of 60 W for 600 s was routinely used during MW ablation. If the heat-generated hyperechoic water vapour did not completely encompass the entire tumour and if the measured temperature did not reach 60°C or remain above 54°C for at least 3 min, prolonged MW emission was applied until the desired temperature was reached. All insertions were performed by either of the two experienced radiologists (P.L. and X.L.Y.) who had collaborated for more than fifteen years in MW ablation of hepatocellular carcinoma. After all insertions, intravenous anaesthesia was administered by a combination of propofol and ketamine via the peripheral vein during standard hemodynamic monitoring. When withdrawing the antenna, the needle track was coagulated to prevent bleeding and tumour cell seeding.
Individual case techniques
In case 1, during the first tumour ablation procedure two thermocouples were placed about 0.5 cm away from the tumour and directly abutting the vessel of the splenic hilum respectively, for real-time temperature monitoring to obtain complete ablation and to avoid thermal-mediated injury during MW ablation. Before ablating the second tumour located in the upper pole of the spleen, a 20-gauge, 32-mm sheathed needle was inserted into the thoracic cavity via an intercostal approach under US guidance. After removal of the needle, normal saline solution at room temperature was infused into the thoracic cavity via the remaining sheath by dripping until a separation of at least 1 cm between the lung and diaphragm was achieved under US guidance. The infused saline solution is defined as representing artificial pleural effusion.
Post-procedural observation and imaging follow up
After MW ablation patients were closely monitored for possible complications such as intraperitoneal haemorrhage, skin burn, side effects such as fever, pleural effusion and pain were also documented. All patients received CEUS examination 1 day after MW ablation to assess the treatment efficacy. If residual tumour was found, further sessions were planned, or else patients entered the follow-up protocol which consisted of contrast-enhanced CT/MRI and/or CEUS at 1, 3 and 6 months after MW ablation and every 6 months thereafter.
Results
The mean age of patients treated was 49.8 ± 11.8 years (32–56 years). The mean tumour size ablated was 2.1 ± 0.7 cm (1.3–2.9 cm). The histological analyses of the biopsy specimens were confirmed to be metastatic carcinoma consistent with the primary cancer. Microwaves were emitted at 60 W for mean 782.0 ± 215.0 s (540–1030 s) to reach temperatures sufficient enough to ensure tumour kill. All tumours were completely ablated in a single session. The hospital stay for four cases was 5–7 days. The patients reported minor left upper quadrant pain after the procedure, and no opiates or analgesics were necessary. No other complications were observed. The coagulation function tests in all four patients were normal after MW ablation.
CEUS showed no enhancement in all the ablation zones in the scan performed after 24 h. Contrast-enhanced CT showed a low-density coagulative zone within the lesion without enhancement (, 1C, 2B). The patients were followed up regularly according to the protocol. During a mean follow up of 22 ± 17.1 months (range 4 to 43 months), the patient in case 3 died of acute bleeding from a gastric tumour 4 months after MW ablation, the patient in case 4 died of multiple metastases and hepatic failure 13 months after MW ablation, without splenic recurrence being detected. The other two patients were both alive. A new metastatic neoplasm was detected at 11 months in case 1 after ablation by imaging examinations, which was successfully treated by another MW ablation and then no recurrence was detected at follow up. The patient in case 2 experienced multiple organic metastases including kidney, brain and recurrence in the lung at 11 months after the ablation. The patient was treated with immunotherapy and thus resection or interventional treatment was not considered. shows the clinical features and outcomes of the four patients.
Table I. Clinical features and outcomes of the four patients.
Discussion
Tumour metastasis to the spleen is thought to be rare, and is more frequent in tumours known to have a high metastatic potential, especially those that have metastasised to several organs via a haematogenous route. Local neoplasm treatment options have traditionally included surgery and radiation therapy. However, the spleen is an important immunological organ and the adverse consequences of its removal have become increasingly apparent over the last decades. Death rates from overwhelming post-splenectomy sepsis have been reported to be up to 600 times greater than those of the general population Citation[3].
Recent trends towards a minimally invasive approach for benign and malignant diseases have emphasised decreased cost and time and reduced morbidity and mortality. Multiple techniques such as splenic embolisation, splenic artery occlusion and transcatheter splenic ablation have been tried to prevent total splenectomy Citation[14], Citation[15]. Local thermal ablation not only reduces tumour burden, it might also reduce necessity for further systemic chemotherapy if it is the only site of metastasis, thereby reducing morbidity for these patients who will frequently and ultimately succumb to progressive disease in other sites. US-guided tissue thermal ablation with microwave is an effective therapeutic technique which has been successfully adopted as a treatment modality of primary and metastatic tumours of the liver and kidney with acceptable low complication rates Citation[11], Citation[16]. A clinical study has shown the feasibility and safety of MW ablation in the management of hypersplenism without sacrificing efficacy Citation[17]. Theoretically, MW ablation for splenic tumours has some potential benefit compared to other ablation methods including RF ablation and cryoablation: (1) MW has a larger ablation zone of active heating and its transmission in tissue is not limited by desiccation and charring. Therefore, intratumoural temperatures can be driven consistently high, which may contribute to larger ablation zone, less treatment time and more complete tumour kill Citation[18]; (2) MW is also less affected by the perfusion median ‘heat-sink’ effect, which may be helpful for treating tumours with rich blood supply including the spleen Citation[19], especially when bleeding occurred immediately following inserting biopsy needle and MW antenna into the spleen, the bleeding ceased soon due to thermal coagulation; (3) In addition, multiple antennae can be used simultaneously to ablate larger tumours Citation[7], Citation[11], Citation[13]; (4) In recent years, internally cooled antenna for MW ablation capable of distributing the heat deeper in the tissue resulted in larger ablated volumes and better hemostasis Citation[12], Citation[13], which may obtain complete tumour necrosis without causing skin burn.
For all these reasons and based on our experience of MW ablation technique Citation[7], Citation[11], Citation[12], Citation[20], we have conducted, to our knowledge, the first study for non-excised, percutaneous MW ablation for splenic metastasis using US guidance to evaluate the technical feasibility and safety. After ablation, complete necrosis was obtained, and the post-ablation survival (mean 22 months) for the four patients is comparable to that of splenectomy treatment as the published studies reported Citation[21], Citation[22]: the median survival 11–19.5 months. Noticeably, in order to avoid the potential risks, several important steps should be followed: (1) the high vascularisation of the spleen produces extremely high intra-abdominal temperature with the risk of thermal injury of the adjacent viscera. For accurate assessment of the treatment efficacy and to avoid overheating, a thermal monitoring system was used in our treatment with one to two thermocouples placed about 0.5 cm away from the tumour and abutting the hilar vessel, thus no residual tumour and major complications occurred; (2) insertion of the needle at the lower pole of the spleen or establishment of artificial pleural effusion before ablation is preferred to reduce pulmonary complications and referred pain; (3) the puncture site must be kept away from large vessels at the hilum of the spleen.
The limited follow up and the fact that only four patients were treated are major limitations to our study. The application of MW energy to the spleen is in many ways, including tissue property and blood supply, different from that performed on the liver parenchyma, therefore appropriate treatment time and power still needs to be further defined. Additionally, in our study the following inclusion criteria were met: single splenic tumour of 3 cm or smaller; three or fewer multiple lesions with a maximum diameter of 2 cm or less; no extensively extrasplenic metastases, and the general condition of the patient permits MW ablation, which is essential for the complete and safe ablation of tumours. Further rational inclusion criteria still need to be established.
Conclusion
In summary, microwave ablation preliminarily appears to be a safe technique for treatment of splenic metastases. The true role of this method still remains to be determined, and further studies are warranted to observe the therapeutic outcome and define the appropriate inclusion and exclusion criteria.
Declaration of interest: The paper was supported by the National Scientific Foundation Committee of China (30825010). The authors alone are responsible for the content and writing of the paper.
References
- Morgenster L, Rosemberg J, Geller SA. Tumors of the spleen. World J Surg 1985; 9: 468–476
- Klein B, Stein M, Kuten A, Steiner M, Barshalom D, Robinson E, Gal D. Splenomegaly and solitary spleen metastasis in solid tumors. Cancer 1987; 60: 100–102
- Traub A, Giebink GS, Smith C, Kuni CC, Brekke ML, Edlund D, Perry JF. Splenic reticuloendothelial function after splenectomy, spleen repair, and spleen autotransplantation. N Engl J Med 1987; 317: 1559–1564
- Uranues S, Kronberger L, Kraft-Kine J. Partial splenic resection using the TA-Stapler. Am J Surg 1994; 168: 49–53
- Bradshaw PH, Thomas CG, Jr. Partial splenectomy and overwhelming infection in rats. J Surg Res 1982; 32: 173–175
- Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: Part 1, indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. Am J Roentgenol 2005; 185: 64–71
- Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, Xiao Q. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005; 235: 299–307
- Kim HR, Cheon SH, Lee KH, Ahn JR, Jeung HC, Lee SS, Chung HC, Noh SH, Rha SY. Efficacy and feasibility of radiofrequency ablation for liver metastases from gastric adenocarcinoma. Int J Hyperthermia 2010; 26: 305–315
- Marangio A, Prati U, Luinetti O, Brunetti E, Fìlice C. Radiofrequency ablation of colorectal splenic metastasis. Am J Roentgenol 2002; 178: 1481–1482
- Wood BJ, Bates S. Radiofrequency thermal ablation of a splenic metastasis. J Vasc Interv Radiol 2001; 12: 261–263
- Liang P, Wang Y, Zhang D, Yu X, Gao Y, Ni X. Ultrasound-guided percutaneous microwave ablation of small renal cancer: Initial experience. J Urol 2008; 180: 844–888
- Wang Y, Sun Y, Feng L, Gao Y, Ni X, Liang P. Internally cooled antenna for microwave ablation: Results in ex vivo and in vivo porcine livers. Eur J Radiol 2008; 67: 357–361
- Liu FY, Yu XL, Liang P, Wang Y, Zhou P, Yu J. Comparison of percutaneous 915 MHz microwave ablation and 2450 MHz microwave ablation in large hepatocellular carcinoma. Int J Hyperthermia 2010; 26: 448–455
- Kaiho T, Miyazaki M, Iinuma K, Ito H, Koyama T, Nakagawa K, Nakajima N. Long-term prognosis of idiopathic thrombocytopenic purpura treated by partial splenic embolization. Nippon Geka Gakkai Zasshi 1993; 94: 383–393
- Guzzetta PC, Stolar CH, Potter BM, Broadman L, Ruley EJ. Partial splenic ablation in preparation for renal transplantation in children. J Pediatr Surg 1983; 18: 800–804
- Shibata T, Murakami T, Ogata N. Percutaneous microwave coagulation therapy for patients with primary and metastatic hepatic tumors during interruption of hepatic blood flow. Cancer 2000; 88: 302–311
- Duan YQ, Gao YY, Ni XX, Wang Y, Feng L, Liang P. Changes in peripheral lymphocyte subsets in patients after partial microwave ablation of the spleen for secondary splenomegaly and hypersplenism: A preliminary study. Int J Hyperthermia 2007; 23: 467–472
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: Principles and applications. RadioGraphics 2005; 25: S69–83
- Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT, Jr. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005; 236: 132–139
- Wang Y, Liang P, Yu X, Cheng Z, Yu J, Dong J. Ultrasound-guided percutaneous microwave ablation of adrenal metastasis: Preliminary results. Int J Hyperthermia 2009; 25: 455–461
- Sauer J, Sobolewski K, Dommisch K. Splenic metastases – not a frequent problem, but an underestimate location of metastases: Epidemiology and course. J Cancer Res Clin Oncol 2009; 135: 667–671
- de Wilt JH, McCarthy WH, Thompson JF. Surgical treatment of splenic metastases in patients with melanoma. J Am Coll Surg 2003; 197: 38–43
