Abstract
The effects of heat, especially long-term heat exposure, are complex and incompletely understood and few studies have analysed the immunological consequences of such exposures. In the present study we analysed how long-term hyperthermia modified the pulmonary immune responses, especially recruitment of neutrophils to sites of inflammation, infection and injury. Using our mouse model of long-term whole body hyperthermia (continuous 5-day passive febrile range hyperthermia (5d-FRH)) we found that bacterial lipopolysaccharide (LPS) challenge greatly increased neutrophil accumulation in bronchoalveolar lavage and lung parenchyma in 5d-FRH exposed mice in comparison to LPS-treated controls. Moreover, the effect was sustained, and persisted during the post-exposure recovery period, and LPS challenge on days 5–7 post-recovery also exhibited similarly augmented neutrophil response. Lung lavage from 5d-FRH mice, either immediately or up to 7 days post-exposure, showed significantly increased levels of ELR + CXC chemokines, KC or LIX in response to LPS challenge, indicating that enhanced chemokines could contribute to the increased recruitment of neutrophils to the lung. However, an in vivo neutrophil migration assay following 5d-FRH and during the post-exposure recovery period also showed persistently enhanced neutrophil influx in response to a fixed chemotactic gradient generated by recombinant human IL-8, suggesting that additional mechanisms besides increased ELR + CXC chemokines contributed to the augmented neutrophil response caused by 5d-FRH exposure. These previously unappreciated profound and lasting effects of long-term hyperthermia may have important consequences and may help explain the increased risk of respiratory illnesses in active duty personnel and returning veterans.
Introduction
The effects of heat stress are complex, profound, and incompletely understood. Acute exposure to heat shock or mild whole body hyperthermia elicits a myriad of effects, including activation of the heat shock response and induction of heat shock protein (HSP) genes [Citation1–3]. Several studies, including those from our group, have indicated that heat shock and/or hyperthermia can also modify host immune responses including cytokine-chemokine gene expression and mobilisation of lymphocytes and neutrophils [Citation4–16].
We have shown that exposing mice to whole body hyperthermia in the febrile range (febrile-range hyperthermia(FRH) core temperature ∼39.5°C) augments recruitment of neutrophils to sites of infection, inflammation, and injury via multiple converging mechanisms, including: (a) increased generation of chemokines and GM-CSF; (b) induction of G-CSF and expansion of the circulating neutrophil pool; and (c) increased endothelial capacity for neutrophil transendothelial migration [Citation9–11, Citation[17]. These actions of FRH greatly accelerate pathogen clearance but also increase collateral tissue injury and the net outcome depends on the nature of the pathologic process and the tissue affected Citation[9], Citation[10], Citation[18]. For example, FRH exposure improved survival in a mouse model of Klebsiella pneumoniae peritonitis Citation[18] but worsened survival in the pneumonia model with the same pathogen Citation[9] despite similar enhancement of pathogen clearance indicating increased vulnerability of the lung to collateral tissue injury caused by neutrophil-dependent inflammation. In fact, the observed occurrence of acute respiratory distress syndrome (ARDS) in almost a quarter of patients with acute heat stroke Citation[19] and the increased susceptibility of the pulmonary epithelium to apoptosis following hyperthermia Citation[20], provide further support that the lung is at relatively increased risk of injury in the setting of hyperthermia.
Compared with the acute effects of short-term heat exposure, little is known about chronic exposure to hyperthermia. Although long-term heat exposure results in heat acclimation (HA) and acquired thermal tolerance (ATT) Citation[21], Citation[22], the immunological consequences of such exposure are poorly understood. A survey study of troops participating in Operation Bright Star in Egypt showed 47% of 1454 deployed troops reported a respiratory illness Citation[23]. A survey of returning troops participating in Operation Enduring Freedom/Operation Iraqi Freedom showed both asthmatic and non-asthmatic participants had increased respiratory symptoms of wheezing, cough, sputum production, chest pain/tightness, and allergy symptoms during deployment compared to pre-deployment Citation[24]. These studies not only indicate that long-term hyperthermia exerts important immunomodulatory effects but also implicate the vulnerability of the lung and the respiratory tract to its adverse effects.
In the present study we used our recently developed mouse model of long-term hyperthermia comprising a continuous 5-day exposure to passive FRH (5d-FRH) Citation[22] and a well-characterised model of lung inflammation induced by endotoxin inhalation Citation[5], Citation[9] to determine the consequences of long-term hyperthermia on regulation of inflammation in the lung. We found that such exposure caused a persistent alteration in the lung immune response characterised by a greatly increased capacity for bacterial lipopolysaccharide (LPS)-induced neutrophil accumulation in bronchoalveolar lavage fluid (BALF) and lung parenchyma, in part through increased expression of ELR + CXC chemokines. Moreover, the effects were sustained and lasted for several days (5–7 days) during the recovery period after cessation of hyperthermia exposure. These previously unappreciated profound and lasting effects of long-term hyperthermia may have important consequences and may help explain the increased risk of respiratory illnesses in active duty personnel and returning veterans.
Materials and methods
5d-FRH exposure
Male CD-1 mice weighing 30–35 g were purchased from Charles River (Wilmington, MA) and housed in the Animal Care Facility in the Veteran's Administration Medical Center, Baltimore, under Association for Assessment and Accreditation of Laboratory Animal Care (AALAC) approved conditions and under the supervision of a full-time veterinarian. Mice were adapted to standard plastic cages for at least 4 days before the study and were used within 4 weeks of arrival. To avoid the influence of diurnal cycling, all experiments were started at approximately the same time each day (between 8:00 and 10:00 AM). Mice were implanted with intraperitoneal telemetric thermistors (Data Safety International; St. Paul, MN; ETA-F10) 10 days prior to FRH exposure. A sterilised ETA-F10 transmitter was placed into the peritoneal cavity and subcutaneous electrodes secured under isoflurane anaesthesia as described in the manufacturer's protocol. The mice received 0.1 mg/kg buprenorphine analgesia subcutaneously every 12 hours (s.c. q12h) for 2 postoperative days, housed one mouse per cage and were provided with food and water ad libitum immediately after surgery and allowed to recover for 10 days at 24–25°C ambient temperature. FRH was imposed by transferring the mice in the standard cages into modified Air Shields™ infant incubators containing DSI data receivers with air temperature set to 37°C and core temperature monitored using the DSI Automated Data Acquisition System for 5 days (5d-FRH). Normothermic controls were housed at standard room temperature (24–25°C). Following the 5d-FRH-exposure protocol, mice were immediately (0d post-5d-FRH) challenged with intra-tracheal (i.t.) LPS or IL-8 (see below) or were allowed to recover at standard room temperature for up to 7 days prior to LPS or IL-8 challenge. Except for the ambient temperature, handling of normothermic control and 5d-FRH mice was identical. All procedures were approved by the Baltimore VA and the University of Maryland, Baltimore, Animal Care and Use Committee.
Intra-tracheal LPS/IL-8 instillation
Mice were challenged with LPS (50 µg LPS in 50 µL sterile PBS) or with 50 µL sterile PBS i.t. as described before Citation[5], Citation[8], Citation[9] either immediately (0 days post-exposure recovery) or during the post-exposure recovery period as indicated. All LPS instillations were for 24 h at standard room temperature following which the mice were euthanised and lungs were either lavaged for analysis of cell and cytokine composition or inflation-fixed for confocal immunofluorescence analysis as described earlier Citation[5], Citation[8–10]].
To analyse how FRH modified the in vivo capacity for chemokine-directed trans-alveolar migration (TAM) of neutrophils, mice were challenged with 1 µg recombinant human IL-8 (rhIL8) (R&D Systems, Minneapolis, MN) in 50 µL sterile PBS using the same protocol as for LPS, except the mice were euthanised 4 h later at room temperature and analysed for lung lavage composition as above.
Bronchoalveolar lavage fluid (BALF) cell and cytokine composition
Cells were collected by centrifugation and total cell counts and differential cell counts of Diff-Quick™-stained cytopreparations were performed manually using a haemocytometer by two blinded observers using morphologic criteria as described earlier Citation[9], Citation[10]. Mouse KC/CXCL1, MIP-2/CXCL2, and LIX/CXCL5 were measured by ELISA in the cell-free supernatants as we have previously described Citation[5], Citation[8–10]].
Immunofluorescence confocal microscopy
After euthanasia, the anterior chest wall was resected, the trachea cannulated with an 18-gauge blunt needle, and the lungs were inflated in situ with 4% v/v paraformaldehyde at 20 cm H2O pressure. The trachea and lungs were embedded en bloc in paraffin and 25 µm sections were cut immunostained with biotinylated rat anti-mouse Gr-1 (AbD Serotec, Oxford) to identify neutrophils and rabbit anti-VE-cadherin (Sigma, St Louis, MO) to identify endothelium. Non-specific signal was reduced by sequentially blocking with 1% donkey serum (v/v) in PBST for 30 min and with a commercial avidin/biotin blocking kit (Vector, Burlingame, CA) according to the manufacturer's instructions, then sequentially incubated overnight with primary antibody and a 1:500 dilution of appropriate secondary antibody for 1 h, and mounted. The immunostained sections were visualised using an Olympus microscope and Fluo View confocal software (Olympus America, Center Valley, PA). 1024 × 1024-pixel z series images (1 uM step size) for each fluorophore were obtained. The number and localisation of the neutrophils was analysed using the Neurolucida™ software. The localisation of the neutrophils relative to the vascular endothelium was determined using a modification of the criteria described by Woodfin et al. Citation[25]. In brief, if endothelial staining was observed on both sides of the long axis of a neutrophil, the cell was classified as intravascular. If endothelial staining was absent from either side of a neutrophil, it was classified as extravascular or extravasating.
Data analysis
Data are presented as mean ± SE. Differences between more than two groups were analysed by one-way analysis of variance (ANOVA); post-hoc analysis was conducted using the Tukey honestly significant difference (HSD) test.
Results
Effect of ambient temperature on core temperature of mice exposed to FRH
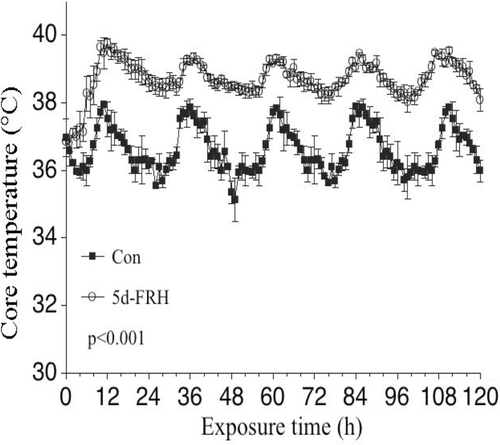
Core temperature of FRH-exposed and normothermic controls was remotely monitored in at least one mouse per group using the Data Sciences International Automated Data Acquisition System (St Paul, MN) every 20 s for 120 h and hourly averages were calculated. The core temperature exhibited a circadian pattern in both control and FRH exposed mice (), but core temperature in the FRH mice was approximately 2°C higher than the normothermic controls and this difference was maintained for the entire 5-day exposure. Upon removal to room temperature after 5d-FRH, core temperature of the mice returned to baseline levels within 60–90 min (data not shown) Citation[22].
Figure 1. Core temperature during passive 5d-FRH: Mice implanted with i.p. sensors were housed at 25°C (Control) or 37°C (FRH) ambient temperature for 5 days while continuously monitoring core temperature. The mean temperature for each hour was calculated. Two experiments, each with four mice per group, were pooled. Data are mean ± SE. Core temperature in FRH mice was greater than control with p < 0.001 by repeated measures ANOVA.
5d-FRH markedly enhanced neutrophil recruitment to the lung following i.t. LPS challenge
We have earlier shown that exposure to acute FRH for 24 h augmented neutrophil accumulation in BALF following i.t. LPS Citation[9]. To determine whether 5d-FRH had similar effects, we determined neutrophil content of BALF collected 24 h after i.t. LPS in 5d-FRH exposed and normothermic control mice (). As previously reported, i.t. LPS for 24 h caused enhanced neutrophil accumulation in BALF, but the effect was markedly increased by approximately 2-fold in the 5d-FRH exposed group (2.55 × 106 versuss ∼1.32 × 106, p < 0.001). More interestingly, the effect of 5d-FRH on LPS-induced BALF neutrophilia persisted for several days after cessation of 5d-FRH exposure and mice challenged with LPS for 24 h even on day 2, day 3 or day 5 post-5d-FRH showed significantly higher count of neutrophils in the BALF (3.72 × 106, 2.49 × 106 and 2.38 × 106, respectively, p < 0.05 with respect to LPS-challenged normothermic controls).
Figure 2. 5d-FRH exposure increases neutrophil recruitment to lung after i.t. LPS challenge. Mice were exposed to 5d-FRH and then transferred to room temperature for post exposure recovery. LPS-treated normothermic controls or 5d-FRH-exposed mice received 50 µg LPS i.t. either immediately (0 days) or as indicated whereas LPS-untreated mice received sterile PBS and euthanised after 24 h at room temperature. (A) lungs were lavaged and total neutrophil content determined by manual counting. Mean ± SE of 4 mice per group. * and # denotes p < 0.05 versus PBS- or LPS-treated controls, respectively. (B) lungs were inflation/fixed, stained for neutrophils (Gr-1, red) and endothelium (VE-cadherin, green) and analysed by confocal microscopy. Representative fields of three mice per group are shown. (C) Confocal images were analysed for number of total, intravascular (IN) and extravasating (OUT) neutrophils per 60× field. Mean of three fields per mouse, three mice per group plotted.
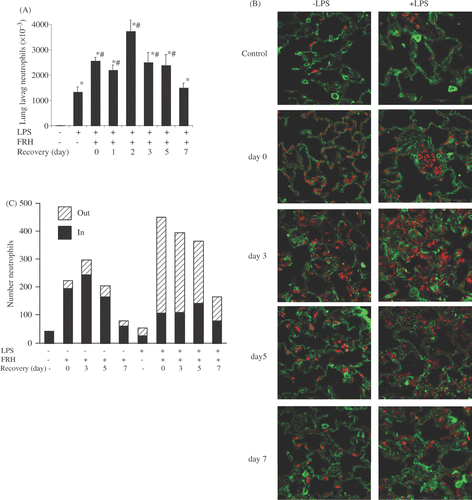
To further analyse the effects of 5d-FRH and recovery on neutrophil recruitment from the pulmonary microvasculature, normothermic and 5d-FRH-exposed mice were treated with or without i.t. LPS for 24 h and lungs were inflation-fixed, immunostained with antibodies against Gr-1 and VE-cadherin to identify neutrophils and endothelium, respectively, and analysed by immunofluorescence confocal microscopy (). Prior to i.t. LPS instillation, neutrophil sequestration in the pulmonary vasculature was substantially higher in the 5d-FRH mice compared with normothermic controls (, compare panel 0- with C-) and the effect persisted for at least 5 days post-5d-FRH exposure (, compare panels 5- and 7- with 0-). In normothermic mice, LPS instillation also increased neutrophil counts in the pulmonary microvasculature but the effect was modest (, compare panels C− with C+) and not as distinct as observed with BALF analysis (). However, similar to BALF analysis, LPS instillation greatly enhanced neutrophil accumulation in the terminal airspaces in 5d-FRH-exposed mice (, compare panels 0+ with C+) and the effects paralleled neutrophil sequestration during the post-exposure recovery period (, panels 3+, 5+ and 7+).
To further define the effect of 5d-FRH exposure and recovery on neutrophil recruitment in lung, we quantified neutrophil intravascular retention and extravasation in the confocal images using a previously described protocol Citation[25] (). Neutrophils were classified as intravascular if they were completely contained within the blood vessel. Otherwise they were classified as extravasating/extravascular. This analysis further showed that the neutrophil content in lung was increased several fold following 5d-FRH, that ∼80% of these cells were intravascular, and that this effect lasted for at least 5–7 days after 5d-FRH exposure. In control mice, i.t. LPS caused a modest increase in the total neutrophil content but, most importantly, increased the proportion of total neutrophils that had extravasated from ∼0% to 50%. 5d-FRH exposure markedly increased the total number of neutrophils in the lung by several fold till day 5–7 of recovery but the proportion of extravasated neutrophils was around 20% in the absence of LPS challenge. LPS instillation further enhanced the lung neutrophil content by approximately two-fold and also enhanced the extravascular or extravasating proportion to about 75% which was comparable throughout the recovery period.
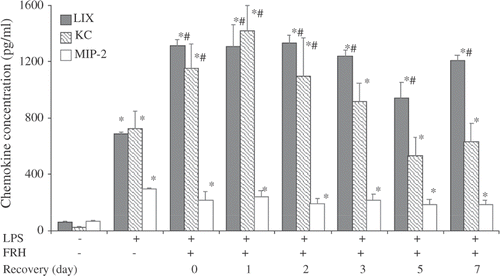
5d-FRH-exposure greatly enhanced LPS-induced CXC chemokine levels in the BALF
We have previously shown that FRH concurrent with i.t. LPS Citation[8], Citation[9] or hyperoxia Citation[10] augmented CXC chemokine levels in BALF and that this effect is dependent upon the stress-activated transcription factor heat shock factor-1 (HSF1) Citation[8], Citation[26], Citation[27]. To analyse the effect 5d-FRH exposure and its potential contribution to the incremental neutrophil accumulation, we measured endogenous ELR + CXC chemokines, KC (CXCL1), MIP-2 (CXCL2), and LIX (CXCL5) levels in BALF of control and 5d-FRH exposed mice 24 h after i.t. LPS instillation (). As expected, LPS instillation increased BALF levels of all three ELR + CXC chemokines in controls and in 5d-FRH-exposed mice the levels of KC and LIX (but not MIP-2) were increased further by 1.6- and 1.9-fold, respectively. Furthermore, the effect of 5d-FRH exposure on LIX expression persisted throughout the 7-day recovery period, whereas, LPS-induced KC expression returned to pre-5d-FRH-exposure levels by the third day of recovery. In the absence of i.t. LPS, 5d-FRH-exposed and control mice had similarly low BALF levels of the three ELR + CXC chemokines (data not shown).
Figure 3. 5d-FRH exposure has a profound and lasting effect on LPS-induced CXC chemokine levels in lung: Mice were treated as indicated above (), lungs lavaged, and KC, LIX and MIP-2 assayed by ELISA. Data are mean + SE, of four to six mice per group. * and # denotes p < 0.05 versus PBS or LPS-treated controls, respectively.
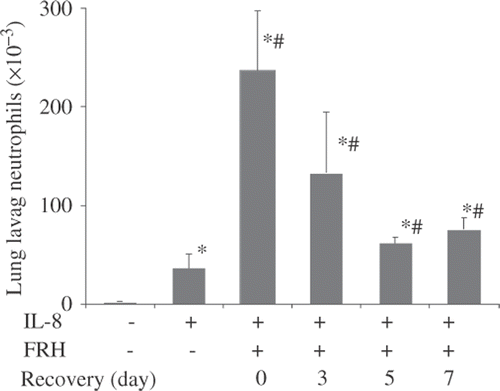
IL8-directed transalveolar migration of neutrophils is also enhanced after 5d-FRH
Using an in vivo IL-8-directed neutrophil TAM assay we have recently found that FRH for 16–24 h profoundly augmented subsequent accumulation of neutrophils in BALF after i.t. IL-8 Citation[28]. Using the same assay we found that 1 µg rhIL-8 i.t. caused about 3.3-fold more neutrophil accumulation in the BALF of 5d-FRH mice than in normothermic mice (∼245 × 103 versus 74 × 103 cells/mL, p < 0.001) (). Moreover, the effect persisted during the recovery period and although it waned during recovery, it was still significantly greater after 7 days of post-5d-FRH exposure. Most importantly, i.t. rhIL-8 for 4 h did not alter BALF levels of KC, LIX, or MIP-2 in controls or 5d-FRH exposed mice (data not shown) suggesting the enhanced recruitment of neutrophils after 5d-FRH was not only due to enhanced LIX and KC levels but also due to other contributing mechanisms.
Figure 4. 5d-FRH exposure increases capacity for IL-8-directed neutrophil transalveolar migration. Mice were treated as indicated above () except 1 µg rhIL-8 was instilled i.t. instead of LPS and mice were euthanised for lung lavage 4 h later and neutrophils counted. Mean ± SE of four mice per group. * and # denotes p < 0.05 versus PBS- or LPS-treated controls, respectively.
Discussion
Neutrophils are among the earliest leukocyte responders to infection, inflammation, and injury and play an essential role in containing infections through release of cytotoxic effector molecules Citation[29], Citation[30]. Unfortunately, the same effector molecules can also cause substantial collateral tissue injury, especially to highly susceptible sites such as the lungs Citation[31]. Neutrophil accumulation and enhanced neutrophilia in BALF has been correlated with poor prognosis in septic ARDS and acute lung injury (ALI) and neutrophil-mediated cytotoxicity has been cited as the major contributor to ALI and ARDS [Citation32–35].
Our group has previously shown that acute, concurrent exposure to FRH exerts multiple actions that profoundly increase neutrophil recruitment, enhance pathogen clearance and augment lethal lung injury in mouse models of pneumonia and hyperoxia Citation[9], Citation[10]. We showed that acute FRH exposure converted non-lethal lung inflammation induced by i.t. instillation of LPS in normothermic mice to a lethal, neutrophil-dependent lung injury Citation[9] suggesting that the increase in core temperature that occurs during febrile illnesses can increase collateral tissue injury in susceptible tissues, such as in the lung. In contrast, long-term heat exposure initiates adaptive changes including ATT and HA, a process of adaptive physiological changes that abates physiological strain, sustains physical and cognitive performance capabilities and protects against severe heat-related illnesses Citation[21], Citation[36], Citation[37]. In a mouse model we showed that following exposure to 5d-FRH mice exhibited improved heat elimination and exercise tolerance during subsequent heat exposure and increased basal HSP72 expression Citation[22], similar to what we observed with human HA Citation[21].
In the present study we extend our previous studies of 5d-FRH and the immunological consequences of concurrent FRH by showing that exposing mice to a chronic hyperthermia protocol that mimics human HA exhibit greatly enhanced neutrophil accumulation in response to a proinflammatory stimuli. Our present results indicate that 5d-FRH can have a persistent and sustained alteration in the lung immune response by profoundly enhancing the number of neutrophils in the pulmonary vasculature as well as their ability to migrate to sites of infection and injury. Using two independent methods, cell count in the BALF () and by confocal microscopy of lung tissue sections () we have shown that 5d-FRH markedly enhanced neutrophil recruitment to the lung and the influx was further augmented in response to a proinflammatory stimulus. Reutersham et al. Citation[38] showed that neutrophil recruitment following LPS inhalation occurs through a stepwise process, beginning with increased neutrophil sequestration within the lung vasculature that is detectable within the first hour after LPS instillation and culminating in accumulation of neutrophils in the bronchoalveolar space by 12 to 24 h post-LPS. Confocal imaging demonstrated that mice exposed to 5d-FRH exhibited greatly increased neutrophil sequestration within the lung vasculature (, day 0), and by 24 h after LPS instillation, the total neutrophil content in lung doubled but the number of neutrophils remaining in lung vasculature had decreased by over half (, day 0+LPS and ). Furthermore, mice exposed to 5d-FRH and allowed to recover for 3 or 5 days exhibited similar patterns of neutrophil sequestration and migration ( and C) suggesting that the effect was persistent and lasted for several days after cessation of FRH exposure. This augmented neutrophil recruitment was accompanied with an increase in ELR+ CXC chemokine LIX and KC levels in the BALF () indicating that an enhanced chemotactic gradient could be the contributing factor. However, neutrophil influx in the lung was increased in the 5d-FRH-exposed mice even in the absence of LPS challenge and increased CXC chemokine expression suggesting that additional mechanisms might also contribute to the process. To test this possibility we performed an in vivo IL-8-directed neutrophil TAM assay and found that neutrophil influx in the lung against a fixed chemotactic gradient generated with 1 µg rhIL-8 i.t. was significantly higher in 5d-FRH mice than normothermic controls () and similar to LPS instillation, the effect was persistent during the post-exposure recovery period. These studies indicate that in addition to increased CXC chemokine levels, other mechanisms involving both the neutrophils and the vascular endothelia might contribute to the enhanced neutrophil influx in the lung of 5d-FRH-exposed mice.
Our present results are unique and novel in two respects: first, no earlier study has demonstrated such a profound effect of hyperthermia on neutrophil recruitment to the pulmonary vasculature, and second, the persistent and lasting nature of the effect that could modify neutrophil priming and recruitment 5-7 days after the exposure. The fact that the effect was persistent and lasted for several days before returning or ‘resetting’ to normal baseline indicated that although reversible, the recovery after 5d-FRH was slow and prolonged during which the host displayed an altered inflammatory response to proinflammatory agonists. Enhanced neutrophil recruitment and neutrophil-mediated inflammation, loss of endothelial barrier function and epithelial injury are considered major contributors of ARDS and acute lung injury Citation[39], Citation[40]. It is likely that the increased incidences of multi-symptom illnesses [Citation41–44], immune abnormalities [Citation45–48], and respiratory illness including bronchitis and asthma Citation[23], Citation[24], Citation[49], Citation[50] reported in military personnel and returning veterans deployed in high temperature environments like the Gulf region might be due to environmental/exertional hyperthermia endured during deployment. The present study uniquely demonstrates that exposure to long-term hyperthermia can have profound and persistent consequences for innate immune function and regulation of inflammation, but the full spectrum of immunologic effects and mechanisms responsible remain to be elucidated. The importance of understanding the potential health effects of chronic exposure to hyperthermia and its long-term consequences is underscored by its relevance to large numbers of individuals who are exposed to environmental/exertional hyperthermia, including athletes, deployed troops and veterans.
Conclusion
Our studies show that 5d-FRH has a profound and distinct effect on neutrophil recruitment to the lung in response to a proinflammatory agonist. Furthermore, the effects are persistent and require several days before ‘resetting’ to normal during which the host displays a dysregulated response to proinflammatory agonists. Thus, long-term heat exposure that induces ATT and HA and protects against subsequent stress can be maladaptive in certain clinical contexts including infection, injury, and inflammatory disorders. Our studies underscore the complex effect of hyperthermia on host responses and the importance of understanding the immunological consequences especially on subsequent immune surveillance and/or sensitivity to infections and inflammatory disorders.
Declaration of interest: This study was supported by US National Institutes of Health grants GM069431 (ISS) and GM066855, HL69057 and HL085256 (JDH), and by Veterans Affairs Merit Review grants to JDH and ISS. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lindquist S. The heat-shock response. Ann Rev Biochem 1986; 55: 1151–1191
- Morimoto RI. Cells in stress: Transcriptional activation of heat shock genes. Science 1993; 259: 1409–1410
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: Regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem 1997; 32: 17–29
- Cahill CM, Lin HS, Price BD, Bruce JL, Calderwood SK. Potential role of heat shock transcription factor in the expression of inflammatory cytokines. Adv Exp Med Biol 1997; 400B: 625–630
- Cooper ZA, Ghosh A, Gupta A, Maity T, Benjamin IJ, Vogel SN, Hasday JD, Singh IS. Febrile-range temperature modifies cytokine gene expression in LPS-stimulated macrophages by differentially modifying NF-{kappa}B recruitment to cytokine gene promoters. Am J Physiol Cell Physiol 2010; 298: C171–181
- Singh IS, Viscardi RM, Kalvakolanu I, Calderwood S, Hasday JD. Inhibition of tumor necrosis factor-alpha transcription in macrophages exposed to febrile range temperature. A possible role for heat shock factor-1 as a negative transcriptional regulator. J Biol Chem 2000; 275: 9841–9848
- Singh IS, He JR, Calderwood S, Hasday JD. A high affinity HSF-1 binding site in the 5'-untranslated region of the murine tumor necrosis factor-alpha gene is a transcriptional repressor. J Biol Chem 2002; 277: 4981–4988
- Singh IS, Gupta A, Nagarsekar A, Cooper Z, Manka C, Hester L, Benjamin IJ, He JR, Hasday JD. Heat shock co-activates interleukin-8 transcription. Am J Respir Cell Mol Biol 2008; 39: 235–242
- Rice P, Martin E, He JR, Frank M, DeTolla L, Hester L, O'Neill T, Manka C, Benjamin I, Nagarsekar A, et al. Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental gram-negative bacterial pneumonia. J Immunol 2005; 174: 3676–3685
- Hasday JD, Garrison A, Singh IS, Standiford T, Ellis GS, Rao S, He JR, Rice P, Frank M, Goldblum SE, et al. Febrile-range hyperthermia augments pulmonary neutrophil recruitment and amplifies pulmonary oxygen toxicity. Am J Pathol 2003; 162: 2005–2017
- Ellis GS, Carlson DE, Hester L, He JR, Bagby GJ, Singh IS, Hasday JD. G-CSF, but not corticosterone, mediates circulating neutrophilia induced by febrile-range hyperthermia. J Appl Physiol 2005; 98: 1799–1804
- Chen Q, Fisher DT, Clancy KA, Gauguet JM, Wang WC, Unger E, Rose-John S, von Andrian UH, Baumann H, Evans SS. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol 2006; 7: 1299–1308
- Appenheimer MM, Chen Q, Girard RA, Wang WC, Evans SS. Impact of fever-range thermal stress on lymphocyte-endothelial adhesion and lymphocyte trafficking. Immunol Invest 2005; 34: 295–323
- Pritchard MT, Li Z, Repasky EA. Nitric oxide production is regulated by fever-range thermal stimulation of murine macrophages. J Leukoc Biol 2005; 78: 630–638
- Repasky E, Issels R. Physiological consequences of hyperthermia: Heat, heat shock proteins and the immune response. Int J Hyperthermia 2002; 18: 486–489
- Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood 2001; 97: 2727–2733
- Hasday JD, Bannerman D, Sakarya S, Cross AS, Singh IS, Howard D, Drysdale BE, Goldblum SE. Exposure to febrile temperature modifies endothelial cell response to tumor necrosis factor-alpha. J Appl Physiol 2001; 90: 90–98
- Jiang Q, Cross AS, Singh IS, Chen TT, Viscardi RM, Hasday JD. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun 2000; 68: 1265–1270
- el-Kassimi FA, Al-Mashhadani S, Abdullah AK, Akhtar J. Adult respiratory distress syndrome and disseminated intravascular coagulation complicating heat stroke. Chest 1986; 90: 571–574
- Lipke AB, Matute-Bello G, Herrero R, Kurahashi K, Wong VA, Mongovin SM, Martin TR. Febrile-range hyperthermia augments lipopolysaccharide-induced lung injury by a mechanism of enhanced alveolar epithelial apoptosis. J Immunol 2010; 184: 3801–3813
- McClung JP, Hasday JD, He JR, Montain SJ, Cheuvront SN, Sawka MN, Singh IS. Exercise-heat acclimation in humans alters baseline levels and ex vivo heat inducibility of HSP72 and HSP90 in peripheral blood mononuclear cells. Am J Physiol Regul Integr Comp Physiol 2008; 294: R185–191
- Sareh H, Tulapurkar ME, Shah NG, Singh IS, Hasday JD. Response of mice to continuous 5-day passive hyperthermia resembles human heat acclimation. Cell Stress Chaperones 2011; 16: 297–307
- Riddle MS, Halvorson HA, Shiau D, Althoff J, Monteville MR, Shaheen H, Horvath EP, Armstrong AW. Acute gastrointestinal infection, respiratory illness, and noncombat injury among US military personnel during Operation Bright Star 2005, in Northern Egypt. J Travel Med 2007; 14: 392–401
- Roop SA, Niven AS, Calvin BE, Bader J, Zacher LL. The prevalence and impact of respiratory symptoms in asthmatics and nonasthmatics during deployment. Mil Med 2007; 172: 1264–1269
- Woodfin A, Voisin MB, Imhof BA, Dejana E, Engelhardt B, Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood 2009; 113: 6246–6257
- Nagarsekar A, Hasday JD, Singh IS. CXC chemokines: A new family of heat-shock proteins?. Immunol Invest 2005; 34: 381–398
- Maity TK, Henry MM, Tulapurkar ME, Shah NG, Hasday JD, Singh IS. Distinct, gene-specific effect of heat shock on heat shock factor-1 recruitment and gene expression of CXC chemokine genes. Cytokine 2011; 54: 61–67
- Almutairy EA, Shah NJ, Tulapurkar ME, Hasday JD. Febrile-range hyperthermia (FRH) augments neutrophil recruitment to lung via modulation of lung endothelium and neutrophils. Am J Resp Crit Care Med 2009; 179: A4012
- Nathan C. Neutrophils and immunity: Challenges and opportunities. Nat Rev Immunol 2006; 6: 173–182
- Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol 2010; 31: 318–324
- Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care 2001; 7: 1–7
- Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury: Prognostic and pathogenetic significance. Am J Respir Crit Care Med 1997; 155: 1187–1205
- Abraham E. Neutrophils and acute lung injury. Crit Care Med 2003; 31: S195–199
- Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest 2002; 110: 1703–1716
- Martin TR. Neutrophils and lung injury: Getting it right. J Clin Invest 2002; 110: 1603–1605
- Sawka MN, Young AJ, Cadarette BS, Levine L, Pandolf KB. Influence of heat stress and acclimation on maximal aerobic power. Eur J Appl Physiol Occup Physiol 1985; 53: 294–298
- Nielsen B, Strange S, Christensen NJ, Warberg J, Saltin B. Acute and adaptive responses in humans to exercise in a warm, humid environment. Pflugers Arch 1997; 434: 49–56
- Reutershan J, Basit A, Galkina EV, Ley K. Sequential recruitment of neutrophils into lung and bronchoalveolar lavage fluid in LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 2005; 289: L807–815
- Matthay MA, Zemans RL. The acute respiratory distress syndrome: Pathogenesis and treatment. Annu Rev Pathol 2011; 6: 147–163
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342: 1334–1349
- Proctor SP, Heeren T, White RF, Wolfe J, Borgos MS, Davis JD, Pepper L, Clapp R, Sutker PB, Vasterling JJ, et al. Health status of Persian Gulf War veterans: Self-reported symptoms, environmental exposures and the effect of stress. Int J Epidemiol 1998; 27: 1000–1010
- Blanchard MS, Eisen SA, Alpern R, Karlinsky J, Toomey R, Reda DJ, Murphy FM, Jackson LW, Kang HK. Chronic multisymptom illness complex in Gulf War I veterans 10 years later. Am J Epidemiol 2006; 163: 66–75
- Research Advisory Committee on Gulf War Veterans’ Illnesses. Gulf War illness and the health of Gulf War veterans: Scientific findings and recommendations. US Department of Veterans Affairs, Washington, DC 2008
- Iowa Persian Gulf Study Group. Self-reported illness and health status among Gulf War veterans. A population-based study. JAMA 1997; 277: 238–245
- Everson MP, Kotler S, Blackburn WD, Jr. Stress and immune dysfunction in Gulf War veterans. Ann N Y Acad Sci 1999; 876: 413–418
- Everson MP, Shi K, Aldridge P, Bartolucci AA, Blackburn WD. Immunological responses are not abnormal in symptomatic Gulf War veterans. Ann N Y Acad Sci 2002; 966: 327–342
- Everson MP, Shi K, Aldrige P, Bartolucci AA, Blackburn WD, Jr. Is there immune dysregulation in symptomatic Gulf War veterans?. Z Rheumatol 2000; 59S2: II/124–126
- Vojdani A, Thrasher JD. Cellular and humoral immune abnormalities in Gulf War veterans. Environ Health Perspect 2004; 112: 840–846
- Karlinsky JB, Blanchard M, Alpern R, Eisen SA, Kang H, Murphy FM, Reda DJ. Late prevalence of respiratory symptoms and pulmonary function abnormalities in Gulf War I Veterans. Arch Intern Med 2004; 164: 2488–2491
- Gray GC, Blankenship TL, Gackstetter G. History of respiratory illness at the US Naval Academy. Mil Med 2001; 166: 581–586
