Abstract
Purpose: The aim of this report was to determine the impact of hyperthermia (HT) on preoperative radiochemotherapy for locally advanced rectal cancer.
Materials and methods: Between 1996 and 2007, 235 patients with locally advanced rectal cancer were treated with concurrent preoperative radiochemotherapy with or without HT. The total dose of radiotherapy was 39.6 Gy for 109 patients (group A) and 45 Gy for 126 patients (group B). Two or three cycles of chemotherapy were administered. Hyperthermia was given immediately after radiotherapy.
Results: In the HT subgroup of group A, more patients achieved down-staging of T stage when compared to the non-HT subgroup (57.9% versus 38%, p = 0.047). For the cN+ subgroup of all patients, the number of patients with ypN+ were significantly less in the HT subgroup (25% versus 50%, p = 0.022). In group A, HT appeared to reduce distant metastasis, increase disease-free survival, and improve overall survival.
Conclusions: HT seemed to increase the response of both primary tumour and lymph nodes to preoperative radiochemotherapy in patients with locally advanced rectal cancer. The relationship between increased response by HT and survival should be confirmed by a large prospective randomised trial.
Introduction
Adjuvant treatments to improve local control have been developed for patients with locally advanced rectal cancer because local failure occurs in 16%–29% of patients after surgery alone Citation[1]. Radiotherapy (RT) alone was given to patients before or after surgery in the past Citation[2–4]; however, concurrent chemotherapy is currently used to enhance the effect of RT. When compared to preoperative RT alone, preoperative radiochemotherapy (RCT) enhances the pathological response and improves local control in patients with resectable stage II and III rectal cancer Citation[5]. Preoperative RCT followed by total mesorectal excision (TME) is considered the standard therapy based on a German randomised controlled trial Citation[6].
Another strategy to increase local control is to add hyperthermia (HT) to RT alone or RCT. Hyperthermia, as a potent radiosensitiser, has been shown to increase not only response rates but also clinical outcomes to RT in various cancers, except rectal cancer Citation[7]. With respect to rectal cancer, HT has been reported to enhance the efficacy of RT in human colon cancer cells transplanted into nude mice by changing the expression of apoptosis genes Citation[8] and increase the tumour response to RT based on a Cochrane meta-analysis of five randomised controlled trials Citation[9].
The most commonly used agent for adjuvant chemotherapy for rectal cancer, 5-fluorouracil (5-FU), has also been reported to be affected positively by HT Citation[10–12]. Therefore, 5-FU is expected to further increase the effect of RCT when combined with HT in patients with locally advanced rectal cancer. Hyperthermia has been reported to be safely combined with preoperative RCT and increase the tumour response in patients with rectal cancer Citation[13–17], but there have been no reports on the impact on local control or survival rates.
We have combined HT with preoperative RCT for patients with rectal cancer since 1996. The aim of this report was to investigate the effect of HT on the response and survival rates of patients with locally advanced rectal cancer who were treated with preoperative RCT.
Materials and methods
Patients
Between 1996 and 2007, 498 patients were treated with RCT preoperatively at Yeungnam University Medical Centre. Of these patients, there were 235 eligible patients, who had histologically confirmed, locally advanced (cT3-4N0/+) rectal adenocarcinoma. The tumours were located within 10 cm from the anal verge, and curative surgery was performed after the completion of preoperative treatment. To verify the role of HT on preoperative RCT in patients with locally advanced rectal cancer, we used the following exclusion criteria: metachronous or synchronous double primary cancers; rectosigmoid or sigmoid tumours (above 10 cm from the anal verge); early distal rectal tumours (cT1-2N0); distant metastasis at the time of diagnosis or at the time of surgery; incomplete RT; treatment with other chemotherapeutic agents; or patients who declined surgery or who underwent surgery at another hospital.
To exclude ineligible patients, pretreatment staging work-ups were reviewed. Pretreatment computed tomography (CT) images were re-evaluated for cT and cN stage. If CT images were not available, radiological reports at the time of diagnosis were used for staging. Transrectal ultrasonography (TRUS), if performed, was used for T staging. Metastatic lymph nodes were considered to be present if the diameter exceeded 5 mm in the shorter axis. Post-operative pathological stage was assigned according to the American Joint Committee on Cancer (AJCC) 2002 system.
Treatment
Preoperative treatment consisted of RT, chemotherapy, and HT. Whole pelvic irradiation was given using a 3- or 4-field technique with 6 MV or 10 MV photons. Initially, the total dose of RT was 39.6 Gy in 22 daily fractions of 1.8 Gy over a period of 4.5 weeks (N = 109, group A); beginning in 2003 the total dose of whole pelvic irradiation was escalated to 45 Gy in 25 fractions (N = 126, group B).
Two to three cycles of chemotherapy were administered before surgery. First and second cycles of chemotherapy were administered concurrently with RT. All patients received 5-FU and leucovorin; 5-FU (425 mg/m2/day) was continuously infused for 5 days during the first and last weeks of RT, and leucovorin (20 mg/kg) was infused on each day of chemotherapy. Mitomycin C (10 mg/m2) was infused on day 1 in 186 patients; 105 patients in the HT group and 81 patients in the non-HT group.
Hyperthermia was considered in patients referred for preoperative RCT due to locally advanced rectal cancer. Hyperthermia was given to 38 patients in group A and 60 patients in group B. Patients who consented were treated with HT but those who did not consent (N = 29) or who had thick subcutaneous fat tissue (>2 cm) were not (N = 82). Patients who had prior surgery at the abdomen or pelvis (N = 2) or cardiac problems including atrial fibrillation and old myocardiac infarction (N = 3) were not considered for HT. Twenty-one patients of the non-HT subgroup of group A were those who were treated before or around the time of installation of a HT machine.
Hyperthermia was delivered twice a week during RT using an 8-MHz radiofrequency capacitive heating device (Cancermia GHT-RF8; Green Cross Medical, Yongin, Korea). Each hyperthermic session was started immediately after RT and continued for 40–60 min per session; 40 min in 26 patients before 2000, and thereafter 60 min in 72 patients. The median number of HT treatments was 9 (range, 1–11) for all patients, 7 (range, 1–10) for group A, and 9 (range, 1–11) for group B. We used both the 25-cm top and bottom electrodes to cover the radiation field. To prevent skin burn, circulating distilled water in the bolus was cooled to the temperature of 5–10°C but precooling of the skin and subcutaneous tissues was not performed. Applied minimal and maximal powers were 766 ± 207 W (range, 223–1267) and 975 ± 202 W (range, 427–1610). Intrarectal temperature was measured in 72 patients using a thermocouple (Sensor Tech, Trenton, NJ); 37 in group A and 35 in group B. Temperature was measured 1–9 times in each patient and the highest temperatures were 39.5° ± 0.8°C (range, 37.9°–42.4°C) for all patients, 40° ± 0.9°C for group A, and 39.5° ± 0.7°C for group B.
Surgery was planned 4–6 weeks after the completion of RT. The type of surgery was determined by the surgeon and total mesorectal excision was routinely performed. Adjuvant chemotherapy was administered in 224 patients. The same regimen as the preoperative treatment, except mitomycin C, was delivered to 191 patients for up to 11 cycles. Thirty-six patients received oral 5-FU agents. Post-operative RT was given to 11 patients in whom the tumour invaded adjacent organs or the pelvic wall at the time of surgery.
Assessment of response
Complete remission (CR) was defined as the absence of residual tumour cells in the surgical specimen regardless of the presence of mucin pools. Partial remission (PR) was defined as a tumour size reduction of ≥50% when comparing the size of the tumour on the surgical specimen to the pretreatment tumour size, which was measured by the preoperative imaging studies, such as colonoscopy, barium enema, or CT images. Tumours achieving CR or PR were considered to have responded to treatment.
Endpoints and statistical analysis
We analysed the effect of HT on the response rates, failure patterns based on the first failure sites, and survival rates in groups A and B. Local failure was defined as recurrences at the anastomosis site, presacral area, and regional lymphatic area in the pelvic cavity and distant failure as the recurrences outside the pelvic cavity.
Comparisons of patients and tumours characteristics were done with χ2 test, Fisher's exact test, and t-test, as indicated. Overall survival (OS), cancer-specific survival (CSS), disease-free survival (DFS), local relapse-free survival (LRFS), and distant metastasis-free survival (DMFS) rates were calculated from the first day of RCT to the date of the event using the Kaplan-Meier method. The log-rank test was used for the analysis of the factors affecting survival rates. Statistical evaluations were performed using the PASW statistics 18 software (SPSS, Chicago, IL). A P value <0.05 was considered as significant.
Results
Patient and tumour characteristics before and after RCT
For all patients the median age was 60 years (range, 18–83 years) and 137 (58.3%) were men. The mean tumour size was 5.1 ± 1.9 cm in 218 patients in whom the tumour size was measurable. The clinical stage was cT3N0 for 152 (64.7%), cT3N+ for 75 (31.9%), cT4N0 for 3 (1.3%), and cT4N+ for 5 patients (2.1%).
shows the distribution of various characteristics before RCT according to HT and the total dose of RT. Males were more prevalent in the HT subgroup (p < 0.001), and in each group. The age, cT stage, and mean tumour size were not different according to HT. The cN stage did not differ in all patients and group B, but the HT subgroup of group A had more cN+ stage patients (p = 0.065).
Table I. Patient and tumour characteristics before preoperative radiochemotherapy.
shows the distribution of various characteristics after RCT. None of the characteristics, except the ypT stage in the HT subgroup of group A, differed according to HT.
Table II. Patient and tumour characteristics after preoperative radiochemotherapy.
Response and survival rates
The median follow-up period was 63 months (range, 2–172 months) for all patients. To the date of last follow up 71 patients (30.2%) died and 55 patients (23.4%) developed recurrences, including 13 patients (5.5%) with local failures and 45 patients (19.1%) with distant metastases. The OS, CSS, DFS, LRFS, and DMFS were 73.9%, 83.0%, 75.1%, 93.9%, and 79.8% at 5 years, respectively.
shows the relationships between the tumour response with the changes in T and N stage. Tumour which responded to treatment showed a higher down-staging rate of T stage in all patients (p = 0.013) and a lower rate of pN+ in cN0 patients (p = 0.03). However, tumour response was not related to the change of pN stage in cN+ patients.
Table III. Relationships between the tumour response with the changes in T and N stage.
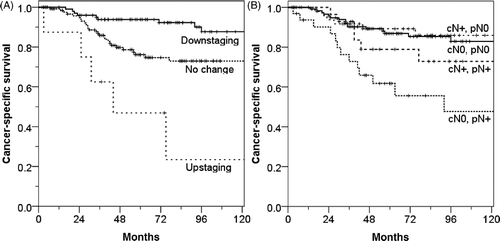
Post-operative AJCC stage and the changes in T and N stage were significantly related to OS, CSS, DFS, and DMFS (p < 0.05). LRFS curves also showed similar pattern although they were not statistically significant. shows CSS curves according to the changes of T and N stage. However, tumour response was not related to any survival rates.
Response according to hyperthermia
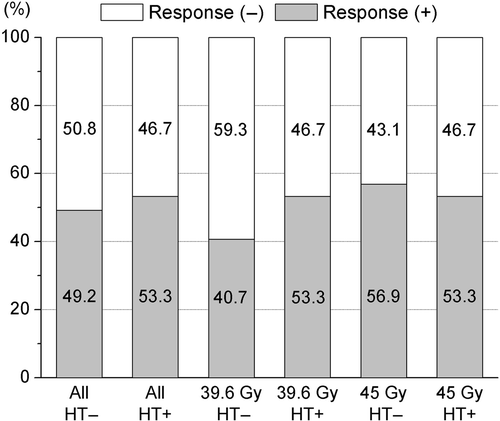
The tumour response rate was 50.9% for the 214 evaluable patients; the tumour size of 21 patients was not described in the pathological reports. shows the response rate of each group. The response rate of group B (69/125, 55.2%) was higher than group A (40/89, 44.9%). However, the HT subgroup of group A achieved a similar response rate (16/30, 53.3%) as group B, while the non-HT subgroup of group A had the lowest response rate (24/59, 40.7%).
Figure 2. Response (CR+PR) rate of tumor size according to hyperthermia (HT) and a total dose of radiotherapy. The overall response rate was 50.6% for the 214 evaluable patients.
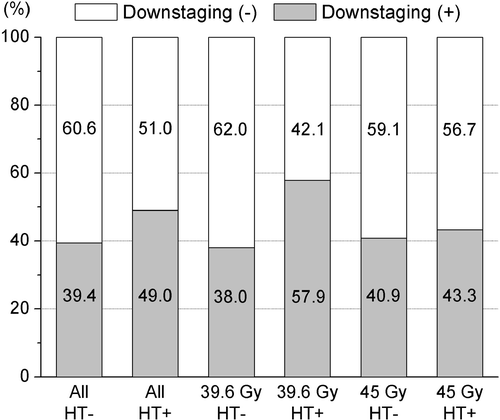
The overall down-staging rate of the T stage was 43.4% for all 235 patients. shows the down-staging rate for each group. HT significantly increased the down-staging rate of the T stage in group A (p = 0.047), but not in group B.
Figure 3. Downstaging rates of T stage. The overall downstaging rate was 43.4%. Hyperthermia (HT) significantly increased the downstaging rate in the 39.6 Gy group (p = 0.047), but not in the 45 Gy group.
The changes in lymph node status are shown in . Patients with cN0 had ypN+ in 20.6% of all patients and the change from cN0 to ypN+ was not influenced by adding HT. However, HT significantly down-staged lymph node status (p = 0.022) and the rates of ypN0 were higher by 20%–30% in the HT subgroup in groups A and B.
Table IV. Changes in lymph node status after preoperative radiochemotherapy according to hyperthermia.
Survival rates according to hyperthermia
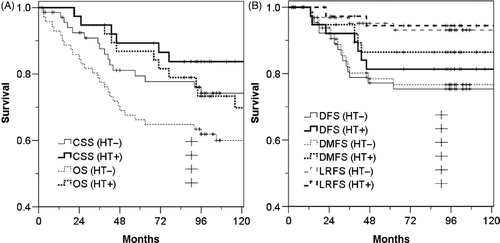
For group A, the median follow-up period was 102 months (range, 2–173 months). The OS, CSS, DFS, LRFS, and DMFS were 73.4%, 83.1% 78.8%, 94.8%, and 81.6% at 5 years, respectively. The curves for OS, CSS, DFS, and DMFS in the HT subgroup remained above of the corresponding curves in the non-HT subgroup, but LRFS was not affected by HT ().
Figure 4. Survival curves of the 39.6 Gy group according to hyperthermia (HT). (A) Overall survival (OS, p = 0.103) and cancer-specific survival (CSS, p = 0.254), and (B) disease-free survival (DFS, 0.471), local relapse-free survival (LRFS, 0.785) and distant metastasis-free survival (DMFS, p = 0.236).
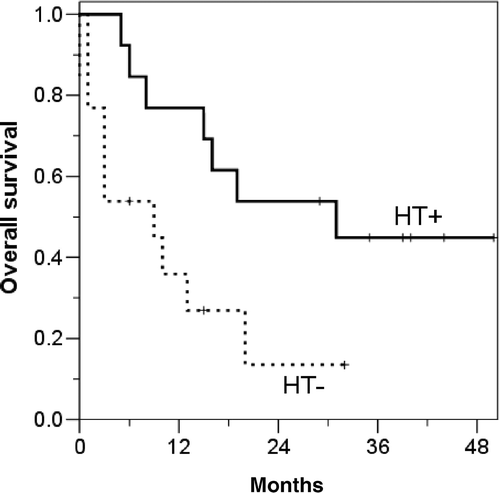
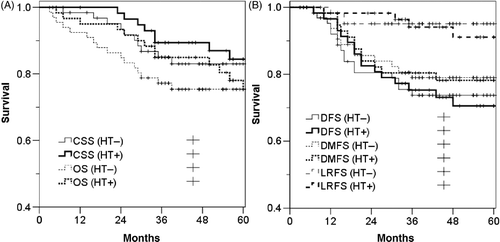
For group B, the median follow-up period was 53 months (range, 3–93 months). The OS, CSS, DFS, LRFS, and DMFS at 5 years were 74.4%, 83.2%, 72.1%, 93.1%, and 78.5%, respectively. The curves for OS and CSS in the HT subgroup remained above the corresponding curves in the non-HT subgroup, but other survival rates were not affected by HT (). However, among 26 patients who experienced distant metastasis, patients previously treated with HT significantly survived longer after the detection of distant metastasis when compared to patients who had never been treated with HT (, p = 0.026).
Figure 5. Survival curves of the 45 Gy group according to hyperthermia (HT). (A) Overall survival (OS, p = 0.598) and cancer-specific survival (CSS, p = 0.682), and (B) disease-free survival (DFS, p = 0.799), local relapse-free survival (LRFS, p = 0.688) and distant metastasis-free survival (DMFS, p = 0.944).
Discussion
Hyperthermia has been reported to enhance the response to preoperative RCT in patients with rectal cancer. Furuta et al. Citation[13] evaluated the effect of adding HT to preoperative RCT (40.5 Gy and a 5-FU suppository) in patients with lower rectal cancer; the study revealed that patients treated with HT achieved better response rates with respect to tumour size reduction on barium enema, the ratio of residual tumour, and the argyrophilic nucleolar organizer regions (AgNOR) score of the surgical specimen. Our results also showed that the down-staging rates of T and N stage in the HT subgroup were significantly higher for group A and all patients, respectively.
Comparing the distributions of T and N stage before and after treatment clarifies the effect of HT. In group A, while most patients had cT3 before preoperative RCT, more patients in the HT subgroup achieved a higher down-staging rate of T stage with a difference of 20% when compared to the non-HT subgroup (57.9% versus 38%, p = 0.047) and gained a higher rate of ypT0-2 (55.3% versus 38%, p = 0.064; and ). With respect to the change in lymph node status in all patients, the rate of cN+ was similar between the HT and non-HT subgroups; the HT subgroup achieved a significantly lower rate of ypN+ ().
Although tumour response to preoperative RCT was better in the HT subgroup, local control rate was not different according to the use of HT in the current study. Since a considerable reduction in local recurrence rates to <10% has been accomplished with advances in surgical technique and adjuvant RCT in patients with locally advanced rectal cancer Citation[5], Citation[6], there might be little room for improvement with local control by HT. However, based on the facts that overall or disease-free survival rates of male patients with primary or recurrent rectal cancer were worse, and the narrow male pelvis hinders securing a wide resection margin or applying enough large applicators for intraoperative radiotherapy, male patients may have more risk of pelvic recurrence Citation[18–20]. Although the proportion of male patients in the HT group were about twice that of the non-HT group, the rates of local control at 10 years were 87.1% for the non-HT group and 93.9% for the HT group. So, even though it was not statistically significant, it is possible for HT to have had a positive impact on local control. In addition, HT may still be a good option for certain patients who have locally advanced, unresectable, or recurrent rectal cancer in whom it is not easy to perform a R0 resection Citation[14], Citation[16].
In contrast to the misunderstanding that HT may facilitate distant metastasis by increasing tumour blood flow, HT did not increase distant metastasis in the current study. In group A, reduced distant metastasis seemed to translate into a better DFS, CSS and OS; the non-significant difference might be due to the small sample size. Such benefits of HT may have contributed to down-staging of N stage since ypN status after preoperative RCT is closely related to distant metastasis Citation[21]. In this case regional lymph nodes as well as the primary tumour are the targets of HT. In group B, CSS of the HT subgroup seemed to be better, although DFS was not different according to the use of HT. This discrepancy may be caused by the fact that the patients in the HT subgroup significantly survived longer after the detection of distant metastasis than those in the non-HT subgroup (). Although we cannot ignore the possibility of selection bias, this might be a result of increased host immunity by HT. Recently, mild HT has been reported to have a positive effect on anti-tumour immunity by increasing the activity of immune effector cells including T cells, NK cells and macrophages Citation[22].
Hyperthermia, as a chemosensitiser, has the potential to improve the effect of chemotherapy combined with preoperative RT. Maeta et al. Citation[12] showed that mild HT could enhance the uptake of 5-FU and the rate of conversion to reactive metabolites. The intracellular uptake of 5-FU increased two- to five-fold at 39°–42°C when compared to 37°C, and was greatest at 39°C. The production of active metabolites was also enhanced through the up-regulation of thymidylate synthase activity, which was greatest at 39°C, but inhibited at 42°C. On the basis of these results, Anscher et al. Citation[16] administered a continuous 5-FU (250 mg/m2/day 7 days per week) infusion throughout RT with weekly HT, with a target T90 (temperature achieved by 90% of measured points) of 39°–40°C in patients with locally advanced, unresectable, or recurrent rectal cancer. Therefore, this kind of treatment deserves to be investigated.
Our measured intrarectal temperature was 39.5° ± 0.8°C (range, 37.9°–42.4°C), which can be classified as mild HT. As it is difficult to obtain temperatures above 42.5°C in the clinical setting, there have been many studies on the changes of the tumour microenvironment after mild HT (temperature range, 39.5°–42.5°C) Citation[23]. Some reported that the increases of tumour blood flow and tumour oxygenation persisted for 24–48 h after mild HT Citation[24–27]. Furthermore, Thrall et al. Citation[28] found that an increase in oxygenation in tumours persisted during a course of fractionated thermoradiotherapy in a canine sarcoma.
Although invasive intra-tumoural temperature measurement is recommended, it is not easy to perform on every patient Citation[29]. Instead, a minimally invasive technique using endoluminal thermometry catheters placed in the rectum, bladder, and vagina has been used for patients with rectal cancer Citation[17], Citation[30]. The thermal parameters, T90 and cumulative minutes with T90 ≥ 40.5°C, were significantly related to the tumour response to preoperative hyperthermic radiochemotherapy in one study, and T90 and Tmax (maximal temperature achieved at each HT session) were related to the tumour response in another study Citation[17], Citation[30]. In the current study, the effects of HT on responses and survival rates were better in group A than in group B and the mean value of highest temperature was higher in group A. However, since the temperature difference between groups A and B was not significant and measurements were not performed in all HT sessions, we cannot draw any conclusions. Therefore, the temperature needs to be measured in all HT sessions to analyse the relationship between temperature and clinical outcomes.
In summary, HT appeared to increase the response of the primary tumour and lymph nodes to preoperative RCT in patients with locally advanced rectal cancer, and patients with down-staging showed better survival rates. However, the relationship between increased response by HT and survival should be confirmed by a large prospective randomised trial.
Declaration of interest: This research was supported by Yeungnam University research grants in 2008. The authors alone are responsible for the content and writing of the paper.
References
- McCall JL, Cox MR, Wattchow DA. Analysis of local recurrence rates after surgery alone for rectal cancer. Int J Colorectal Dis 1995; 10: 126–132
- Party MRCRCW. Randomised trial of surgery alone versus surgery followed by radiotherapy for mobile cancer of the rectum. Medical Research Council Rectal Cancer Working Party. Lancet 1996; 348: 1610–1614
- Pahlman L, Glimelius B. Pre- or postoperative radiotherapy in rectal and rectosigmoid carcinoma. Report from a randomized multicenter trial. Ann Surg 1990; 211: 187–195
- Trial SRC. Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med 1997; 336: 980–987
- Ceelen WP, Van Nieuwenhove Y, Fierens K. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev 2009; CD006041
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–1740
- Horsman MR, Overgaard J. Hyperthermia: A potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol) 2007; 19: 418–426
- Liang H, Zhan HJ, Wang BG, Pan Y, Hao XS. Change in expression of apoptosis genes after hyperthermia, chemotherapy and radiotherapy in human colon cancer transplanted into nude mice. World J Gastroenterol 2007; 13: 4365–4371
- De Haas-Kock DF, Buijsen J, Pijls-Johannesma M, Lutgens L, Lammering G, van Mastrigt GA, De Ruysscher DK, Lambin P, van der Zee J. Concomitant hyperthermia and radiation therapy for treating locally advanced rectal cancer. Cochrane Database Syst Rev 2009; CD006269
- Kido Y, Kuwano H, Maehara Y, Mori M, Matsuoka H, Sugimachi K. Increased cytotoxicity of low-dose, long-duration exposure to 5-fluorouracil of V-79 cells with hyperthermia. Cancer Chemother Pharmacol 1991; 28: 251–254
- Matsuoka H, Sugimachi K, Abe R, Ueo H, Akiyoshi T. Enhancement of cytotoxicity by hyperthermia after a long-term culture with 5-fluorouracil in transformed cells. Anticancer Res 1992; 12: 1621–1625
- Maeta M, Sawata T, Kaibara N. Effects of hyperthermia on the metabolism of 5-fluorouracil in vitro. Int J Hyperthermia 1993; 9: 105–113
- Furuta K, Konishi F, Kanazawa K, Saito K, Sugawara T. Synergistic effects of hyperthermia in preoperative radiochemotherapy for rectal carcinoma. Dis Colon Rectum 1997; 40: 1303–1312
- Rau B, Wust P, Hohenberger P, Loffel J, Hunerbein M, Below C, Gellermann J, Speidel A, Vogl T, Riess H, et al. Preoperative hyperthermia combined with radiochemotherapy in locally advanced rectal cancer: A phase II clinical trial. Ann Surg 1998; 227: 380–389
- Wust P, Rau B, Gellerman J, Pegios W, Loffel J, Riess H, Felix R, Schlag PM. Radiochemotherapy and hyperthermia in the treatment of rectal cancer. Recent Results Cancer Res 1998; 146: 175–191
- Anscher MS, Lee C, Hurwitz H, Tyler D, Prosnitz LR, Jowell P, Rosner G, Samulski T, Dewhirst MW. A pilot study of preoperative continuous infusion 5-fluorouracil, external microwave hyperthermia, and external beam radiotherapy for treatment of locally advanced, unresectable, or recurrent rectal cancer. Int J Radiat Oncol Biol Phys 2000; 47: 719–724
- Maluta S, Romano M, Dall'oglio S, Genna M, Oliani C, Pioli F, Gabbani M, Marciai N, Palazzi M. Regional hyperthermia added to intensified preoperative chemo-radiation in locally advanced adenocarcinoma of middle and lower rectum. Int J Hyperthermia 2010; 26: 108–117
- Blumberg D, Paty PB, Picon AI, Guillem JG, Klimstra DS, Minsky BD, Quan SH, Cohen AM. Stage I rectal cancer: Identification of high-risk patients. J Am Coll Surg 1998; 186: 574–579, discussion: 579–580
- Reerink O, Mulder NH, Botke G, Sluiter WJ, Szabo BG, Plukker JT, Verschueren RC, Hospers GA. Treatment of locally recurrent rectal cancer, results and prognostic factors. Eur J Surg Oncol 2004; 30: 954–958
- Diaz-Gonzalez JA, Calvo FA, Cortes J, Garcia-Sabrido JL, Gomez-Espi M, Del Valle E, Munoz-Jimenez F, Alvarez E. Prognostic factors for disease-free survival in patients with T3-4 or N+ rectal cancer treated with preoperative chemoradiation therapy, surgery, and intraoperative irradiation. Int J Radiat Oncol Biol Phys 2006; 64: 1122–1128
- Bujko K, Michalski W, Kepka L, Nowacki MP, Nasierowska-Guttmejer A, Tokar P, Dymecki D, Pawlak M, Lesniak T, Richter P, et al. Association between pathologic response in metastatic lymph nodes after preoperative chemoradiotherapy and risk of distant metastases in rectal cancer: An analysis of outcomes in a randomized trial. Int J Radiat Oncol Biol Phys 2007; 67: 369–377
- Muthana M, Multhoff G, Pockley AG. Tumour infiltrating host cells and their significance for hyperthermia. Int J Hyperthermia 2010; 26: 247–255
- Vaupel PW, Kelleher DK. Pathophysiological and vascular characteristics of tumours and their importance for hyperthermia: Heterogeneity is the key issue. Int J Hyperthermia 2010; 26: 211–223
- Shakil A, Osborn JL, Song CW. Changes in oxygenation status and blood flow in a rat tumor model by mild temperature hyperthermia. Int J Radiat Oncol Biol Phys 1999; 43: 859–865
- Vujaskovic Z, Poulson JM, Gaskin AA, Thrall DE, Page RL, Charles HC, MacFall JR, Brizel DM, Meyer RE, Prescott DM, et al. Temperature-dependent changes in physiologic parameters of spontaneous canine soft tissue sarcomas after combined radiotherapy and hyperthermia treatment. Int J Radiat Oncol Biol Phys 2000; 46: 179–185
- Song CW, Park H, Griffin RJ. Improvement of tumor oxygenation by mild hyperthermia. Radiat Res 2001; 155: 515–528
- Viglianti BL, Lora–Michiels M, Poulson JM, Lan L, Yu D, Sanders L, Craciunescu O, Vujaskovic Z, Thrall DE, Macfall J, et al. Dynamic contrast-enhanced magnetic resonance imaging as a predictor of clinical outcome in canine spontaneous soft tissue sarcomas treated with thermoradiotherapy. Clin Cancer Res 2009; 15: 4993–5001
- Thrall DE, Larue SM, Pruitt AF, Case B, Dewhirst MW. Changes in tumour oxygenation during fractionated hyperthermia and radiation therapy in spontaneous canine sarcomas. Int J Hyperthermia 2006; 22: 365–373
- Wust P, Cho CH, Hildebrandt B, Gellermann J. Thermal monitoring: Invasive, minimal-invasive and non-invasive approaches. Int J Hyperthermia 2006; 22: 255–262
- Rau B, Wust P, Tilly W, Gellermann J, Harder C, Riess H, Budach V, Felix R, Schlag PM. Preoperative radiochemotherapy in locally advanced or recurrent rectal cancer: Regional radiofrequency hyperthermia correlates with clinical parameters. Int J Radiat Oncol Biol Phys 2000; 48: 381–391