Abstract
Purpose: To evaluate the efficacy and safety of microwave ablation combined with transcatheter arterial chemoembolization for unresectable large-sized hepotocellular carcinoma.
Materials and methods: Institutional review board approval and informed consent were obtained. Between May 2004 and December 2006, 34 consecutive patients with large unresectable hepatocellular carcinoma (>5 cm) were alternately enrolled in one of two treatment groups: group 1 (n = 18), in which TACE was performed alone, and group 2 (n = 16), in which percutaneous ablation of HCC with microwave ablation was performed 2–4 weeks after TACE. All patients were followed up for 2–28 months to observe long-term therapeutic effects and complications in both groups. Tumor reduction rates, median survival time, and cumulative survival rates in both groups were calculated by using the unpaired Student t test and Kaplan-Meier method.
Results: Follow-up images showed reduction in tumor size was seen in 21 patients (61.7%; 7/18 in group 1, 14/16 in group 2), survival rates were better in group 2 than in group 1 (P = 0.003), during the median follow-up of 8 months, 10 patients (62.5%) remained alive in group 2, whereas 6 patients (33.3%) remained alive in group 1, the mean survival times were 6.13 months ± 0.83 in group 1 and 11.61 months ± 1.59 in group 2.
Conclusion: MWA combined with transcatheter arterial chemoembolization appears to be an effective and promising approach for the treatment of large-sized unresectable hepotocellular carcinoma. However, large-scale randomized clinical trials are needed to determine the future role of this treatment.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver, ranking fifth in frequency in the world Citation[1] and is also one of the most difficult types of cancer to treat. HCC is an insidious disease, with no particular or specific signs and symptoms of manifestation and whose behavior is usually unpredictable. Its natural history is also dependent on functional impairment of the underlying liver disease which often limits the application of therapeutic opportunities and influences survival. The spontaneous course of the unresectable disease has recently been evaluated in a meta-analysis which analyzed the survival rates of the placebo and untreated arms of several RCTs on HCC patients, showing that the 1- and 2-year survival is extremely heterogeneous and that the 1-year survival was much higher in RCTs including only BCLC B or C patients (34%) than in those also including BCLC D patients (11%) Citation[2]. Surgical resection is considered the most effective treatments, but it has a high recurrence rate Citation[3], Citation[4]. Unfortunately, hepatic resection of HCC in patients with cirrhosis is associated with significant preoperative mortality and morbidity Citation[5], Citation[6], the advantage of hepatic resection is only marginal in patients with huge HCC Citation[7]. Severe cirrhosis, multiple lesions often precludes liver resection or liver transplant in patients with liver tumors, it is only suitable for 30% patients with liver tumors Citation[8], Citation[9].
Advances in imaging have led to an increase in the use of minimally invasive technologies such as radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, high-intensity focused ultrasound (HIFU) have been used for the local ablation of liver lesions Citation[10–13]. Those minimally invasive technologies are principally performed in patients with small-volume HCCs no larger than 3–4 cm in diameter Citation[14], Citation[15]. Therefore transcatheter arterial chemoembolization (TACE) is one of the most widely used primary treatments for unresectable large-sized HCC. However, the treatment efficacy of TACE is influenced by many factors, such as blood supply, the size of tumors, and the ultra-selectivity of the catheter and else, it is almost impossible to achieve complete killing the tumor cells by TACE alone. After TACE, the remaining viable tumor cells may cause local recurrence and distant metastasis Citation[16], Citation[17]. For these reasons, TACE combination with ablative therapies has recently been used to exterminate residual tumor cells after effective TACE treatment Citation[18–21]. In clinical practice a multimodal approach that combines various techniques is used, either as first-line therapy or as a rescue (second-line) approach after the failure of a monotherapy. Multidisciplinary and multimodal approaches (with concomitant and/or sequential therapy) can be successful for converting patients with unresectable disease into surgical candidates and can stabilize disease as patients await transplantation Citation[22].
Microwave ablation (MWA) is an effective local thermal ablation technique for treating HCC which exhibit many advantages over other alternatives to resection Citation[23–31]. MWA is an appealing alternative for patients for whom surgery poses a risk, and as an effective conservative approach to HCC in cirrhosis in China because of the minimal damage to liver function, relative lack of complications, and low mortality, as well as the promising clinical results Citation[27–31]. The efficacy of combining transcatheter arterial chemoembolization with subsequent percutaneous microwave coagulation therapy was assessed in hepatocellular carcinomas (HCC) measuring >2.0 cm but <3.0 cm in greatest dimension Citation[32].
In this study, we hypothesized that microwave ablation combined with TACE would be more effective than TACE alone in the treatment of large-sized HCC in patients. Thus, the purpose of our study was to evaluate the use of microwave ablation combined with TACE in the treatment of large-sized HCC in patients. To our knowledge, this study was the first study about MWA combined with TACE for unresectable large-sized hepotocellular carcinoma (HCC).
Materials and methods
Patients
Between May 2004 and December 2006, 34 consecutive patients with large unresectable hepatocellular carcinoma (>5 cm) were enrolled in a prospective controlled clinical protocol that was approved by the ethics committee at Chinese PLA General Hospital. Patients in whom lesions were not suitable for surgical resection were referred to this trial. The selection criteria for enrollment in our study were as follows: presence of multiple lesions; tumor proximity to major vascular structures, which precluded the resection of a tumor-free margin; or presence of severe cirrhosis with an insufficient hepatic functional reserve to tolerate conventional HCC resection. HCC diagnosis confirmed at US-guided fine-needle biopsy or made on the basis of both the characteristic findings of HCC lesions shown at imaging and a high level (more than 200 ng mL−1) of serum α-fetoprotein. A detailed written description of the procedure was provided to all patients before enrollment. Informed consent was obtained from all patients at the time of enrollment.
All patients had stable hematogenic parameters (patients with platelets count <40,000/mm3, INR > 1.75, PTT > 40 s were excluded from the procedure); no active infection; no history of hepatic encephalopathy; and few or no ascites detected at Doppler US. Patients with extrahepatic metastases, or severe coagulation disorders were excluded from this study.
Patients were consecutively enrolled at clinical presentation and were alternately assigned to one of the following two treatment groups: group 1, the TACE group (n = 18), in which TACE alone was performed; or group 2, the combined treatment group (n = 16), in which transcutaneous ablation of HCC with microwave ablation was performed 2–4 weeks after TACE. There were two fewer patients in group 2 than in group 1, because one patient in group 1 refused to undergo microwave ablation after TACE. Patient ranged in age from 36 to 83 years. There were 29 men and 5 women. The mean diameter of the largest tumor was 6.74 ± 1.45 cm (range 5.1–10.6 cm); The mean liver tumor number was 2.1 ± 1.4 (range 1–6) per patient. Patient characteristics assessed in both groups are shown in the .
Table I. Patient characteristics in transarterial chemoembolization and microwave ablation combined with transcatheter arterial embolization groups.
All patients had hepatitis B or C–related liver cirrhosis. HCC diagnosis was confirmed by means of US-guided percutaneous biopsy with an 18-gauge cutting needle in all patients. The histological grade of each tumor was determined according to the general rules for the clinical and pathological study of primary liver cancer Citation[7]. Six tumors (17.6%; 3/18 in group 1, 3/16 in group 2) were well-differentiated, 23 (67.6%; 12/18 in group 1, 11/16 in group 2) moderately differentiated, and 5 (14.7%; 3/18 in group 1, 2/16 in group 2) poorly differentiated. According to the tumor, node metastasis (TNM) classification Citation[34], 10 (29.4%; 5/18 in group 1, 5/16 in group 2) patients corresponded to stage II (T2N0M0), 11 (32.3%; 6/18 in group 1, 5/16 in group 2) to stage IIIA (T3N0M0), and 13 (38.3%; 7/18 in group 1, 6/16 in group 2) to IIIC (T3N1M0).
TACE procedures
TACE was performed in all patients by two senior interventional radiologists, each of whom had more than 8 years of clinical experience. After the introduction of a 5-F pigtail catheter through the femoral artery under local anesthesia, an angiographic survey of the abdominal vessels was performed. Superselective cannulation of artery supplying the tumor was performed whenever possible. Depending on the size, location, and arterial supply of the tumor and its satellite lesions, An emulsion, which was mixed of either 80–120 mg of cisplatin (Qilu Pharmaceutical Factory, Jinan, China) or 40–60 mg of adriamycin (Main Luck Pharmaceutical, Shenzhen, China) in 10–20 mL of iodized oil (Lipiodol; Huaihai Pharmaceutical Factory, Shanghai, China), was slowly injected with fluoroscopic guidance. The 18 patients (nine patients each in groups 1 and 2) treated between May 2004 and December 2005 received adriamycin, and the remaining 16 patients (9 patients in group 1, 7 patients in group 2), who were treated after December 2005, received cisplatin. The reason for this change in regimen was a local shortage of adriamycin. Embolization of tumor-feeding vessels was performed with 1-mm3 particles of gelatin sponge (Gelfoam; 3 rd Pharmaceutical Factory of Nanjing, Nanjing, China) in all patients. After embolization, devascularization was confirmed with an additional angiographic study of the hepatic artery. The median number of courses of TACE performed was 1.6 (range, 1–3 courses) per patient in the TACE group and 1.3 (range, 1–2 courses) per patient in the group undergoing combined treatment. When there was progressive disease, development of extrahepatic diseases, severe life-threatening complications or evidence of insufficient liver function after TACE, TACE treatment was stopped. In group 2, TACE was performed as neoadjuvant therapy prior to Microwave Ablation, so the interval between TACE and Microwave Ablation should be very short (2–4 weeks).
Microwave ablation procedure
Microwave ablation was always performed after TACE. The interval between TACE and microwave ablation was at least 2 weeks (range, 2–4 weeks).All treatments were performed at our institution. Tumors were treated by means of microwave ablation with KY-2000 system (Kangyou Industry Company, Nanjing, China) with a frequency of 2450 MHz delivering a maximum power of 100 W through 15-gauge cooled-shaft needle antennas. After induction of general anesthesia, patients were placed in a modified flank position. A single antenna or multiple antennas were positioned in the tumor using US guidance depending on the tumor size Citation[29]. Percutaneous placement of the antenna was applied by any one of the three experienced radiologists for all tumors. A detailed protocol was worked out for each patient on an individual basis before treatment and included the placement of the antennas, power output setting, emission time, and appropriate approach Citation[29]. Our general strategy was to perform multiple overlapping ablations to treat the tumor fully in a single session and defined successful ablation as a lack of enhancement or resolution of the liver tumor. In general, for tumors less than 1.7 cm in diameter, a single antenna was used; for tumors 1.7 cm or larger, multiple antennae were required. An output setting between 40 W–60 W was used during ablations. During ablation, the region of ablation was monitored with US. The treatment session was ended if the hyperechoic region on gray-scale US covered the entire target region. For tumors larger than 30 mm, antennae were first inserted into the deeper region of lesions. If the hyperechoic region covered the deeper region of lesion on US after a series of microwave emission, antennae were withdrawn gradually and microwave emission was restarted and stopped until the hyperechoic region covered the lesion along the axis of antennae. A total of 3-5 antennae insertions in one or two sessions were needed for tumors larger than 30 mm. A thermal monitoring system was used during treatment. With US guidance, one to three thermal couples were placed at different sites 0.5 cm outside the tumor to monitor temperature throughout the procedure. If the measured temperature at 0.5 cm outside the tumor did not reach 60°C by the end of 300 seconds and did not remain at 54°C for at least 3 minutes, a prolonged emission time was required depending on the temperature, monitored dynamically. General anesthesia was achieved by using a combination of two anesthetics, propofol (Diprivan; Zeneca Pharmaceuticals, Wilmington, Del) and ketamine (Shuanghe Pharmaceuticals, Beijing, China), which were administered by two anesthetists to all patients by means of the peripheral veins.
After the procedure, all patients recovered in the postanesthesia care unit, and then all patients were observed for 3 nights in the hospital.
Follow-up
No patients were lost to follow-up. Follow up every 3–6 months was computed as starting from the beginning of treatment and was maintained until death in both groups. Patients underwent a uniform follow-up regimen, consisting of clinical examination, blood analysis including AFP and imaging techniques (ultrasound, spiral CT or MRI, alternatively) to observe long-term therapeutic effectiveness of treatment and complications related to TACE and MWA. modified response evaluation criteria in solid tumors (mRECIST) were used to assess the patients before and after treatment. Results were analyzed when all patients died due to disease progression in both groups. At this point, follow-up time ranged from 2 to 28 months. Survival was estimated from the date of initial treatment.
Follow-up imaging techniques were used to locate the target lesion and plan the interventional procedure before treatment and to detect evidence of residual or recurrent tumor in treated lesions, to assess changes in tumor size with time, and to monitor for the development of new hepatic lesions after treatment. The device used for Doppler US was Sequoia 512 (Siemens, Germany) with 3.5–5.0-MHz curved-array multifrequency transducers equipped for Cadence CPS software. Tumor size and pulsatile color flow within the tumor was detected in all patients before and after treatment. The contrast agent used in this study was SonoVue® (Bracco, Milan, Italy) for contrast-enhanced ultrasonographic imaging (CEUS). Contrast-enhanced CT was performed with multi–detector row CT (Lightspeed 16; GE Medical Systems, Milwaukee, Wis) with a section thickness of 5 mm, a 1.35:1.0 pitch, 120 kV, and 250 mA. Biphasic contrast-enhanced scans were obtained as follows: 100 mL of a nonionic contrast agent (iopromide, Ultravist 300; Schering, Berlin, Germany) was administered by means of a power injector at a rate of 3–4 mL/sec. Contrast-enhanced MR imaging was performed by using a 1.5-T unit (Signa Echo-Speed, GE Medical Systems). The following sequences were used: a spin-echo T1-weighted sequence with 500/15 (repetition time msec/echo time msec), a matrix of 256 – 192, and two signals acquired; a fat-suppressed T2- weighted respiratory-triggered fast spin echo sequence with 3000–4000/102, a matrix of 256 – 256, and three signals acquired; and a fat-suppressed spin-echo T1-weighted sequence with 500/15, a matrix of 256 – 192, and two signals acquired.
Images were interpreted jointly by three senior radiologists who were blinded to the treatment method used, and a consensus was reached for each patient. Any enhancement in the zone of ablation after contrast administration was considered evidence of incomplete treatment. When incomplete necrosis or local recurrence was confirmed with use of imaging modalities or post treatment biopsy, as long as the patient still met the requirements for those minimally invasive treatments, patients were considered for new treatment sessions.
Statistical analysis
All the data are reported as the mean ± standard deviation. The differences between data in these two groups were analyzed by using the Fisher exact test and an Independent sample t test. A cumulative survival rate was calculated by using the Kaplan-Meier method, in which the difference between the two groups was evaluated by using a log-rank test. Statistical analysis was done with SPSS package (SPSS Inc., Chicago Ill., USA) and Statistical significance was defined at a P value of less than 0.05.
Results
Tumor response
Measurements of the treated tumors were recorded from post procedural Doppler US examinations. During the follow-up period, reduction in tumor size was seen in 21 patients (61.7%; 7/18 in group 1, 14/16 in group 2), stable tumor size was seen in 7 patients (20.6%; 6/18 in group 1, 2/16 in group 2), and increase in tumor size was seen in 6 patients (17.6%; 6/18 in group 1, none in group 2).
All patients had an increase in the level of serum α-fetoprotein (AFP) concentration before therapy, 23 patients (67.6%; 12/18 in group 1, 11/16 in group 2) had high concentration of AFP (>400 ng/ml). There was a decrease to the normal level of AFP concentration in 22 patients (64.7%; 9/18 in group 1, 12/16 in group 2) after TACE or MWA ablation.
Follow-up imaging
All patients in both groups underwent Doppler sonography. Pulsatile flow signals within treated tumor were detected in 29 patients (85.3%; 15/18in group 1, 14/16 in group 2) before initial treatment. Follow-up contrast-enhanced ultrasonographic imaging was performed in 25 patients (73.5%, 14/18 in group 1 and 11/16 in group 2). Tumors appeared as a hypoechoic, isoechoic, or hyperechoic round or oval lesion and appeared hyperechoic relative to liver parenchyma during the early phase and hypoechoic during the later phase on contrast-enhanced ultrasonographic imaging before initial treatment. During microwave ablation, the echo of the tumors rapidly increased and covered the whole tumors at the end of treatment, at the same time, small bubbles could be seen diffusing form the tumors to the surround structure. After MWA ablation, the intratumoural flow signals absolutely disappear, the acupuncture pathway at the central of the tumor was hyperechoic, the whole tumor or the whole treated area was gradually occupied by a heterogeneous echogenic area. However, there were still pulsatile flow signals in 10 (55.6%) of 18 patients treated with TACE. Doppler US was not sufficiently sensitive to detect the intratumoural pulsatile flow signals in 5 patients (14.7%,3/18 in group 1, 2/16 in group 2) with poor tumor blood supply before and after treatment, however, contrast-enhanced ultrasonographic imaging was performed and able to reveal the obvious reduction of tumor blood supply of the 3/18 in group 1 and the destruction of the tumor blood vessels of 2/16 in group 2, as shown in .
Figure 1. A 12 cm HCC tumor occupying segment six of the right lobe of the liver in a 53-years-old patient underwent one course of TACE and three sessions of microwave ablation for HCC. Compared with tumor size before ablation, an obvious shrinkage and absences of contrast enhancement within the treated region was observed in the lesion treated with TACE plus microwave ablation. (a, b) before TACE; (c) 3 weeks after TACE and just before microwave ablation; (d, e) 3 weeks after TACE, just before ablation, tumor blood supply was reduced, but contrast enhancement still remained in some areas within the tumor; (f, g) After the microwave ablation session, no evidence of contrast enhancement was observed in the treated HCC, the tumor became completely necrotic.
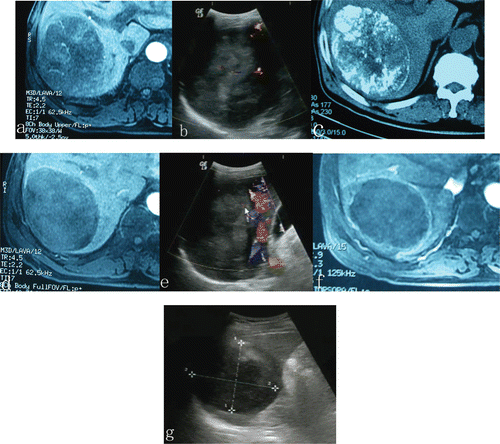
Follow-up CT was performed in 28 patients (82.4%, 17/18 in group 1 and 11/16 in group 2). Due to retained iodized oil in the tumors had a high attenuation at CT, no valid assessment about the effectiveness of MWA could be made within this patient subset. Dynamic contrast-enhanced MR imaging was performed in 14 patients (41.2%, 8/18 in group 1 and 6/16 in group 2). Dynamic contrast-enhanced MR images revealed an obvious reduction of tumor blood supply after TACE in 8/18 in group 1, but tumor vascularity still remained, particularly in the portal venous phase. Comparing with the dynamic contrast-enhanced MR images before MWA, there were absences of contrast enhancement within the treated region in five patients of six patients after MWA.
Survival
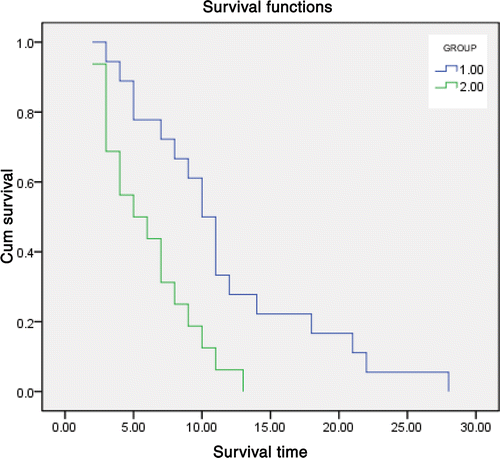
Survival rates were better in group 2 than in group 1 (P = 0.003), as shown in . During the median follow-up of 8 months (range, 2–28 months), 10 patients (62.5%) remained alive and 6 (37.5%) died as a result of disease progression in group 2, whereas 6 patients (33.3%) remained alive and 12 (66.7%) died as a result of disease progression in group 1. The median survival time was 10 months for patients treated in group 2 and 6 months for patients treated in group 1. The 6-month survival rate was 75% in group 2 and 50% in group 1, and the 1-year survival rate was 33.3% and 11.1%, the 18-month survival rate was 18.7% in group 2 and 0% in group 1, and the 2-year survival rate was 6.25% and 0%, respectively. For those patients who died during the follow-up period, mean survival times were 6.13 months ± 0.83 in group 1 and 11.61 months ± 1.59 in group 2.
Figure 2. Graph shows cumulative survival curves, calculated with the Kaplan-Meier method, for patients treated with either TACE alone (green line, group 1, n = 18) or TACE and microwave ablation (blue line, group 2, n = 16). Patients treated with TACE and ablation had substantially higher survival rates than those treated with TACE alone (P = 0.003). The median survival time was 10 months for patients treated in group 2 and 6 months for patients treated in group 1. The 6-month survival rate was 75% in group 2 and 50% in group 1, and the 1-year survival rate was 33.3% and 11.1%, The 18-month survival rate was 18.7% in group 2 and 0% in group 1, and the 2-year survival rate was 6.25% and 0%, respectively. For those patients who died during the follow-up period, mean survival times were 6.13 months ± 0.83 in group 1 and 11.61 months ± 1.59 in group 2.
Side effects and complications
Generally, the patients tolerated the TACE and MWA procedure well. No fatal or major complications related to those treatments were observed. A transient and mild body temperature increase due to the presumably necrotic liver tissue persisted for approximately 3 days in 15 patients (88.2%) after microwave ablation. Transient mild pain or abdominal discomfort was common during treatment and for 24–48 hours afterward. Only 10 patients required additional analgesia, administered orally for up to 3 days. Liver function tests showed a marked increase in serum (transaminase) levels in all patients at 24 hours after MWA, but always returned to normal by 3–20 days thereafter. Pleural effusion was seen in 8 of the 17 patients, however, no respiratory symptoms were present, hospital admission was not necessary, and the effusions resolved in a few days. No patients had skin burns, tumor bleeding or large blood vessel rupture after microwave ablation. All patients who underwent MWA recovered in the postanesthesia care unit, and observed for 3 nights in the hospital. Follow-up did not show any long-term sequelae or worsening of the liver function in any patients after the combined protocol. After TACE, all patients had transient impairment of hepatic function. Eighteen (60%) of the 30 patients developed a self-limited postembolization syndrome of fever, abdominal pain, and nausea. All patients discharged on the same day after the procedure.
Discussion
HCC is one of the most frequent primary malignant tumors worldwide. TACE is widely performed in patients with unresectable HCC. The embolization of the hepatic artery during TACE reduces the blood flow, creates ischemia and increases the contact time between the chemotherapeutic agent and the tumor cells, resulting in an increased local effect on the neoplasm with only slight damage to the surrounding liver tissue Citation[35]. However, patients with tumors greater than 5 cm have a higher prevalence of extracapsular tumor invasion into the liver parenchyma, more frequent intrahepatic dissemination and worse survival rates compare with those with smaller tumors Citation[7]. For these reasons, the control of large nonnodular lesions with TACE alone is still a challenging problem Citation[36].
MWA is one of the thermal ablation techniques, which have been widely used in China for treatment of hepatocellular carcinoma Citation[27–31], results of our previous clinical studies have shown that the 5-year survival rate of MW ablation is comparable to that of hepatectomy for small hepatic cancer Citation[30]. Comparing with RF ablation, MWA has a number of theoretical advantages including a larger zone of active heating, higher temperatures, and the capacity for ablation with several, simultaneously active antennas Citation[37], Citation[38]. However, additional hepatic perfusion is a major factor limiting the size of the coagulation areas and preservation of viable cells surrounding larger vessels for MWA as well as other ablation techniques. Some survival cells may increase the risk of tumor recurrence.
MWA with TACE as a combined therapy may be a possible therapeutic option in the future, especially for unresectable large-sized hepotocellular carcinoma (HCC). Repeated TACE can reduce the blood flow, creates ischemia, increases the chemotherapeutic agent local effect on the tumor cells and increases the sensitivity of neoplastic cells to hyperthermia, resulting in synergy with to the thermal ablation effect and increasing the range of therapeutic isotherms of local ablation Citation[39]. Veltri A et al has treated 46 patients affected by unresectable non-early HCC and suggest a relatively high complete local response and promising midterm clinical success with TACE followed by RFA Citation[40]. Zangos S et al. have reported an effective treatment of large-sized HCC with repeated TACE before MR-guided laser-induced thermotherapy (LITT), which extends the indication for MR-guided LITT and extends the indication for MR-guided LITT Citation[41]. Similarly, Wu F et al reported the combination of high-intensity focused ultrasound ablation and TACE is a promising approach in patients with advanced-stage HCC Citation[42]. To our knowledge, this study was the first study about MWA combined with TACE for unresectable large-sized hepotocellular carcinoma (HCC).
In this study, reduction in tumor size was seen in 87.5% of patients underwent MWA combined with TACE, while only 38.9% of patients underwent TACE alone. A decrease in the AFP level was seen in 75% and 50% of patients underwent MWA combined with TACE and TACE alone, respectively. The median survival time was 10 months for patients treated in group 2 and 6 months for patients treated in group 1. Survival rates were better in group 2 than in group 1. The survival rates for both groups are low compared to other studies Citation[16], Citation[43–46], There is a huge discrepancy between survival rates found in different studies, and this is assumed to be caused by variation between populations, which explains why a local study directly comparing different treatment methods within the same population provides the only valid means to assess the survival benefits of a new treatment method. In our own study, the poor survival rate could be caused by two factors. First, patients enrolled in this study had advanced-stage HCC. The mean diameter of the largest tumor was 6.74 ± 1.45 cm (range 5.1–10.6 cm); the mean liver tumor number was 2.1 ± 1.4 (range 1–6) per patient. Portal vein involvement, the histological grade of each tumor and TNM classification might contribute to the poor survival rate. Second, all patients had HBV/HCV related cirrhosis. Objective treatment response was not obtained with repeated TACE and/or MWA, as normal liver function was very difficult to maintain after TACE and/or MWA. Although the survival rates for both groups are low, the different treatment might contribute to the median survival difference between MWA combined with TACE and TACE alone. The intratumoural flow signals absolutely disappear after TACE and MWA, whereas there were still pulsatile flow signals in 55.6% patients treated with TACE alone. CT is commonly used as the standard imaging technique for evaluating the therapeutic response in patients with HCC after TACE. The pattern and distribution of iodized oil in the tumor are useful for assessing the therapeutic effects of TACE Citation[47]. Because of the high attenuation of iodized oil in the embolized tumors, dynamic contrast-enhanced CT was not applied to detect the residual or recurrent tumors in this study. Contrast-enhanced sonography and MRI can be useful in the diagnosis of HCC and the detection of residual or recurrent tumors after TACE and MWA. Complete tumor necrosis did not show contrast enhancement on Contrast-enhanced sonography and MRI. Any focal and nodular peripheral enhancement in the ablated lesion should be considered indicative of residual or recurrent tumor. Due to the relatively strict inclusion criteria, careful operation and nurse, all patients tolerated the TACE and MWA procedure well and no fatal or major complications related to those treatments were observed in this study.
There were some limitations that may affect clinical value of our study. First, due to only 34 patients were included in this study, more patients should be recruited for better assessment of treatment safety and efficacy of TACE and MWA. Second, in the current study, all TACE and MWA procedures were performed by the same group in our hospital, multicentric prospective randomized controlled trials are needed to confirm the safe and effect of MWA combined with TACE for large-sized unresectable HCCs. Thirdly, Sorafenib is currently established as a drug for prolonging the survival in HCC patients with metastatic disease or TACE-refractory disease who are not suitable candidates for local treatments Citation[48]. In the future, investigation of sorafenib or other molecular-targeted therapies is also expected in the adjuvant or prophylactic setting after TACE and MWA.
Conclusion
In summary, our results demonstrate that MWA combined with TACE appears to be an effective and promising approach for the treatment of large-sized unresectable HCCs. However, large-scale randomized clinical trials are needed to determine the future role of this treatment.
Declaration of interest: The authors alone are responsible for the content and writing of the paper.
References
- Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999; 19(3)271–285, Review
- Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010; 51(4)1274–1283
- Shimada M, Yamashita Y, Hamatsu T, Rikimaru T, Hasegawa H, Gion T, Shirabe K, Takenaka K, Sugimachi K. Surgical indications for advanced hepatocellular carcinoma. Hepatogastroenterology. Jul–Aug, 2000; 47(34)1095–1099
- Takenaka K, Kawahara N, Yamamoto K, Kajiyama K, Maeda T, Itasaka H, Shirabe K, Nishizaki T, Yanaga K, Sugimachi K. Results of 280 liver resections for hepatocellular carcinoma. Arch Surg Jan, 1996; 131(1)71–76
- Teh SH, Christein J, Donohue J, Que F, Kendrick M, Farnell M, Cha S, Kamath P, Kim R, Nagorney DM. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: Model of End-Stage Liver Disease (MELD) score predicts perioperative mortality. J Gastrointest Surg 2005; 9: 1207–1215, discussion 1215
- Benzoni E, Molaro R, Cedolini C, Favero A, Cojutti A, Lorenzin D, Intini S, Adani GL, Baccarani U, Bresadola F, et al. Liver resection for HCC: Analysis of causes and risk factors linked to postoperative complications. Hepatogastroenterology 2007; 54: 186–189
- Mok KT, Wang BW, Lo GH, Liang HL, Liu SI, Chou NH, Tsai CC, Chen IS, Yeh MH, Chen YC. Multimodality management of hepatocellular carcinoma larger than 10 cm. J Am Coll Surg. Nov, 2003; 197(5)730–738
- Belghiti J, Kianmanesh R. Surgical treatment of hepatocellular carcinoma. HPB, 2005; 7: 42–49
- Llovet JM, Fuster J, Bruix J. The Barcelona approach: Diagnosis, staging and treatment of hepatocellular carcinoma. Liver Transplantation, 2004; 10: S115–S120
- Beaugrand M, Seror O. Transcutaneous treatments of hepatocellular carcinoma in patients with cirrhosis: Present status and future developments. Curr Pharm Des. 2007; 13(32)3274–3278
- Wang ZL, Liang P, Dong BW, Yu XL, Yu DJ. Prognostic Factors and Recurrence of Small Hepatocellular Carcinoma after Hepatic Resection or Microwave Ablation: A Retrospective Study. J Gastrointest Surg. Feb, 2008; 12(2)327–337
- Hinshaw JL, Lee FT, Jr. Cryoablation for liver cancer. Tech Vasc Interv Radiol. Mar, 2007; 10(1)47–57
- Marquet F, Pernot M, Aubry JF, Tanter M, Montaldo G, Fink M. In-vivo non-invasive motion tracking and correction in High Intensity Focused Ultrasound therapy. Conf Proc IEEE Eng Med Biol Soc. 2006; 1: 688–691
- Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: A unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000; 174: 323–331
- Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology 2000; 217: 633–646
- Farinati F, De Maria N, Marafin C, et al. Unresectable hepatocellular carcinoma in cirrhosis: Survival, prognostic factors, and unexpected side effects after transcatheter arterial chemoembolization. Dig Dis Sci 1996; 41: 2332–2339
- Okada S. Transcatheter arterial embolization for advanced hepatocellular carcinoma: The controversy continues. Hepatology 1998; 27: 1743–1744
- Pacella CM, Bizzarri G, Cecconi P, Caspani B, Magnolfi F, Bianchini A, Anelli V, Pacella S, Rossi Z. Hepatocellular carcinoma: Long-term results of combined treatment with laser thermal ablation and transcatheter arterial chemoembolization. Radiology 2001; 219: 669–678
- Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, Li KQ, Jin CB, Xie FL, Su HB. Advanced hepatocellular carcinoma: Treatment with high-intensity focusedultrasound ablation combined with transcatheter arterial embolization. Radiology. May, 2005; 235(2)345–346
- Zangos S, Eichler K, Balzer JO, Straub R, Hammerstingl R, Herzog C, Lehnert T, Heller M, Thalhammer A, Mack MG, et al. Large-sized hepatocellular carcinoma (HCC): A neoadjuvant treatment protocol with repetitive transarterial chemoembolization (TACE) before percutaneous MR-guided laser-induced thermotherapy (LITT). Eur Radiol. Feb, 2007; 17(2)553–563
- Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol. Mar, 2006; 16(3)661–669
- Cabibbo G, Latteri F, Antonucci M, Craxì A. Multimodal approaches to the treatment of hepatocellular carcinoma. Nat Clin Pract Gastroenterol Hepatol. Mar, 2009; 6(3)159–169
- Giuseppe Cabibbo, Antonio Craxì. Needle track seeding following percutaneous procedures for hepatocellular carcinoma. World J Hepatol. Oct 31, 2009; 1(1)62–66
- Wang W, Shi J, Xie WF. Transarterial chemoembolization in combination with percutaneous ablation therapy in unresectable hepatocellular carcinoma: A meta-analysis. Liver Int. 2010; 30(5)741–749
- Seki T, Wakabayashi M, Nakagawa T, Imamura M, Tamai T, Nishimura A, Yamashiki N, Okamura A, Inoue K. Percutaneous microwave coagulation therapy for patients with small hepatocellular carinoma: Comparison with percutaneous ethanol injection therapy. Cancer 1999; 85: 1694–1702
- Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: Comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology 2002; 223: 331–337
- Ren H, Liang P, Yu X, Wang Y, Lu T, Li X. Treatment of liver tumours adjacent to hepatic hilum with percutaneous microwave ablation combined with ethanol injection: A pilot study. Int J Hyperthermia.2011, 27(3)249–254
- Liu FY, Yu XL, Liang P, Wang Y, Zhou P, Yu J. Comparison of percutaneous 915 MHz microwave ablation and 2450 MHz microwave ablation in large hepatocellular carcinoma. Int J Hyperthermia.2010, 26(5)448–455
- Dong B, Liang P, Yu X, Su L, Yu D, Cheng Z, Zhang J. Percutaneous sonographically guided microwave coagulation therapy for hepatocellular carcinoma: Results in 234 patients. AJR 2003; 180: 1547–1555
- Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, Xiao Q. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology 2005; 235: 299–307
- Lu MD, Xu HX, Xie XY, Yin XY, Chen JW, Kuang M, Xu ZF, Liu GJ, Zheng YL. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: A retrospective comparative study. J Gastroenterol 2005; 40: 1054–1060
- Seki T, Tamai T, Nakagawa T, Imamura M, Nishimura A, Yamashiki N, Ikeda K, Inoue K. Combination therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinoma. Cancer. Sep 15, 2000; 89(6)1245–1251
- The general rules for the clinical and pathological study of primary liver cancer. Liver Cancer Study Group of Japan. Jpn J Surg 1989;19:98–129
- Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual6th. Springer-Verlag, New York 2003
- Tancredi T, McCuskey PA, Kan Z, Wallace S. Changes in rat liver microcirculation after experimental hepatic arterial embolization: Comparison of different embolic agents. Radiology. Apr, 1999; 211(1)177–181
- Yamakado K, Nakatsuka A, Ohmori S, Shiraki K, Nakano T, Ikoma J, Adachi Y, Takeda K. Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: Treatment response based on tumor size and morphology. J Vasc Interv Radiol. Dec, 2002; 13(12)1225–1232
- Liang P, Wang Y. Microwave ablation of hepatocellular carcinoma. Oncology. 2007; 72(Suppl 1)124–131
- Iannitti DA, Martin RC, Simon CJ, Hope WW, Newcomb WL, McMasters KM, Dupuy D. Hepatic tumor ablation with clustered microwave antennae: The US Phase II Trial. HPB (Oxford)2007, 9(2)120–124
- Goldberg SN, Dupuy DE. Image-guided radiofrequency tumor ablation: Challenges and opportunities, part I. J Vasc Interv Radiol 2001; 12(9)1021–1032
- Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol. Mar, 2006; 16(3)661–669
- Zangos S, Eichler K, Balzer JO, Straub R, Hammerstingl R, Herzog C, Lehnert T, Heller M, Thalhammer A, Mack MG, et al. Large-sized hepatocellular carcinoma (HCC): A neoadjuvant treatment protocol with repetitive transarterial chemoembolization (TACE) before percutaneous MR-guided laser-induced thermotherapy (LITT). Eur Radiol. Feb, 2007; 17(2)553–563
- Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, Li KQ, Jin CB, Xie FL, Su HB. Advanced hepatocellular carcinoma: Treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology. May, 2005; 235(2)659–667
- keda K, Kumada H, Saitoh S, Arase Y, Chayama K. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma. Cancer 1991; 68: 2150–2154
- Bruix J, Castells A, Montanyà X, Calvet X, Brú C, Ayuso C, Jover L, García L, Vilana R, Boix L, et al. Phase II study of transarterial embolization in European patients with hepatocellular carcinoma: Need for controlled trials. Hepatology 1994; 20: 643–650
- Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002; 359: 1734–1739
- Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002; 35: 1164–1171
- Lim HS, Jeong YY, Kang HK, Kim JK, Park JG. Imaging features of hepatocellular carcinoma after transcatheter arterial chemoembolization and radiofrequency ablation. AJR Am J Roentgenol. Oct, 2006; 187(4)W341–349
- Junji Furuse. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Biologics. Dec, 2008; 2(4)779–788
