Abstract
Purpose: The purpose of this study was to explore the effects of MIH and radiotherapy alone or combined on metastatic breast cancer and the underlying mechanisms.
Materials and methods: A murine 4T1 metastatic breast cancer model was established and randomly assigned into four treatment groups: C (control), R (radiotherapy), MIH, and MIH + R. Tumour volume, lung metastasis, the expression of Bax and MMP-9, T cell subsets, serum cytokine levels, and mouse survival were evaluated.
Results: Group MIH + R showed significantly reduced tumour volume, lung metastasis, improved survival and increased Bax expression compared to group R or MIH (P < 0.05). MMP-9 expression in the primary tumour tissue was significantly increased in group R compared to the other groups (P < 0.05), which could be brought down by combined MIH treatment. Group MIH + R showed significantly higher CD4+ T cell percentage as well as CD4+/CD8+ cell ratio than group R (P < 0.05). Group MIH + R showed significantly higher serum levels of TNF-α, IFN-γ and IL-2 than group R (P < 0.05).
Conclusions: MIH not only promotes the tumour-cell killing effect of radiotherapy through Bax-mediated cell death, but also improves cellular immunity in mice under radiotherapy and decreases the potential of radiotherapy to enhance MMP-9 expression, which leads to significant improvement in lung metastasis and overall survival of mice under combined treatment of MIH and R. This study is the first to have explored the effect of combined hyperthermia and radiotherapy on tumour metastasis and the underlying mechanisms. It provides insights into the application of MIH as an adjuvant to radiotherapy for metastatic breast cancer.
Introduction
Hyperthermia is a therapeutic procedure used to raise the temperature of a region of the body affected by cancer. The temperature increase required can be achieved by various methods Citation[1]. Conventional hyperthermia usually cannot precisely target tumours and may result in hyperpyrexia of fat tissues Citation[2]. Magnetic induction hyperthermia (MIH), which was developed to induce localised heating in response to focused radio waves, has become a promising anti-tumour therapy in recent years Citation[3–5]. Thermoseeds are implanted into a tumour, followed by the application of a magnetic field to heat the thermoseeds. As a result, the heat is transferred to the surrounding tumour tissue from the thermoseeds. Compared to conventional hyperthermia, MIH is a repeatable process that can control the local temperature in vivo Citation[3–5]. In the past two decades there has been a great interest in application of hyperthermia in conjunction with irradiation and/or chemotherapy in cancer treatment.
Using cultured 4T1 breast cancer cells, Zwolak et al. established a murine metastatic breast cancer model which would develop distant metastasis to lung and liver from the injection site. This model shows similar characteristics to human breast cancer in tumour growth and metastasis Citation[6]. In the present study we explored the effects of MIH and radiotherapy alone or combined on metastatic breast cancer and the underlying mechanisms using a murine 4T1 metastatic breast cancer model. It provides insights into the application of MIH as an adjuvant to radiotherapy for metastatic breast cancer.
Materials and methods
Equipment and reagents
The magnetic-mediated clinical care prototype machine (frequency 100 kHz, current range 0–60 A, central magnetic induction 130 G (current 60 A, core air gap 130 mm)) was produced by Shenzhen Shuangping Technology (Shenzhen, China). The temperature survey and recording system was from Physitemp Instruments (Clifton, NJ). Other equipment included a Forma 3111 carbon dioxide cell incubator (American Thermo Corporation, Thermo Scientific, Franklin, MA), a DL-CJ-1 N high performance aseptic laboratory bench (Harbin Donglian Electronic Technology), an electronic balance (Mettler-Toledo Instruments), a Vernier caliper, a 3-18 K constant temperature centrifuge (Sigma), and a murine pulse oximeter system (Beijing Zhenglong Instruments, Beijing). The thermoseeds, composed of nickel-copper alloy (72%:27%) with a Curie point of 80°C, a diameter of 0.9 mm and a length of 0.6 cm, were manufactured by Beijing University of Science and Technology in cooperation with the Department of Metal Physics Research of Tsinghua University (Beijing, China). The linear accelerator was purchased from Varian (model 2300, Basel, Switzerland). Goat anti-MMP-9 (sc-6841), rabbit anti-Bax (sc-526) and mouse anti-β-actin (sc-81178) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). For flow cytometry analysis, CD4 and CD8 labelling antibodies conjugated with PE were purchased from Beijing Jinqiao Biotechnology (Beijing). The enzyme-linked immunosorbent assay (ELISA) kits for mouse TNF-α, IFN-γ and IL-2 were purchased from Invitrogen (Carlsbad, CA). BALB/C mice (female, 5 weeks old, weight 18–20 g) were purchased from Central South University (Changsha, China).
Establishment of murine 4T1 metastatic breast cancer model and treatment assignment
The 4T1 human breast cancer cells were purchased from the Chinese Academy of Medical Sciences Institute of Pharmacology (Shanghai, China) and maintained in RPMI-1640 medium supplemented with 10% fo][[;]/ etal bovine serum (FBS) in 5% CO2 at 37°C. The mice were subcutaneously injected in the right flank with 5×106 4T1 cells in 0.1 mL of DMEM. Perpendicular tumour diameters were measured with a Vernier caliper every two days, and tumour volumes were calculated as length × width2 × 0.5. On days 7–10 post 4T1 cell injection, when tumours reached an average diameter of 0.8–1.0 cm (approximately 0.5–0.6 cm3 in volume), mice were randomly assigned into four treatment groups: C, tumour-bearing control; R, radiotherapy; MIH, magnetic induction hyperthermia; MIH + R, magnetic induction hyperthermia plus radiotherapy. Radiotherapy was administered as follows: All mice were lightly anaesthetised by intraperitoneal injection of Avertin (240 mg/kg) and placed on a dedicated Plexiglas tray. The whole body was protected by lead shielding, except for the area of the tumour to be irradiated. According to previous publications Citation[7], we used two fractions of 10 Gy for radiotherapy in the highly metastatic murine 4T1 breast cancer model. Briefly, radiotherapy was delivered to a field including the tumour with 5-mm margins by two fractions of 10 Gy each at a two-day interval, with a 1.0-cm-thick tissue equivalent bolus covering the skin surface of the tumour. The mice were irradiated with 6-MV X-rays using a Varian Clinac 2300 CD linear accelerator (Varian). To evaluate the impact of thermoseeds on radiation dose distribution, an anthropomorphic phantom and three-dimensional treatment planning system were used to simulate irradiation. The absorbed dose caused by the implanted thermoseeds was less than 1.5% Citation[8]. Tumour growth was evaluated until death or sacrifice when tumour dimensions exceeded 5% body weight or mice showed dyspnea, abnormal posture, >20% body weight loss, difficulty with ambulation, or any other clinical sign of metastatic disease causing significant pain or distress, according to institutional guidelines. All animal care, breeding, and testing procedures were approved by the Laboratory Animal Users Committee at Xiangya Hospital, Central South University, Changsha, China.
Implantation of thermoseeds and MIH treatment
On days 7–10 post 4T1 cell injection, when tumours reached an average diameter of 0.8–1.0 cm (approximately 0.5–0.6 cm3 in volume), four thermoseeds were implanted at equal distance in the centre and the edge of the tumour using an 18 gauge spinal needle, with approximately 0.5 cm distance between each two thermoseeds. After implantation, X-rays were used to verify the location and direction of implanted thermoseeds. Right after the implantation, the mice were anaesthetised with 1% barbital sodium (50 mg/kg) and placed in a magnetic field. Their body temperature was measured rectally. Three electric copper-constantan thermocouples (model It-18, Physitemp Instruments, Clifton, NJ) were inserted to separately monitor the temperature at the centre and the edge of the tumour. A nearly fixed current of 250–300 A was applied for 3 min. Then manual adjustment was applied to stabilise the tumour centre temperature at 45°C ± 1°C and the tumour edge temperature at 41°C ± 1°C for 10 min (Supplementary Figure S1). The MIH treatment was conducted for a total of twice at a two-day interval. For the MIH + R treatment, MIH and R treatments were simultaneously conducted. In all experiments with anaesthesia, pulse and breath rates of the mice were monitored with a murine pulse oximeter system (Beijing Zhenglong Instruments). The mice anaesthetised by barbital sodium and Avertin® showed similar pulse and breath rates during treatment (Supplementary Table S1).
Clonogenic lung metastases assay
Twenty-five days after the treatment, nine mice from each treatment group were subject to clonogenic lung metastasis assays as previously described Citation[7]. Briefly, lungs from each individual animal were minced into 1-mm pieces, and digested with 5 mL enzyme cocktail containing 1 mg/mL collagenase IV and 6 units/mL elastase (Sigma) in phosphate buffered saline (PBS) for 1 h at 4°C with rotation. Cell suspensions were filtered through 70-µm nylon cell strainers and washed twice with HBSS, then resuspended in complete medium. Then the cells were cultured in 10-cm tissue culture dishes and treated with 60 µmol/L of 6-thioguanine (Sigma) to allow only the growth of 4T1 cells, which are resistant to this drug Citation[9]. When colonies of growing 4T1 cells became visible (8–14 days), the plates were washed with PBS, fixed with methanol, and stained with crystal violet. The colonies were counted independently by two investigators, blinded to the group to which each mouse belonged, and the total colony number/lungs was calculated for each animal.
Immunohistochemistry
Paraffin-embedded tumour tissues were examined for MMP-9 or Bax expression. The immunostaining for Bax or MMP-9 was performed utilising the streptavidin-biotin-peroxidase method, according to the manufacturer's instructions (Beijing Golden Bridge Biotechnology). In brief, sections (4 µm) of paraffin-embedded specimens were de-paraffinised in xylene, hydrated in a degraded series of ethanol, and heated in 0.01 M citrate buffer for 10 min in a microwave oven. After cooling for 20 min and washing in PBS, endogenous peroxidase was blocked with methanol containing 0.3% hydrogen peroxide for 30 min, followed by incubation with PBS for 30 min. Then the sections were incubated with the anti-Bax or anti-MMP-9 antibody at a dilution of 1:150, and stained using the avidin–biotin complex method. Coloration was developed by DAB containing H2O2, and the sections were counter-stained with haematoxylin. The number of positive cells was counted in 10 high-power view fields and a percentage was calculated as follows: Bax or MMP-9 positive cells/ total tumour cells × 100%.
Western blot analysis
Immunoblotting was performed as described previously with respective antibodies Citation[10]. Briefly, extracted tumour tissues were homogenised and lysed in 0.1% Nonidet P-40 lysis buffer (0.1% Nonidet P-40, 50 mM Tris-HCl (PH 7.4), 150 mM NaCl, and 1 mM EDTA). Equal amounts of proteins for each sample were separated by 10% SDS-polyacrylamide gels and blotted onto a polyvinylidene difluoride microporous membrane (Millipore). Membranes were incubated for 1 h with a 1/1000 dilution of goat anti-MMP-9 (sc-6841), rabbit anti-Bax (sc-526), or mouse anti-β-actin (sc-81178) antibodies (Santa Cruz Biotechnology), and then washed and revealed using secondary antibodies with horseradish peroxidase conjugate (1/5000, 1 h). Peroxidase was revealed with a GE Healthcare ECL kit. Proteins were quantified before being loaded onto the gel, and equal loading of extracts was verified by Ponceau coloration.
Flow cytometry and ELISA
Twenty-five days after the treatment, five mice were randomly selected from each group and sacrificed. Peripheral blood was collected in EDTA-coated tubes. The samples were incubated with CD4 or CD8 antibody at room temperature in the dark for 20 min and shaken once every 3 min, after which the samples were incubated with 1 mL of 1× erythrocyte lysis for 10 min and centrifuged at 2000 rpm for 2 min. The supernatant was discarded and the pellet was washed twice with PBS containing 2% serum and centrifuged at 2000 rpm for 2 min. The supernatant was discarded, and 500 µL of 4% polyformaldehyde was added to the tubes. The samples were then analysed by flow cytometry. ELISA was performed to detect serum TNF-α, IFN-γ and IL-2 levels using blood samples according to the manufacturer instructions (Invitrogen).
Statistical analysis
Statistical analyses were performed with SPSS for Windows 10.0. Numerical data were expressed as means ± standard deviation. Kruskal-Wallis test plus post hoc Wilcoxon rank sum pairwise comparison was used for comparison of the number of lung metastases among treatment groups. For other experiments, one-way ANOVA plus post hoc pairwise comparisons were employed for comparison of numerical variables among treatment groups. Log-rank test was used to compare animal groups in terms of overall survival, defined as time to death or sacrifice. The median and mean survival times within each treatment group were estimated using the Kaplan-Meier product-limit method. The statistical significance level of this study was set at two-sided α = 0.05.
Results
Effect of treatments on tumour volume
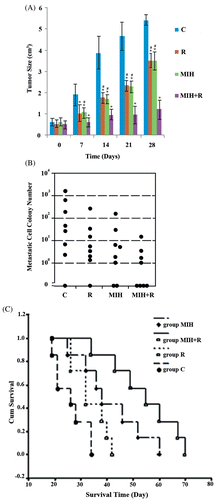
As shown in , compared to the tumour-bearing control (C), radiotherapy (R) and MIH (MIH) alone or combined (MIH + R) could significantly inhibit tumour growth (P < 0.05), with MIH + R being the most effective. Fourteen days after the treatment, the tumour volume in group MIH + R was significantly smaller than that in group R or MIH (P < 0.05).
Figure 1. Tumour volume, lung metastasis and mouse survival after treatments. (A) Tumour volumes on the day of treatment (day 0) and 28 days after the treatment were shown as mean ± standard deviation in histograms. (B) Clonogenic lung metastasis assays were performed 25 days after the treatment. Each symbol represents a single animal (n = 9 per treatment group). (C) Mouse overall survival was defined as time to death or sacrifice. Kaplan-Meier curve was plotted to evaluate mouse survival among treatment groups: C, tumour-bearing control; R, radiotherapy; MIH, magnetic induction hyperthermia; MIH + R, magnetic induction hyperthermia plus radiotherapy. *P < 0.05 compared with group C; #P < 0.05 compared with group MIH + R.
Effect of treatments on tumour lung metastasis
To evaluate the effect of treatments on tumour lung metastasis, clonogenic lung metastases assays were performed 25 days after the treatment. As shown in , lung metastasis in group R, MIH and MIH + R was significantly lower than that in group C (P < 0.05). Compared with group R or MIH, group MIH + R showed significantly less lung metastasis (P < 0.05).
Effect of treatments on mouse survival
To evaluate the overall effect of different treatments on mouse survival, Kaplan-Meier survival analysis and log-rank tests were performed. As shown in , both group MIH and group MIH + R showed significantly increased survival time compared to group C or R (P < 0.05), while there was no significant difference between group C and group R. Group MIH + R had significantly longer survival time than group MIH (P < 0.05), consistent with the results shown in and 1B.
Effect of treatments on Bax expression in the primary tumour
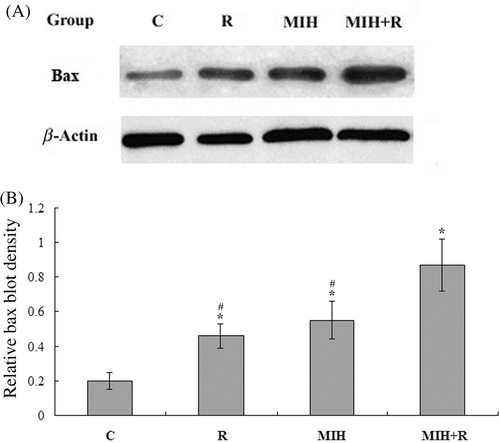
The response of eukaryotic cells to radiotherapy includes cell-cycle arrest and apoptosis. In radiotherapy-induced tumour cell apoptosis, up-regulation of Bax is observed Citation[10], Citation[11]. Thus, we next examined the expression of Bax protein in the primary tumour at the 4T1 cell injection site 25 days after the treatment. As shown in , immunohistochemical analysis showed that the Bax expression in group R, MIH and MIH + R was significantly higher than that in group C (P < 0.05). Compared with groups R or MIH, group MIH + R showed significantly higher Bax expression (P < 0.05). The results were confirmed with western blot analyses ().
Figure 2. Immunohistochemical detection of Bax expression in the primary tumour. (A) Immunohistochemical analyses were performed to determine Bax expression in the primary tumour at the 4T1 cell injection site 25 days after the treatments. Positive staining for Bax was brownish in colour. Magnification, ×200. (B) Bax positive cells were counted and a percentage was calculated as follows: Bax positive cells/total tumour cells × 100%. The Bax immunostaining percentage in the tumour was compared among treatment groups: C, tumour-bearing control; R, radiotherapy; MIH, magnetic induction hyperthermia; MIH + R, magnetic induction hyperthermia plus radiotherapy. *P < 0.05 compared with group C; #P < 0.05 compared with group MIH + R.
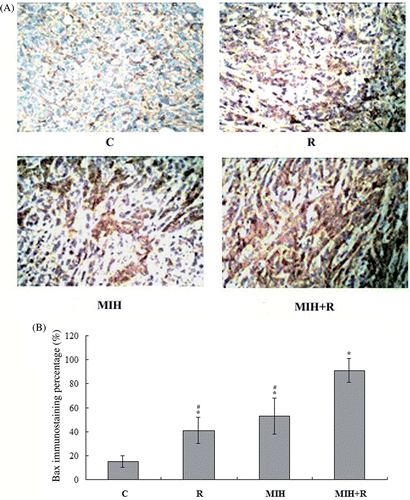
Figure 3. Western blot analysis of Bax expression in the primary tumour. (A) 25 days after the treatments, primary tumour tissue lysates from nine mice in each treatment group were subject to western blot analysis for Bax expression. β-actin blotting was used as a loading control. Representative results are shown in this figure. (B) Bax and β-actin blots were measured by densitometry. The density of the Bax blot was normalised against that of β-actin to obtain a relative Bax blot density. Treatment groups: C, tumour-bearing control; R, radiotherapy; MIH, magnetic induction hyperthermia; MIH + R, magnetic induction hyperthermia plus radiotherapy. *P < 0.05 compared with group C; #P < 0.05 compared with group MIH + R.
Effect of treatments on MMP-9 expression in the primary tumour
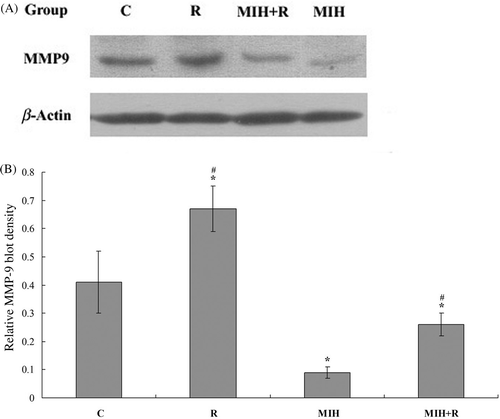
As radiotherapy reportedly enhances the expression of matrix metalloproteinase-9 (MMP-9) Citation[12], an important promoter of breast cancer metastasis Citation[13], we next examined the expression of MMP-9 in the primary tumour at the 4T1 cell injection site 25 days after the treatments. As shown in , immunohistochemical analysis showed that the MMP-9 expression was significantly increased in group R compared to the other groups (P < 0.05). In group MIH + R, the addition of MIH brought the MMP-9 expression down to below the control level (group C). Group MIH showed lower MMP-9 expression than the other groups (P < 0.05). The results were confirmed with western blot analyses ().
Figure 4. Immunohistochemical detection of MMP-9 expression in the primary tumour. (A) Immunohistochemical analyses were performed to determine MMP-9 expression in the primary tumour at the 4T1 cell injection site 25 days after the treatments. Positive staining for MMP-9 was brownish in colour. Magnification, ×200. (B) MMP-9 positive cells were counted and a percentage was calculated as follows: MMP9 positive cells/total tumour cells × 100%. The MMP-9 immunostaining percentage in the tumour was compared among treatment groups: C, tumour-bearing control; R, radiotherapy; MIH, magnetic induction hyperthermia; MIH + R, magnetic induction hyperthermia plus radiotherapy. *P < 0.05 compared with group C; #P < 0.05 compared with group MIH + R.
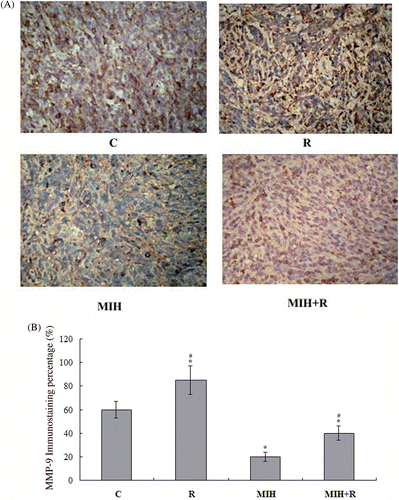
Figure 5. Western blot analysis of MMP-9 expression in the primary tumour. (A) 25 days after the treatments, primary tumour tissue lysates from nine mice in each treatment group were subject to western blot analysis for MMP-9 expression. β-actin blotting was used as a loading control. Representative results are shown in this figure. (B) MMP-9 and β-actin blots were measured by densitometry. The density of the MMP-9 blot was normalised against that of β-actin to obtain a relative MMP-9 blot density. Treatment groups: C, tumour-bearing control; R, radiotherapy; MIH, magnetic induction hyperthermia; MIH + R, magnetic induction hyperthermia plus radiotherapy. *P < 0.05 compared with group C; #P < 0.05 compared with group MIH + R.
Effect of treatments on T cell subsets and cytokine levels in peripheral blood
It has been proposed that hyperthermia may enhance body immunity through the abscopal effect Citation[2]. In this study we explored the effect of MIH on body immunity by examining changes in T cell subsets and cytokine levels in the peripheral blood 25 days after the treatment. As shown in , flow cytometry analysis revealed that compared to the non-tumour-bearing control (group N), groups C and R had significantly lower CD4+ or CD8+ T cell percentage and CD4+/CD8+ cell ratio. Group R showed no significant difference in CD4+ or CD8+ T cell percentage compared to group C. Both group MIH and group MIH + R showed significantly higher CD4+ T cell percentage as well as CD4+/CD8+ cell ratio compared to group C and group R (P < 0.05), suggesting that the cellular immunity was increased by MIH. Moreover, ELISA showed that compared to the non-tumour-bearing control (group N), groups C and R had significantly lower serum levels of TNF-α, IFN-γ and IL-2. Compared to group C, group R had significantly lower levels of serum TNF-α and IFN-γ (P < 0.05). In group MIH + R, the addition of MIH significantly elevated the serum cytokine levels above those in group C (P < 0.05). The anaesthetic agent, Avertin, reportedly causes peritonitis in mice one day after intraperitoneal injection Citation[14], which was still not observable one week after the injection Citation[15]. In our study, no gross lesions could be detected by macroscopic examination of organs in the abdomen when the mice were sacrificed (25–70 days after using Avertin). To investigate whether anaesthetic agents and thermoseed implantation would affect the mouse cellular immunity, tumour-bearing mice were anaesthetised by barbital sodium or Avertin (as described in Materials and methods) with or without thermoseed implantation when the tumour reached an average diameter of 0.8–1.0 cm (approximately 0.5–0.6 cm3 in volume). T cell subsets and cytokine levels in the peripheral blood were examined 25 days after the anaesthesia treatment. No statistically significant difference was noted among the data (Supplementary Table S2), suggesting that Avertin, barbital sodium and thermoseed implantation had no significant impact on the cellular immunity of 4T1 tumour-bearing mice.
Table 1A. T cell subset change in peripheral blood 25 days after treatment (n = 5).
Table 1B. Serum cytokine level change 25 days after treatment (n = 5).
Discussion
It is well established that sustained temperature above 41°C–42°C will cause death of living cells Citation[16–19]. It is thought that hyperthermia alters the function of many structural and enzymatic proteins within cells, which in turn alters cell growth and differentiation and can induce apoptosis Citation[20–24]. As a physical therapy, hyperthermia would have far fewer restrictive side effects than chemotherapy and radiotherapy, and it could be used in combination with these therapies. In the present study we explored the effects of MIH and radiotherapy alone or combined on metastatic breast cancer and the underlying mechanisms, using a murine 4T1 metastatic breast cancer model. We found that combined treatment with MIH and radiotherapy could significantly reduce tumour volume and lung metastasis, and promote survival, compared to MIH or radiotherapy alone.
Up-regulation of Bax, a critical cell death promoter, has been reported along with increased tumour cell apoptosis after radiotherapy or hyperthermia, through a p53-dependent or p53-independent pathway Citation[10], Citation[11], Citation[25]. In this study, the Bax expression was significantly increased by combined treatment with MIH and R, compared to either treatment alone ( and ). This is consistent with the results of tumour volume change among the treatment groups (), suggesting a positive interaction between MIH and R in inducing Bax-mediated tumour cell death.
Radiotherapy can enhance the expression of MMP-9 Citation[12], which reportedly promotes breast cancer metastasis Citation[13]. On the other hand, Sawaji et al. reported that hyperthermia could inhibit secretion of MMP-1 and activation of MMP-2 in various cancer cell lines Citation[19]. In agreement with the above results, we found that the MMP-9 expression in the primary tumour tissue was significantly increased in group R compared to the other groups. Group MIH had the lowest MMP-9 expression among the treatment groups, which may account for the decreased MMP-9 expression in group MIH + R compared to group R ( and ). The results suggest that as an adjuvant therapy, MIH can decrease the potential of radiotherapy to enhance MMP-9 expression.
Compared to the tumour-bearing control (group C), a significant decrease in CD4+/CD8+ cell ratio and serum TNF-α and IFN-γ was noted after radiotherapy (). This is consistent with previous reports that local irradiation could suppress cellular immunity in cancer patients Citation[26–28]. The underlying mechanism is unclear. On the other hand, hyperthermia reportedly enhances body immunity through the abscopal effect, which is a phenomenon that local treatment of a tumour can affect tumour growth at distant sites in the body Citation[2]. It has been proposed that the immune system contributes to this phenomenon Citation[4]. In our study, MIH had the highest CD4+ T cell percentage and CD4+/CD8+ cell ratio among the treatment groups, which accounted for the increased CD4+ T cell percentage and CD4+/CD8+ cell ratio in group MIH + R compared to group R (). Likewise, group MIH had the highest serum cytokine levels among the treatment groups, which accounted for the increased serum cytokine levels in group MIH + R compared to group R (). The results suggest that MIH can improve the cellular immunity in animals under radiotherapy, probably through the abscopal effect.
Metastasis is the major cause of cancer death Citation[29], Citation[30]. Our results indicate that MIH not only promotes the tumour-cell killing effect of radiotherapy through Bax-mediated cell death, but also improves cellular immunity in mice under radiotherapy and decrease the potential of radiotherapy to enhance MMP-9 expression. These findings suffice to explain the significant improvement in lung metastasis and overall survival of mice in group MIH + R (). The effectiveness of hyperthermia combined with radiotherapy on local tumour control has been known for years Citation[1]. However, our study is the first to have explored the effect of combined hyperthermia and radiotherapy on tumour metastasis and the underlying mechanisms, and thus will serve as an important reference for future studies.
The anti-tumour effect of combined radiotherapy and hyperthermia is maximised when these modalities are administered simultaneously, which has been proved in clinical trials Citation[31–36]. However, there are still technical difficulties regarding simultaneous delivery of radiotherapy and hyperthermia in clinical practice. When tumour and normal tissues are irradiated and heated, the normal tissue sensitivity is also increased, and the therapeutic gain factor (TGF) becomes 1 and the advantage of simultaneous administration is lost Citation[31]. To increase the TGF in simultaneous radiotherapy and hyperthermia, it is necessary to confine irradiation and hyperthermia to the tumour as far as possible Citation[31]. In addition, the significant benefits of simultaneous thermoradiotherapy may be limited to superficial tumours, because superficial lesions are the least difficult to heat adequately due to their accessibility and proximity to external energy sources Citation[32]. Nevertheless, clinical trials have shown that simultaneous thermoradiotherapy is technically feasible and safe Citation[32]. Moreover, our study provides solid preclinical evidence that simultaneous delivery of radiotherapy and hyperthermia is feasible and effective for treating metastatic breast cancer.
Conclusions
MIH not only promotes the tumour-cell killing effect of radiotherapy through Bax-mediated cell death, but also improves cellular immunity in mice under radiotherapy and decreases the potential of radiotherapy to enhance MMP-9 expression, which leads to significant improvement in lung metastasis and overall survival of mice under combined treatment of MIH and R. This study provides insights into the application of MIH as an adjuvant to radiotherapy for metastatic breast cancer.
Declaration of interest: This study was supported by the National Nature Science Foundation of China (Grant Numbers 10775085 and 30571779), the Science Committee Fund of Beijing (Grant Number Z07000200540704), and the Yuyuan Fund of Tsinghua University (Grant Number 20240000519). The authors alone are responsible for the content and writing of the paper.
Supplemental references.
Download PDF (169.7 KB)References
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002; 3: 487–497
- Dahl O, Dalene R, Schem BC, Mella O. Status of clinical hyperthermia. Acta Oncol 1999; 38: 863–873
- Moroz P, Jones SK, Gray BN. Magnetically mediated hyperthermia: Current status and future directions. Int J Hyperthermia 2002; 18: 267–284
- Ito A, Honda H, Kobayashi T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: A novel concept of ‘heat-controlled necrosis’ with heat shcok protein expression. Cancer Immunol Immunother 2006; 55: 320–328
- Sorbe B, Roos DI, Karlsson LG. The use of microwave-induced hyperthermia in conjunction with afterloading irradiation of vaginal carcinoma. A preliminary report. Acta Oncol 1990; 29: 1029–1033
- Zwolak P, Jasinski P, Terai K, Gallus NJ, Ericson ME, Clohisy DR, Dudek AZ. Addition of receptor tyrosine kinase inhibitor to radiation increases tumour control in an orthotopic murine model of breast cancer metastasis in bone. Eur J Cancer 2008; 44: 2506–2517
- Pilones KA, Kawashima N, Yang AM, Babb JS, Formenti SC, Demaria S. Invariant natural killer T cells regulate breast cancer response to radiation and CTLA-4 blockade. Clin Cancer Res 2009; 15: 597–606
- Wang H, Lu X, Liu J, Xiang F, Zeng B, Tang J. Impact of metal thermoseeds on radiation dose distribution. Chin J Radiat Oncol 2010; 19: 145–148, [in Chinese. English abstract web link: http://eng.med.wanfangdata.com.cn/PaperDetail.aspx?qkid=zhfszl&qcode=zhfszl201002018)]
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res 1992; 52: 1399–1405
- Liang H, Zhan HJ, Wang BG, Pan Y, Hao XS. Change in expression of apoptosis genes after hyperthermia, chemotherapy and radiotherapy in human colon cancer transplanted into nude mice. World J Gatroenterol 2007; 13: 4365–4371
- Gong B, Chen Q, Endlich B, Mazumder S, Almasan A. Ionizing radiation-induced, Bax-mediated cell death is dependent on activation of cysteine and serine proteases. Cell Growth Differ 1999; 10: 491–502
- Camphausen K, Moses MA, Beecken WD. Radiation therapy to a primary tumor accelerates metastatic growth in mice. Cancer Res 2001; 61: 2207–2211
- Wang X, Lu H, Urvalek AM, KLF8 promotes human breast cancer cell invasion and metastasis by transcriptional activation of MMP9. Oncogene 2010, doi: 10.1038/onc.2010.563
- Zeller W, Meier G, Burki K, Panoussis B. Adverse effects of tribromoethanol as used in the production of transgenic mice. Lab Anim 1997; 32: 407–413
- Papaioannou VE, Fox JG. Efficacy of tribromoethanol anaesthesia in mice. Lab Anim Sci 1993; 43: 189–192
- Cavaliere R, Ciocatto EC, Gionanella BC, Heidelberger C, Johnson RO, Margottini M, Mondovi B, Moricca G, Rossi-Fanelli A. Selective heat sensitivity of cancer cells. Biochemical and clinical studies. Cancer 1967; 20: 1351–1381
- Robinson JE, Wizenberg MJ, McCready WA. Combined hyperthermia and radiation suggest an alternative to heavy particle therapy for reduced oxygen enhancement ratios. Nature 1974; 251: 521–522
- Steeves RA. Hyperthermia in cancer therapy: Where are we today and where are we going?. Bull NY Acad Med 1992; 68: 342–350
- Dewey W. Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 1994; 10: 457–483
- Harmon BV, Takano YS, Winterford CM, Gobe GC. The role of apoptosis in the response of cells and tumours to mild hyperthermia. Int J Radiat Biol 1991; 59: 489–501
- Sellins KS, Cohen JJ. Hyperthermia and induced apoptosis in thymocytes. Radiat Res 1991; 126: 88–95
- Fairbairn JJ, Khan MW, Ward KJ, Loveridge BW, Fairbairn DW, O’Neill LK. Induction of apoptotic cell DNA fragmentation in human cells after treatment with hyperthermia. Cancer Lett 1995; 89: 183–188
- Bertone V, Barni S, Silvotti MG, Freitas I, Mathe G, Pontiggia P. Hyperthermic effects on the human metastatic liver: A TEM study. Anticancer Res 1997; 17: 4713–4716
- Christophi C, Winkworth A, Muralihdaran V, Evans P. The treatment of malignancy by hyperthermia. Surg Oncol 1999; 7: 83–90
- Zhao J, Wang SZ, Tang XF, Liu N, Zhao D, Mao ZY. Analysis of thermochemotherapy-induced apoptosis and the protein expressions of Bcl-2 and Bax in maxillofacial squamous cell carcinomas. Med Oncol 2010, doi: 10.1007/s12032-010-9736-4
- Tarpley JL, Potvin C, Chretien PB. Prolonged depression of cellular immunity in cured laryngopharyngeal cancer patients treated with radiation therapy. Cancer 1975; 35: 638–644
- Yokoyama Y, Sakamoto K, Arai M, Akagi M. Radiation and surgical stress induce a significant impairment in cellular immunity in patients with esophageal cancer. Jpn J Surg 1989; 19: 535–543
- Mellios T, Ko HL, Beuth J. Impact of adjuvant chemo- and radiotherapy on the cellular immune system of breast cancer patients. In Vivo 2010; 24: 227–230
- Hopkin K, Edwards P, Harris A, Klausner R, Peters G, Selby P, Stanley MCancer. Molecular Biology of the CellFourth, B Alberts, A Johnson, J Lewis. Garland Science, New York 2002; 1324–1325
- Pinkas J, Martin SS, Leder P. Bcl-2-mediated cell survival promotes metastasis of EpH4 βMEKDD mammary epithelial cells. Mol Cancer Res 2004; 2: 551–556
- Fuma N, Nomoto Y, Shouji K, Kodaira T, Kamata M, Ito Y. Therapeutic effects of simultaneous intraluminal irradiation and intraluminal hyperthermia on oesophageal carcinoma. Brit J Radiol 2001; 74: 709–714
- Moros EG, Peñagaricano J, Novak P, Straube WL, Myerson RJ. Present and future technology for simultaneous superficial thermoradiotherapy of breast cancer. Int J Hyperthermia 2010; 26: 699–709
- Van Der Zee J, De Bruijne M, Mens JW, Ameziane A, Broekmeyer-Reurink MP, Drizdal T, Linthorst M, Van Rhoon GC. Reirradiation combined with hyperthermia in breast cancer recurrences: Overview of experience in Erasmus MC. Int J Hyperthermia 2010; 26: 638–648
- Zagar TM, Oleson JR, Vujaskovic Z, Dewhirst MW, Craciunescu OI, Blackwell KL, Prosnitz LR, Jones EL. Hyperthermia for locally advanced breast cancer. Int J Hyperthermia 2010; 26: 618–624
- Zagar TM, Oleson JR, Vujaskovic Z, Dewhirst MW, Craciunescu OI, Blackwell KL, Prosnitz LR, Jones EL. Hyperthermia combined with radiation therapy for superficial breast cancer and chest wall recurrence: A review of the randomised data. Int J Hyperthermia 2010; 26: 612–617
- Oldenborg S, Van Os RM, Van Rij CM, Crezee J, Van de Kamer JB, Rutgers EJ, Geijsen ED, Zum Vörde Sive Vörding PJ, Koning CC, Van Tienhoven G. Elective re-irradiation and hyperthermia following resection of persistent locoregional recurrent breast cancer: A retrospective study. Int J Hyperthermia 2010; 26: 136–144
