Abstract
Purpose: Hyperthermia-induced apoptosis is mediated by mitochondrial pathway, and is temporally correlated with alterations in mitochondrial morphology in neuroepithelial cells. In addition, regular exercise up-regulates heat shock proteins (HSPs) that inhibit apoptosis. However, embryo-protective effects of maternal exercise against heat exposure during pregnancy have not been fully understood yet.
Materials and methods: To investigate the role of maternal exercise in protecting embryos from hyperthermia, we measured apoptosis-related factors and HSPs in Hsp70 knockout mouse embryos. Pregnant mice were divided into control, exercise, hyperthermia-after-exercise, and hyperthermia groups. Where appropriate the swimming exercise was performed for 5–10 min/day from embryonic day (ED) 1 to ED 8, and hyperthermia (43°C, 5 min) was induced on ED 8. To characterise the effects of maternal exercise on apoptosis-related factors and HSPs, we performed western blotting and transmission electron microscopy.
Results: Caspase-9, -7, -3 and Bax were down-regulated in the hyperthermia-after-exercise group and Bcl-2, Hsp27 and Hsp110 were up-regulated. The number of apoptotic cells was markedly reduced in the hyperthermia-after-exercise group.
Conclusions: Maternal exercise plays an important role in inhibiting apoptotic cell death in embryos against hyperthermic exposure during pregnancy.
Introduction
Several groups have reported that maternal hyperthermia gives rise to various kinds of congenital malformations. It is well known that hyperthermia-induced embryonic anomalies resulting from apoptotic cell death are closely related to activation of the cytoplasmic caspase cascade Citation[1], Citation[2]. This cellular response involves the mitochondrial pathway, and initiator and effector caspases are implicated in it [3, Citation[4]. Thus, apoptotic signals induced by several teratogens, including hyperthermia, act on mitochondria to induce the release of cytochrome c, which activates Apaf-1 to form apoptosomes. Procaspase-9 is then cleaved and activated to form caspase-9, which activates downstream effector caspase precursor, procaspase-3 Citation[1]. Conversely, Bcl-2 from mitochondria inhibits the release of cytochrome c and Bax and inactivates the caspase cascade, so preventing stress-induced apoptosis Citation[5], Citation[6]. Heat shock proteins (HSPs) function as molecular chaperones and are classified according to their molecular weight into 27, 60, 70, 90 and 110 kDa families, and, among them, Hsp70 is induced by stimuli such as hypoxia, cellular damage, hyperthermia and oxidative stress Citation[7]. The induction of Hsp70 expression protects embryos/fetuses from congenital abnormalities in response to maternal hyperthermia Citation[3]. Embryos lacking the Hspa1a and Hspa1b genes, which are forms of 70 kDa Hsp genes (formerly known as Hsp70) are significantly more sensitive to hyperthermia-induced neural tube and eye defects, and this increased sensitivity is correlated with increased levels of apoptosis Citation[8]. However, biochemical alterations brought about by HSPs and anti-apoptotic factors in response to hyperthermia have not been fully defined.
Physical exercise is known to reduce tissue damage by up-regulating Hsp70 independently of other stressors Citation[9], Citation[10]. Exercise is also beneficial in protecting against oxidative damage by inducing Hsp70 in humans Citation[11] and animals Citation[12], and in reducing apoptosis Citation[13]. Osorio et al. Citation[14] reported that physical exercise in 35°C water during pregnancy was effective in activating antioxidant mechanism, and Lee et al. Citation[15] reported that maternal exercise enhanced short-term memory and neurogenesis in the hippocampus of rat pups. During pregnancy, physical exercise can increase the resting maternal (and perhaps foetal) plasma volume, intervillous space blood volume, cardiac output and placental function Citation[16]. Swimming during pregnancy has been reported to improve maternal strength and foetal health without risk of damage, and is thus recommended Citation[17]. However, the embryo-protective role of maternal exercise, especially in Hsp70 knockout mice (KO), has not been fully investigated. Moreover, the changes in expression of apoptotic and anti-apoptotic factors caused by maternal exercise have not been characterised in Hsp70 KO embryos exposed to hyperthermia.
In the present study we investigated the role of maternal exercise in protecting their offspring against hyperthermic exposure by identifying the changes of apoptotic and anti-apoptotic factors induced in embryos by maternal hyperthermia after exercise in Hsp70 KO mice. In addition we studied the embryo-protective roles of other HSPs in the absence of Hsp70. We also characterised the expression of caspase-9, -7, -3, the apoptosis-related factors, Bax and Bcl-2, and HSPs in embryonic heads by western blotting after 8 days of exercise, and observed morphological changes by transmission electron microscopy.
Materials and methods
Animals and exercise protocol
We used primigravida C57BL/6 strain Hsp70 KO mice (a gift from Dr. David J. Dix at the Research Triangle Park, North Carolina) as experimental animals. The animal care and experimental procedures were approved by the Animal Care Committee of Hanyang University. Time-mated pregnant mice were obtained by caging two female and three male mice overnight. The morning following copulation was designated day 0 of gestation where vaginal plugs were seen. The pregnant mice were subdivided into 4 groups of five mice: control, exercise, hyperthermia-after-exercise, and hyperthermia. The mice swam in a water chamber (diameter 150 cm, height 70 cm, water temperature 35°C) from embryonic day (ED) 1 to ED 8. To allow them to adapt to the swimming, exercise duration was gradually increased from 5 min to 10 min.
Maternal hyperthermia and embryo collection
Where appropriate the time-mated pregnant mice were exposed to hyperthermia following 1 h of rest after the final exercise period at noon on ED 8. They were placed in a 50 mL perforated Falcon tube, and dipped into a 43°C water bath. Using digital thermometer with 2 probes, one probe was inserted into the mouse rectum, and the other dipped in the water bath to check the water temperature during treatment. Once body temperature reached 43°C, the pregnant mouse was maintained at that temperature for a further 5 min. To prevent hypothermia after the heat treatment, the mice were placed in the 38°C incubator immediately after drying with a paper towel. The control and exercise groups also received rectal probes and were dipped for 5 min in a 38°C water bath and placed in a 38°C incubator. The heads of embryos in all groups were collected on ED 8.5. Before preparing their tissues, the embryos were photographed under a stereoscope (Leica M10, Nussloch, Germany) with a digital camera (Nikon D100, Tokyo, Japan).
Western blotting
The heads of embryos on ED 8.5 were homogenised in lysis buffer (Cell Signaling Technology, Danvers, MA) with PMSF at 4°C and centrifuged (13,000× g). The protein content of each sample was determined by the Bradford method Citation[18] with bovine serum albumin as a standard. Protein samples (35µg) were boiled with 5×sample buffer, electrophoresced on polyacrylamide gels, and transferred to a nitrocellulose membrane at 15V over night. The membrane was washed and blocked, and incubated with antibodies to detect caspase-9, -7, -3, Bax and Bcl-2 (1 : 1000; Cell Signaling Technology, Danvers, MA) and Hsp27, Hsp70 and Hsp110 (1 : 1000; Stressgen, Ann Arbor, MI) for 12 h at 4°C. HRP-linked secondary antibody (1 : 5000; Santa Cruz Biotechonology, Santa Cruz, CA) was added for 1 h at room temperature. The membranes were washed and visualised by autoradiography after development with ECL Plus Kit (GE Healthcare Bio-Sciences, Piscataway, NJ). β-actin was used as internal control. Densitometry was performed with gel documentation equipment (Gel Doc 2000, Quantity One, Bio-Rad, Hercules, CA).
Transmission electron microscopy
Tissues were fixed in cold 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. After 4 h of fixation, specimens were post-fixed in 1% OSO4 and dehydrated in graded alcohols. Decreasing concentrations of propylene oxide and increasing Embed 812 (Electron Microscopy Services, Fort Washington, PA) were used. After embedding in pure fresh resin and polymerisation, sections were initially cut 1 µm thick and stained with toluidine blue. Ultrathin sections (60 to 80 nm thick) were made through the prosencephalon, stained with uranyl acetate and lead citrate, and observed with a transmission electron microscope (Hitachi S7600, Tokyo, Japan) at 80 kV.
Statistical analysis
All data were expressed as mean ± SEM and analysed by two-way ANOVA (GraphPad Santiago, CA) using the procedures in SPSS software (SPSS 12.0, Chicago, IL) with Bonferroni post tests; p < 0.05 was considered as statistically significant.
Results
Effects of maternal exercise on survival of ED 8.5 embryos
The absence of a heart beat or the presence of an absorbed gestational sac was taken as indicating a non-living embryo. As shown in , exposure to hyperthermia on ED 8 in Hsp 70 KO embryos reduced survival to 59.2% (p < 0.001) of that of the control group (100%), and maternal exercise combined with hyperthermia increased this to 75.2% (p < 0.05).
Table 1. Effect of regular exercise on embryo survival after maternal hyperthermia, % living embryos/total embryos.
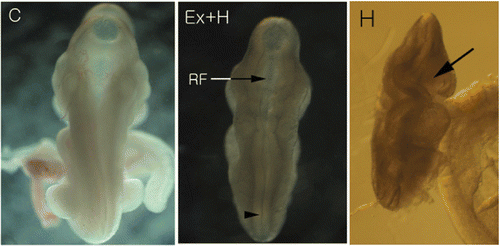
External appearance of ED 8.5 embryo
The control embryo had well-rounded contour and neural tubes was closed. The roof of the rhombencephalon had expanded to cover the typical diamond-shaped rhombencephalic fossa, and well-developing prosencephalons and diencephalons were seen through the rhombencephalic roof. Although the roof of rhombencephalons was not so wide, the general morphology of the hyperthermia-after-exercise group embryo was similar to that of the control embryo. However, the neural tubes of hyperthermia group was open ().
Figure 1. External appearance of ED 8.5 embryos. In the control embryo (C), prosencephalon and diencephalon can be seen through the greatly expanded rhombencephalic roof. Although the roof of its rhombencephalon (RF) is not expanded, the general morphology of the hyperthermia-after-exercise embryo (Ex+H) is similar to that of the control embryo. In addition, distal parts of the neural tube (arrow head) are close to each other. However, the neural tube of the hyperthermia embryo (H) is open (arrow).
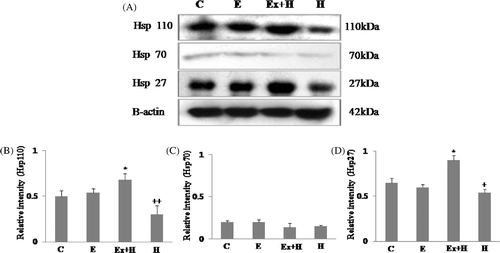
Effects of maternal exercise on expressions of heat shock proteins
Heat shock proteins 27, 70 and 110 were examined. Expression of Hsp27 and Hsp110 was significantly increased in the hyperthermia-after-exercise group compared to the hyperthermia group, and expression of Hsp70 was very limited ().
Figure 2. Western blot analysis of Hsp27, Hsp70 and Hsp110 (A). Hsp27 and Hsp110 proteins are highly expressed in the hyperthermia-after-exercise group compared to the hyperthermia group. Densitometric analyses of western blots for Hsp110 (B), Hsp70 (C) and Hsp27 (D) are shown. Data are means ± SEM (n = 32 (C), 30 (E), 18 (Ex + H), 14 (H)). +p < 0.05 comparing the hyperthermia group and the control group of embryos; ++p < 0.001 comparing the hyperthermia group and the control group of embryos; *p < 0.05 comparing the hyperthermia-after-exercise group and the control group. C, control group; E, exercise group; Ex+H, hyperthermia-after-exercise group; H, hyperthermia group.
Effects of maternal exercise on the expression of apoptosis-related proteins
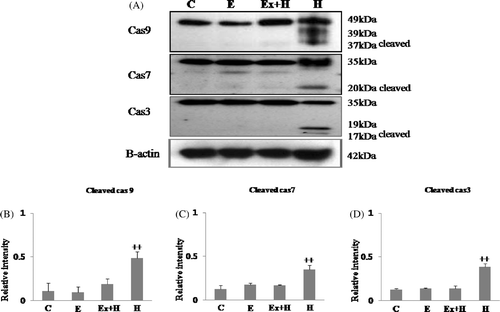
Bax expression was higher in the hyperthermia group than the other groups, whereas it was similar to the control group in the hyperthermia-after-exercise group. Expression of the anti-apoptotic protein, Bcl-2, was significantly reduced in the hyperthermia group. In contrast, the hyperthermia-after-exercise group showed significantly more Bcl-2 expression than the hyperthermia group (). In this study we detected both full-length pro-enzyme and the cleaved active fragments of caspase-9, -7 and -3. Our results showed that maternal exercise substantially decreased the expression of caspase-9, -7 and -3. Procaspase-9, -7 and -3 were detected in all the embryos examined; however, active cleaved caspase-9, -7 and -3 activities were clearly greater in the hyperthermia group than the other groups, and activations of cleaved procaspase-9, -7 and -3 were not detected in the hyperthermia-after-exercise group ().
Figure 3. Western blot analysis of Bcl-2 and Bax (A). The hyperthermia-after-exercise group shows significant up-regulation of Bcl-2 protein compared to the hyperthermia group whereas the opposite is true for Bax. Densitometric analyses of western blots of Bcl-2 (B) and Bax (C) are shown. Data are means ± SEM (n as indicated in ). +p < 0.05 comparing the hyperthermia group with the control group; ++p < 0.001 comparing the hyperthermia group with the control group. C, control group; E, exercise group; Ex+H, hyperthermia-after-exercise group; H, hyperthermia group.
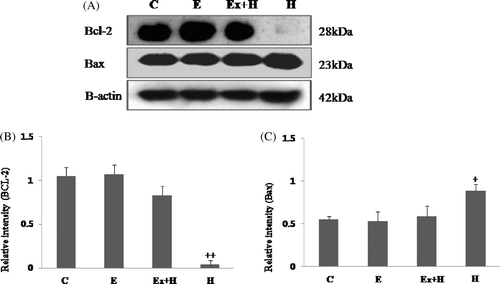
Figure 4. Western blot analysis of cleaved active caspase-9, caspase-7 and caspase-3 (A). Cleaved caspase-9, caspase-7, caspase-3 are up-regulated in the hyperthermia group and down-regulated in the hyperthermia-after-exercise group. Densitometric analyses of western blots of cleaved caspase-9 (B), caspase-7 (C), and caspase-3 (D) are shown. Data are means ± SEM (n as indicated in ). ++p < 0.001 comparing the hyperthermia group and the control group.
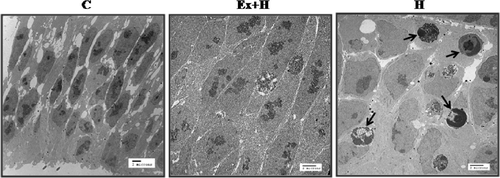
Transmission electron microscopic findings
To examine the effect of maternal exercise on the developing prosencephalon after hyperthermic exposure morphologically, we used transmission electron microscopy. Many apoptosomes were observed in the hyperthermia group and their numbers were diminished in the hyperthermia-after-exercise group ().
Discussion
Previous animal experiments have shown that hard physical exercise during pregnancy results in reduced uterine blood flow and glucose delivery, and increased foetal temperature Citation[19], Citation[20]. The increased central core temperature can in turn affect cell division in the embryo, resulting in congenital malformations. However, low-impact exercise such as swimming during pregnancy does not increase foetal heart rate or maternal body temperature Citation[21], Citation[22]. In one large prospective study with 158 active women (runners, aerobics practitioners, etc.) no association was found between exercise and risk of abortion, congenital malformation or a variety of foeto-maternal problems Citation[16]. In addition, swimming in cool water (34.6° ± 0.4°C) did not affect foetal rat development and resulted in no congenital abnormalities Citation[23]. Based upon these results we made the experimental animals swim in a 35°C water bath for a period of 5 min increasing to 10 min each day for 8 days from ED 1 to ED 8. Maternal exercise such as swimming improves placental function and enhances HSP expression Citation[12], Citation[16]. In addition, it protects against cell death from DNA damage by up-regulation of Hsp70 and Hsp27 through p38 signalling pathway Citation[24], Citation[25]. In the present study we explored the effects of maternal swimming exercise on the expression of HSPs. Because HSP induction lasts only about 24 h, after which normal protein synthesis resumes, we killed the mice and collected embryos on ED8.5. Our results showed that Hsp27 and Hsp110 expression was higher in the hyperthermia-after-exercise group than the hyperthermia group. This suggests that Hsp27 and Hsp110 play a role in protecting embryos from maternal hyperthermia.
Although it is well known that hyperthermia is damaging and induces apoptosis, defences against hyperthermia are not fully understood. Once cells are exposed to several stressors including hyperthermia, heat shock protein genes such as Hsp70 are expressed. Hsp70 functions as a chaperone to inhibit apoptosis Citation[26] by blocking release of Bax from mitochondria, inhibiting the formation of apoptosome complex, activation of Bid, and migration of AIF from mitochondria to the nucleus Citation[8]. Other heat shock proteins such as Hsp27 and Hsp110 are also induced in response to various stresses. Hsp27 is an ATP-independent chaperone, functioning especially in protection against protein aggregation Citation[27]. Exposure of cells to high temperature (43°C for 3 h) stimulates expression of Hsp25/27 and induces a thermoresistant state Citation[28]. Likewise, Hsp110 is regulated by a specific set of stress conditions, most notably hyperthermia Citation[29]. It also confers thermal tolerance to cells when overexpressed, and can prevent the aggregation of denatured proteins in vitro Citation[30]. In the present work, we showed that maternal exercise increased the expressions of Hsp27 and Hsp110 in response to subsequent hyperthermia after exercise. This result suggests that Hsp27 and Hsp110 act in a similar way to Hsp70, as discussed previously Citation[29], Citation[31]. Although we do not know the specific embryo-protective effects of these HSPs, survival in the hyperthermia-after-exercise group (75.2 ± 5.4%) was better than in the hyperthermia group (59.2 ± 6.2%). Therefore Hsp27 and Hsp110 may play an important role in embryo protection in the absence of Hsp70.
Mitochondria-mediated apoptosis is largely dependent on a balance between Bax and Bcl-2. If this balance is disrupted by stresses, cytochrome c is released from mitochondria, the activity of Bax increases, caspases are cleaved and activated and induce apoptosis Citation[32]. Cytochrome c and Bax cooperate to bind Apaf-1 to procaspase-9 to form apoptosomes in the cytoplasm. Apoptosome formation leads to activation of caspase-9 followed in succession by caspase-7 and caspase-3 Citation[33], Citation[34]. Bcl-2, an anti-apoptotic protein, regulates apoptotic signalling by preventing cytochrome c release and inhibiting downstream caspase activation Citation[5]. It also plays a central role in the delivery of apoptotic signals to the mitochondria in stress-induced apoptosis Citation[35]. Maternal exercise inhibits apoptosis by increasing Bcl-2 and decreasing Bax in rats Citation[36]. In addition, exercise increases levels of mitochondrial enzymes regulating oxidative metabolism in mice Citation[37], and improves antioxidant defence against reactive oxygen species (ROS) produced by stress Citation[38], Citation[39]. Mitochondria exposed to hyperthermia produce ROS which induce apotosis Citation[40]. Because Hsp70.1 deficiency inhibits the expression of antioxidant enzymes such as superoxide dismutases (SODs) 1 and 2 Citation[41], and the production of ROS activates downstream of cytochrome c and upstream of caspase-3, apoptotic cell death occurs Citation[42]. To identify the embryo-protective effects of maternal exercise before hyperthermia, we studied the expression of apoptotic and anti-apoptotic factors by western blotting. Expression of Bax was down-regulated in the hyperthermia-after-exercise group compared to the hyperthermia group, and expression of the anti-apoptotic factor Bcl-2 was significantly greater in the hyperthermia-after-exercise group than in the hyperthermia group. In addition, although the level of active cleaved caspase-9, the initiator caspase, was high in the hyperthermia group, cleaved caspase-7 and -3 (effector caspase) were significantly up-regulated only in that group. These results suggest that Hsp70 KO embryos are more sensitive to maternal hyperthermia resulting in decreased survival. In parallel, caspase-9, -7 and -3 were all down-regulated in the hyperthermia-after-exercise group compared to the hyperthermia group. This result suggests that maternal exercise inhibits activation of the caspase cascade and prevents apoptotic cell death in the embryonic brain. In addition, we observed open neural tubes in the hyperthermia group. Although rhombencephalic fossa were not expanded in the hyperthermia-after-exercise group, their neural tubes were closed. In parallel, our TEM study demonstrated reduced apoptotic cell numbers in the developing brains in the hyperthermia-after-exercise group compared to the hyperthermia group. In conclusion, maternal exercise may have an anti-apoptotic effect by activating Hsp27 and Hsp110, and the anti-apoptotic factor Bcl-2, and inhibiting expressions of Bax and caspase-9, -7 and -3, and thus may play an important role in protecting embryos against maternal hyperthermia.
Declaration of interest: This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2007-000-0000-5739). The authors alone are responsible for the content and writing of the paper.
References
- Mirkes PE, Little SA. Teratogen-induced cell death in postimplantation mouse embryos: Differential tissue sensitivity and hallmarks of apoptosis. Cell death Differ 1998; 5: 592–600
- Takle H, McLeod A, Andersen O. Cloning and characterization of the executioner caspases 3, 6, 7 and Hsp70 in hyperthermic Atlantic salmon (Salmo salar) embryos. Comp Biochem Physiol B Mol Bio 2006; 144: 188–198
- Mirkes PE, Cornel LM, Wilson KL, Dilmann WH. Heat shock protein (Hsp70) protects postimplantation murine embryos from the embryolethal effects of hyperthermia. Dev Dyn 1999; 214: 159–170
- Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ 1999; 6: 1028–1042
- Salvesen GS, Dixit VM. Caspases: Intracellular signaling by proteolysis. Cell 1997; 14: 443–446
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997; 275: 1129–1132
- Kregel KC. Heat shock proteins: Modifying factors in physiological stress responses and acquired thermotolerance. J Appl Physiol 2002; 92: 2177–2186
- Barrier M, Dix DJ, Mirkes PE. Inducible 70 kDa heat shock proteins protect embryos from teratogen-induced exencephaly: Analysis using Hspa1a/a1b knockout mice. Birth defects Res A Clin Mol Teratol 2009; 85: 732–740
- Kilgore JL, Musch TI, Ross CR. Physical activity, muscle, and the Hsp70 response. Can J Appl Physiol 1998; 23: 245–260
- Murlasits Z, Cutlip RG, Geronilla KB, Rao MK, Wonderlin WF, Always SE. Resistance training increases heat shock protein levels in skeletal muscle of young and old rats. Exp Gerontol 2006; 41: 398–406
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones 2001; 6: 386–393
- Powers SK, Demirel HA, Vincent HK, Coombes JS, Naito H, Hamilton KL, Shanely RA, Jessup J. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am J Physiol 1998; 275: 1468–1477
- Jin H, Yang R, Li W, Lu H, Ryan AM, Ogasawara AK, Peborgh JV, Paoni NF. Effects of exercise training on cardiac function, gene expression, and apoptosis in rats. Am J Physiol Heart Circ Physiol 2000; 279: H2994–3002
- Osorio RA, Silveira VL, Maldjian S, Morales A, Christofani JS, Russo AK, Silva AC, Picarro IC. Swimming of pregnant rats at different eater temperatures. Comp Biochem Physiol A Mol Integr Physiol 2003; 135: 605–611
- Lee HH, Kim H, Lee JW, Kim YS, Yang HY, Chang HK, Lee TH, Shin MC, Lee MH, Shin MS, et al. Maternal swimming during pregnancy enhances short-term memory and neurogenesis in the hippocampus of rat pups. Brain & Development 2006; 28: 147–154
- Clapp JF III. The effects of maternal exercise on fetal oxygenation and feto-placental growth. Eur J Obstet Gynecol Reprod Biol 2003; 110: S80–S85
- Lynch AM, McDonald S, Magann EF, Evans SF, Choy PL, Dawson B, Blanksby BA, Newnham JP. Effectiveness and safety of a structured swimming program in previously sedentary women during pregnancy. J Matern Fetal Neonatal Med 2003; 14: 163–169
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 1976; 72: 248–254
- Clapp JF III, Rokey R, Treadway JL, Carpenter MW, Artal RM, Warrnes C. Exercise in pregnancy. Med Sci Sports Exerc 1992; 24: S294–300
- Wolfe LA, Mottola MF. Aerobic exercise in pregnancy: An update. Can J Appl Physiol 1993; 18: 119–147
- Manders MA, Sonder GJ, Mulder EJ, Visser GH. The effects of maternal exercise on fetal heart rate and movement patterns. Early Hum Dev 1997; 48: 237–247
- Katz VL, McMurry R, Berry MJ, Cefalo RC. Fetal and uterine responses to immersion and exercise. Obstet Gynecol 1988; 72: 225–230
- Mottola MF, Fitzgerald HM, Wilson NC, Taylor AW. Effect of water temperature on exercise-induced maternal hyperthermia on fetal development in rats. Int J Sports Med 1993; 14: 246–251
- Fehrenbach E, Northoff H. Free radicals, exercise, apoptosis, and heat shock proteins. Exerc Immuno Rev 2001; 7: 66–89
- van Ginneken MM, de Graaf-Roelfsema E, Keizer HA, van Dam KG, Wijnberg ID, van der Kolk JH, van Breda E. Effect of exercise on activation of the p38 mitogen-activated protein kinase pathway, c-Jun NH2 terminal kinase, and heat shock protein 27 in equine skeletal muscle. Am J Vet Res 2006; 67: 837–844
- Christians ES, Zhou Q, Renard J, Benjamin IJ. Heat shock proteins in mammalian development. Semin Cell Dev Biol 2003; 14: 283–290
- Ehmsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J 1997; 16: 221–229
- Geum D, Son GH, Kim K. Phosphorylation-dependent cellular localization and thermoprotective role of heat shock protein 25 in hippocampal progenitor cells. J Biol Chem 2002; 277: 19913–19921
- Easton DP, Kaneko Y, Subjeck JR. The Hsp110 and Grp1 70 stress proteins: Newly recognized relatives of the Hsp70s. Cell Stress Chaperones 2000; 5: 276–290
- Oh HJ, Chen X, Subjeck JR. Hsp110 protects heat-denatured proteins and confers cellular thermoresistance. J Biol Chem 1997; 274: 15712–15715
- O’Reilly AM, Currie RW, Clarke DB. HspB1 (Hsp27) expression and neuroprotection in the retina. Mol Neurobiol 2010; 42: 124–132
- Bowen JM, Gibson RJ, Cummins AG, Keefe DM. Intestinal mucositis: The role of the Bcl-2 family, p53 and caspases in chemotherapy-induced damage. Support Care Cancer 2006; 14: 713–731
- Mirkes PE, Little SA. Cytochrome c release from mitochondria of early postimplantation murine embryos exposed to 4-hydrozycyclophosphamide, heat shock, and staurosporine. Toxicol Appl Pharmacol 2000; 162: 197–206
- Mirkes PE. (2001) Warkany lecture: To die or not to die, the role of apoptosis in normal and abnormal mammalian development. Teratology 2002; 65: 228–239
- Delchev SD, Georgieva KN, Koeva YA, Atanassova PK. Bcl-2/Bax ratio, mitochondrial membranes and aerobic enzyme activity in cardiomyocytes of rats after submaximal training. Folia Med 2006; 48: 50–56
- Siu PM, Bryner RW, Martyn JK, Alway SE. Apoptotic adaptations from exercise training in skeletal and cardiac muscles. FASEB J 2004; 18: 1150–1152
- Nakao C, Ookawara T, Kizaki T, Oh-Ishi S, Miyazaki H, Haga S, Sato Y, Ji LL, Ohno H. Effects of swimming training on three superoxide dismutase isoenzymes in mouse tissues. J Appl Physiol 2000; 88: 649–654
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine, second edition. Oxford University Press, New York 1989
- Ji LL. Exercise-induced modulation of antioxidant defense. Ann N Y Acad Sci 2002; 959: 82–92
- Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today 1994; 15: 7–10
- Choi S, Park KA, Lee HJ, Park MS, Lee JH, Park KC, Kim MH, Lee SH, Seo JS, Yoon BW. Expression of Cu/Zn SOD protein is suppressed in Hsp70.1 knockout mice. J Biochem Mol Biol 2005; 38: 111–114
- Li CY, Lee JS, Ko YG, Kim JI, Seo JS. Heat shock protein 70 inhibits apoptosis downstream of cytochrome c release and upstream of caspase-3 activation. J Biol Chem 2000; 275: 25665–25671