Abstract
Purpose: The aim of this study was to evaluate the feasibility, safety and therapeutic effects of ultrasound (US)-guided high intensity focused US (HIFU) ablation in the treatment of extra-abdominal desmoid tumours.
Materials and methods: From May 2006 to May 2010, ten consecutive patients with pathologically proven extra-abdominal desmoid tumours were treated by US-guided HIFU ablation. Eight patients with multiple recurrent tumours were treated with a palliative aim, two patients with new solitary tumours were treated with a curative aim. The mean size of the largest tumour was 9.2 cm (range 5.9–12.8 cm). An acoustic power of 300–500 W was used according to the echogenic changes after energy exposure, intermittent HIFU exposure of 2–3 s was applied until the planned target area became hyperechoic on US. Outcome of HIFU ablation was observed by serial contrast-enhanced imaging examinations during follow up.
Results: HIFU ablation was successfully performed without major complications. Large volume coagulation necrosis was obtained in all patients. During a mean follow up of 30 months (range 8–55 months), the treated tumours (n = 25) shrank significantly (>50% in volume). Complete tumour necrosis was observed in the two patients with solitary new tumours. Two patients received repeat HIFU ablation for enlarged residual tumours. No tumour spread along the treated area was observed in any patient.
Conclusion: US-guided HIFU ablation could be used as an effective minimally invasive therapy for local control of extra-abdominal desmoid tumours.
Introduction
Desmoid tumours, also called aggressive fibromatosis, are rare fibrous neoplasms originating from the musculoaponeurotic structure. Histologically they are characterised by proliferation of fibroblasts and myofibroblasts with abundant production of intercellular collagen Citation[1]. Desmoid tumours are classified into intra- and extra-abdominal type, the latter principally involves the trunk and extremities. Desmoid tumours have a locally infiltrative growth pattern, lesions may intertwine extensively with the surrounding tissues and are difficult to remove Citation[2].
Although this tumour never metastasises, management of extra-abdominal desmoid tumours is a challenge. Surgical excision has been the traditional main therapy for extra-abdominal desmoid tumours; a large rim of normal-appearing tissue needs to be resected because tumours infiltrate beyond palpable margins at surgery, and is defined on imaging. However, local recurrence is common, even after apparent complete surgical excision Citation[2], Citation[3]. Reported local recurrence rate can be as high as 60% Citation[4]. Due to lack of normal soft tissue, wide excision with sufficient normal margin may be extremely difficult for recurrent lesions, many lesions are inoperable because of involvement of significant neurovascular structures Citation[2], Citation[3].
Radiation therapy has been used in patients with inoperable desmoid tumours, local recurrence, or incompletely excised lesions Citation[3], Citation[5], Citation[6]. It can improve local control but may be associated with post-irradiation fibrosis, joint contracture, and neuropathy. Also, response to radiation can be extremely slow, complete resolution may require several months Citation[7]. Chemotherapy has been used for inoperable progressive lesions in patients in whom surgery or radiotherapy would cause morbidity Citation[7], Citation[8]. However, it was associated with cytotoxic adverse effects while the response rate was disappointing.
High intensity focused ultrasound (HIFU) ablation is a conformal extracorporeal treatment which can yield thermal coagulation necrosis of the target lesion without surgical resection or insertion of applicators Citation[9–13]. The focused ultrasound (US) energy can pass harmlessly through overlying tissues to the target area, the rate of energy deposition at the target tissue far exceeds the rate of heat dissipation, resulting in rapid temperature rise and subsequent coagulation necrosis. Under real-time imaging guidance, large volume well-delineated ablation zones can be induced at depth in the focal area, through the intact skin. The ablation procedure is repeatable as long as an acoustic pathway is present Citation[12]. In the past decade, HIFU ablation has been successfully used for treatment of various malignant tumours; significant coagulative necrosis of the tumour has been obtained Citation[9–13]. However, to our knowledge, there has been no report of HIFU ablation for the treatment of desmoid tumours so far. Thus, the purpose of this study was to evaluate the therapeutic efficacy of HIFU ablation for the treatment of extra-abdominal desmoid tumours.
Materials and methods
Patients
From May 2006 to May 2010, ten patients (seven men, three women) with pathologically proven extra-abdominal desmoid tumours received US-guided HIFU ablation in our hospital. The study was approved by the institutional ethics committee. Informed consent was obtained at enrolment.
The inclusion criteria for HIFU ablation were as follows: 1) inoperable tumour or patient refusal of surgery; 2) the tumour could be shown on US; 3) the tumour was located more than 2 mm from the skin surface. Exclusion criteria were as follows: 1) extensive scarring in the proposed path of the HIFU beam; 2) skin in the pathway of the HIFU beam had become hard and fibrous due to previous radiation therapy.
The age of the patients ranged from 5 to 57 years (mean 22 years). The diagnosis of desmoid tumours was made by US-guided 16 gauge cutting-needle biopsies before HIFU ablation. Eight patients had multiple recurrent tumours in the area of previous surgical resection such as the leg, buttock, groin and chest wall, and the tumours were considered inoperable by experienced surgeons. Two patients had new solitary tumours and refused surgery. For the two patients that received no treatment before HIFU ablation, one had a tumour located in the abdominal wall, the other had a tumour located in the right buttock (). Before HIFU ablation, all patients received colour Doppler US and contrast-enhanced magnetic resonance imaging (MRI) examination to evaluate the extent of the tumour. The mean size of the largest tumour (the maximum diameter in three orthogonal directions) was 9.2 cm (range, 5.9–12.8 cm). The tumours were seen as hypoechoic nodules on US, which showed substantial enhancement on contrast-enhanced MRI.
Table I. Patient and tumour characteristics.
Equipment
A HIFU system (model JC; Chongqing Haifu Technology, Chongqing, China) was used in this study. The system can be operated by using one of several therapeutic transducers with different focal lengths. In this study, the focused US energy is produced from a 20-cm diameter therapeutic transducer with a focal length of 140 mm operating at a frequency of 0.8 MHz. The focal region is 9.8 mm in length along the beam axis and 1.3 mm in the transverse direction. In the centre of the HIFU transducer, a 3.5–5.0 MHz convex diagnostic US probe is mounted coaxially to provide real-time imaging for targeting. The integrated transducer is immersed in a water reservoir filled with degassed water maintained at a temperature of 12–15°C. Through computer control, the integrated transducer can be moved smoothly in six directions, including three orthogonal directions (x, y and z), rotation along the US beam axis (θ), and rotation along the long (γ)or short(φ) axis of the bed.
HIFU ablation procedures
For better observation and treatment of possible complications of HIFU ablation, all patients were treated as inpatients in our department. HIFU ablation was performed under general anaesthesia. The patients were carefully placed in an appropriate position – either prone, supine or on the side according to the tumour location – so that the skin overlying the lesion to be treated could be easily placed in contact with the degassed water.
For patients with multiple recurrent tumour nodules, HIFU ablation was performed with a palliative aim. If the tumour nodule was not adjacent to major nerves such as the sciatic nerve, the planned ablation area enveloped the entire tumour. If the tumour nodule was adjacent to major nerves, the planned area enveloped the tumour as much as possible but evaded the major nerves. For patients with an untreated tumour in the abdominal wall and right buttock, HIFU ablation was performed with a curative aim, the tumour and a margin of at least 1 cm were ablated.
First, real-time US was used to target the tumour, the area to be treated was divided into sections with 5 mm of separation perpendicular to the transducer surface on US. HIFU energy was intermittently applied with an acoustic power varying from 300 to 500 W; each exposure lasted 2–3 s to ablate one target spot. A power output of 300 W was used initially, if the treated area did not become hyperechoic on US after the energy exposure, the power output was increased at an increment of 40 W until the treated spot became hyperechoic on US. After ablation of the spot, the transducer was moved, and a nearby spot was treated. This process was repeated section by section, in successive sweeps from the deep to shallow regions until cavitation and bubble formation occurred (the target area became hyperechoic on US). To avoid excessive skin heating, the focused beam did not pass through the scars if possible, energy exposure was intermittently applied, and the skin overlying the tumour was observed on US during the intervals. All HIFU treatments were performed by the same physician (W.W.).
Post-treatment observation and follow up
After HIFU ablation, patients were carefully observed for possible complications such as skin burn and side effects such as pain and fever, the function of the affected limb was also observed. If there was severe pain after HIFU ablation, oral non-steroidal analgesic or morphine injection was administered depending on the pain severity. During the follow-up period, the treated area was examined by colour Doppler US and contrast-enhanced MRI 1 and 3 months after HIFU ablation and then at 4–6 month intervals to assess the therapeutic response or tumour progression. Areas which did not enhance after contrast administration on MRI were considered to represent necrotic tissue. Enhancing areas were assumed to represent viable tumour. If residual tumour enlarged significantly which caused symptoms or recurrent tumour was detected, another HIFU ablation session was planned if the tumour still met the inclusion criteria.
Results
HIFU ablation was successfully performed in all patients, a total of 25 tumour nodules were treated during HIFU ablation. Eight patients received one HIFU ablation. To avoid possible compartment syndrome, two patients with multiple recurrent tumours received two HIFU ablations separated by one month as scheduled. After HIFU ablation, the skin overlying the treated area became swollen in all patients. One patient had a first-degree skin burn 2 cm in diameter requiring no treatment. The function of the affected limb was not impaired in patients with tumour nodules adjacent to the sciatic nerve. All patients complained of mild pain in the treated area which subsided in 1–3 days requiring no analgesics. Four patients were afebrile after treatment, six patients had low-grade fever (less than 38.5°C) which persisted for 1–3 days after HIFU ablation. No major complications were encountered after HIFU ablation. Recovery after HIFU ablation was speedy. Patients with tumour involvement of the lower extremity were allowed to walk the next day after HIFU ablation. All patients were discharged within three days of treatment. According to contrast-enhanced MRI 1 month after scheduled treatment, the mean ablated volume was 89.5% of the tumour (range, 78–100%).
The patients were closely followed up until January 2011. During a mean follow up of 30 months (range, 8–55 months), the treated area shrank significantly over time (). No tumour spread along the treated area was observed in any patient. In the eight patients with inoperable recurrent tumours, two received further HIFU ablation session due to enlargement of residual tumours; significant coagulation necrosis was also obtained without complications. Because tumour progression was effectively suppressed by HIFU ablation and no recurrence was observed after 9–55 months of follow up, the patients with recurrent tumours received no other treatments. Persistent absence of contrast enhancement was observed in the treated area in the two patients with a solitary new tumour in the right buttock or abdominal wall, suggesting complete tumour necrosis ().
Figure 1. A 7-year-old girl with multiple recurrent desmoid tumour nodules in the left leg and buttock, who received three surgical resections before HIFU ablation. Further resection was considered impossible. HIFU ablation was performed with a palliative aim. (A) Before HIFU ablation, there were multiple tumour nodules (arrows) which showed high enhancement on coronal T1-weighted contrast-enhanced MRI. (B) One month after HIFU ablation, almost all treated tumours (arrows) showed no enhancement. (C) Nine months after HIFU ablation, the treated area (arrow) showed no enhancement and shrank significantly. (D) Sixteen months after HIFU ablation, the treated area (arrow) showed no enhancement and shrank further.
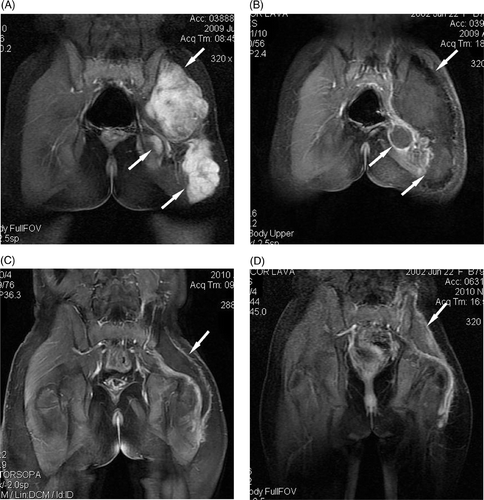
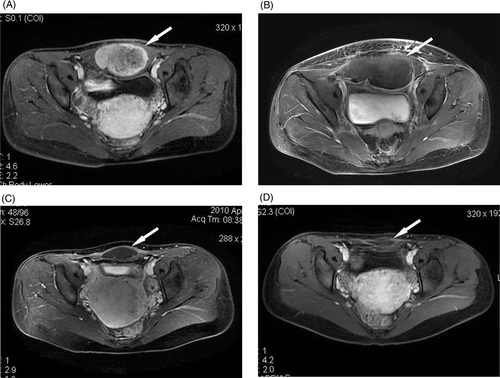
Figure 2. A 28-year-old woman with an abdominal wall desmoid tumour receiving no treatment before HIFU ablation. (A) Before HIFU ablation, the tumour (arrow) showed high enhancement on transverse T1-weighted contrast-enhanced MRI. (B) Four days after HIFU ablation, the treated area (arrow) showed no contrast enhancement, the skin overlying the treated area was swollen. (C) Ten months after HIFU ablation, no contrast enhancement was observed in the treated area (arrow), which shrank significantly. (D) Eighteen months after HIFU ablation, no enhancement was observed in the treated area (arrow), which shrank further.
Discussion
In this study, HIFU was used for the first time to treat extra-abdominal desmoid tumours. HIFU ablation was performed as a palliative treatment for patients with recurrent inoperable tumour or as a curative option for patients refusing surgery. Because the echogenic change during HIFU ablation bears no relationship with the temperature change or extent of ablation Citation[12], Citation[15], to ensure treatment efficacy, the HIFU energy was intermittently applied until cavitation and bubble formation occurred inside the tumour. At this time, the tumour became hyperechoic on US, indicating coagulation necrosis Citation[10], Citation[11], Citation[13], Citation[14].
Though HIFU ablation could not achieve complete tumour eradication in all recurrent tumours, it did obtain significant debulking effect and suppressed the local progression of the tumour, thereby preserving the affected limbs of the patients and improving the quality of life. As a repeatable minimally invasive therapy, HIFU ablation can induce large volume coagulation necrosis in a single session and has a speedy post-operative recovery. Therefore, it is easily accepted by the patients and may become a promising treatment for local control of recurrent desmoid tumours. This technique may also have great potential for the treatment of other soft tissue masses, especially inoperable ones.
HIFU ablation was performed with a curative aim in the two patients with new untreated tumours not involving major neurovascular structures. The tumours were ablated with a margin of at least 1 cm to obtain in situ ‘radical resection’. After HIFU ablation, the entire tumour showed no enhancement on contrast-enhanced MRI which gradually shrank over time, no local recurrence was encountered during follow up. The results suggested that HIFU ablation may also be potentially curative for new desmoid tumours not involving major neurovascular structures.
Tumour spread along the incision is common in extra-abdominal desmoid tumours after incomplete resection, which often precludes further surgery. Loss of integrity of fascial compartment due to surgery is the main cause for tumour spread. Because HIFU ablation requires no incision, no tissue needs to be sacrificed, the integrity of the fascial compartment is preserved. Therefore, the likelihood of tumour spread is low, which may be an advantage for HIFU ablation. As can be seen in this study, no tumour spread along the treated area was observed in all patients.
This study has some limitations. First, it was not a randomised controlled trial with surgical resection or radiation therapy, the patient number was small and the follow up was short for some patients. Second, the therapeutic efficacy and safety of HIFU ablation is influenced by the acoustic pathway. If there is extensive scarring in the proposed pathway of the HIFU beam or if the skin has become hard and fibrous due to high-dose radiation therapy, it may destroy the focus of the beam and absorb excessive energy during HIFU ablation, causing irreversible skin burn. Third, measures to prevent neurovascular damage are based on the operator's experience in this study. Because diagnostic ultrasound does not visualise the nerve effectively, more reliable methods such as MRI guidance should be employed to clearly depict the neurovascular structure and temperature rise inside the tumour, thereby preventing nerve damage while enlarging the ablation area.
Conclusion
In conclusion, our preliminary results showed US-guided HIFU ablation could be used as an effective minimally invasive therapy for local control of extra-abdominal desmoid tumours. The authors alone are responsible for the content and writing of the paper.
Declaration of interest: A declaration of interest statement reporting no conflict has been inserted. Please confirm the statement is accurate.
References
- Enzinger FM, Weiss SW. Soft Tissue Tumorsthird. Mosby, St Louis, MO 1994; 201–229
- Shields CJ, Winter DC, Kirwan WO, Redmond HP. Desmoid tumours. Eur J Surg Oncol 2001; 27: 701–706
- Lewis JJ, Boland PJ, Leung DHY, Woodruff JM, Brennan MF. The enigma of desmoid tumors. Ann Surg 1999; 229: 866–868
- Eich GF, Hoeffel JC, Tschappeler H, Gassner I, Willi UV. Fibrous tumors in children: Imaging features of a heterogeneous group of disorders. Pediatr Radiol 1998; 28: 500–509
- Goy BW, Lee SP, Eilber F, Dorey F, Eckardt J, Fu YS, Juillard GJ, Selch MT. The role of adjuvant radiotherapy in the treatment of resectable desmoid tumors. Int J Radiat Oncol Biol Phys 1997; 39: 659–665
- Kamath SS, Parsons JT, Marcus RB, Zlotecki RA, Scarborough MT. Radiotherapy for local control of aggressive fibromatosis. Int J Radiat Oncol Biol Phys 1996; 36: 325–328
- Pritchard DJ, Nascimento AG, Peterson IA. Local control of extra-abdominal desmoid tumors. J Bone Joint Surg 1996; 78: 848–854
- Skapek SX, Hawk BJ, Hoffer FA, Dahl GV, Granowetter L, Gebhardt MC, Ferguson WS, Grier HE. Combination chemotherapy using vinblastine and methotrexate for the treatment of progressive desmoid tumor in children. J Clin Oncol 1998; 16: 3021–3027
- Kennedy JE, Wu F, ter Haar GR, Gleeson FV, Phillips RR, Middleton MR. High-intensity focused ultrasound for the treatment of liver tumors. Ultrasonics 2004; 42: 931–935
- Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, Li KQ, Jin CB, Xie FL, Su HB. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer. Radiology 2005; 236: 1034–1040
- Chen W, Zhu H, Zhang L, Li K, Su H, Jin C, Zhou K, Bai J, Wu F, Wang Z. Primary bone malignancy: Effective treatment with high-intensity focused ultrasound ablation. Radiology 2010; 255: 967–978
- Haar GT, Coussios C. High intensity focused ultrasound: Past, present and future. Int J Hyperthermia 2007; 23: 85–87
- Wang Yang, Wang Wei, Wang Yuexiang, Tang Jie. Ultrasound-guided high-intensity focused ultrasound treatment for needle-track seeding of hepatocellular carcinoma: Preliminary results. Int J Hyperthermia 2010; 26: 441–447
- Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu H, Wang ZB. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med Biol 2001; 27: 1099–1106
- Tempany CM, McDannold NJ, Hynynen K, Jolesz FA. Focused ultrasound surgery in oncology: Overview and principles. Radiology 2011; 259: 39–56
