Abstract
Purpose: The goal of this study was to evaluate the relationship between previously demonstrated thermosensitising effects of the block copolymer, Pluronic, and heat shock protein 70 (Hsp70) expression in an experimental colorectal cancer model in vitro and in vivo.
Materials and methods: Rat colorectal carcinoma cells were treated with low-grade hyperthermia (43°C) alone or in combination with Pluronics L10 (3 mg/mL), L61 (0.3 mg/mL), or L64 (0.5 mg/mL) for 20 min. Adinosine triphosphate (ATP) levels and cell viability were determined using standard assays. Hsp70 expression was quantified by western blot for cells treated with L10, L61, and L64 at doses specified above and Pluronic P85 (10 mg/mL) alone and in combination with heat. BDIX rats with flank tumours were used to study the effect of L61 and hyperthermia on Hsp70 expression in vivo.
Results: In vitro, treatment with L10, L61, and L64 plus low-grade hyperthermia lead to depletion of ATP levels to between 8 and 66% of untreated control after 24 h. Maximum expression of Hsp70 was observed at 9 h following hyperthermia alone. The combination of low-grade hyperthermia and Pluronic treatment reduced Hsp70 expression for up to 6 hours, and L10 appeared to completely inhibit the Hsp70 expression. In vivo, Hsp70 expression was increased 5 h after hyperthermia in BDIX rat tumour models and no Hsp70 expression was observed in L61 pre-treated and control groups.
Conclusion: Pluronic effectively improves hyperthermic and low-grade hyperthermic treatment in part due to reduction of Hsp70 expression.
Introduction
Hyperthermia treatment using image-guided radiofrequency (RF) ablation has been extensively investigated in the past decade as an alternative treatment for unresectable hepatic [Citation1–4], pancreatic Citation[5], Citation[6], renal Citation[7], Citation[8], and lung Citation[9] tumours. RF ablation is minimally invasive and comparatively low in risk among other percutaneous approaches. This focal hyperthermia technique is designed to obliterate the tumour by heating the tissue to temperatures higher than the normal body temperature (>50°C) Citation[10]. The increase in tissue temperature results in cell membrane destruction, protein denaturation, and local necrosis around the RF probe. Despite demonstrated advantages, the application of RF ablation by itself can be incomplete and can lead to tumour recurrence, largely in marginal areas due to the cooling effect of nearby blood vessels Citation[11], Citation[12] and due to the endogenous protective mechanisms primarily from heat shock protein 70 (Hsp70) [Citation13–15].
Up-regulation of Hsp70 is clinically relevant because it enhances thermotolerance of tumour tissues Citation[16] and results in decreased apoptosis and necrosis, which facilitates cancer survival. Hsp70s are omnipresent, chaperone molecules that assist in protein folding in the active confirmation and are expressed rapidly as a result of cellular stresses such as hyperthermia, hypoxia, ionising radiation, and the like. Activation of Hsp70 transcription Citation[17] as well as the chaperoning function Citation[18] has been shown to be Adinosine triphosphate (ATP)-dependent. The anti-apoptotic effect of Hsp70 in cancer cells is evidenced by both caspase-dependent and -independent pathways [Citation19–25]. As a result, Hsp70 has emerged as a potential target for cancer therapy to increase the efficacy of adjuvant treatments.
As a relatively non-toxic sensitising agent, Pluronic has shown positive effects on low-grade hyperthermia and results in an increased sensitisation effect for heat [Citation26–29]. Pluronic is a triblock copolymer composed of hydrophilic poly(ethylene oxide) (PEO) and hydrophobic poly(propylene oxide) (PPO) in the form of PEOx-PPOy-PEOx (where the x and y values differ to each type of Pluronic leading to structure-dependent properties) Citation[30]. The chemosensitisation effect of Pluronic has been recognised as an effective means of causing an elevation of cancer cell apoptosis by modulating the fluidity of the cell membrane, depleting intracellular ATP Citation[31], and changing the P-glycoprotein drug efflux pump in multidrug resistant (MDR) cells [Citation32–34].
In the RF ablation scenario, since the marginal tumour regrowth may be due in part to the up-regulation of Hsp70 [Citation13–15], we hypothesised that the biological activity of Pluronic can be dependent on its ability to inhibit Hsp70 expression by a yet unidentified mechanism. Prior studies in our group have demonstrated the thermal sensitisation effect of Pluronic P85 in hyperthermia both in vitro and in vivo Citation[35]. Accordingly, the purpose of the current study was to further investigate the effect of various Pluronics (L10, L61, L64, P85) combined with hyperthermia (45°C) and low-grade hyperthermia (43°C) on ATP depletion, cell death, and expression of Hsp70.
Materials and methods
Formulation of Pluronic solutions
Pluronics L10, L61, L64, and P85 (molecular weight (Mw) of 3200, 2000, 2900, and 4600 Da, and PPO/PEO units of 49.7/7.3, 31/4.55, 30/26.36, and 39.66/52.27, respectively Citation[36]) were used. Pluronic P85 and L61 were generously donated by BASF (Shreveport, LA). Pluronic L10 and L64 were purchased from Sigma Aldrich (Milwaukee, WI). Pluronic stock solutions were prepared by dissolving each polymer in RPMI medium overnight at 4°C at concentrations of 10 mg/mL, 0.3 mg/mL, 0.5 mg/mL, and 3 mg/mL for P85, L61, L64, and L10 respectively. The optimum doses of Pluronic were obtained based on the mitochondrial succinate dehydrogenase (WST-1) assay. Solutions were filtered with a sterile 0.22 µm syringe filter (Millipore, MA) and test solutions stored at 4°C until use.
Cell culture
Rat DHD/K12/TRb colorectal adenocarcinoma cells (European Collection of Cell Cultures, Salisbury, UK) originating from 1,2-dimethylhydrazine-induced colon adenocarcinoma in BDIX rats, were cultured in complete RPMI 1640 (10% fetal bovine serum, 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA). Cells were cultured at 37°C and 5% CO2 in a humidified atmosphere. Cells were passaged at 90% confluence. Cells were detached with 0.25% trypsin-EDTA 24 h before treatment and plated onto flat bottom, tissue culture-treated, opaque walled, 96-well plates with 2 × 104 cells in each well.
Cell treatment
After 24 h of incubation, medium was aspirated and cells were incubated with 100 µL of Pluronic test solutions (10 mg/mL, 0.3 mg/mL, 0.5 mg/mL, 3 mg/mL for P85, L61, L64, and L10) for 20 min at 37°C. For Pluronic plus low-grade hyperthermia treatment, cells were exposed to Pluronic test solutions as above and received 43°C for 20 min. At the endpoint, test solutions were removed, cells were washed with incomplete RPMI and wells were replenished with complete RPMI. Plates were returned to a 37°C humidified incubator for a set time and analysed as described below.
In vitro cell viability
Changes in ATP levels were measured using a standard luciferin-luciferase assay (CellTiter-Glo® luminescent cell viability assay, Promega, Madison, WI). This assay measures the luminescence resulting from the ATP-driven luciferase/luciferin reaction. The measured signal corresponds to the relative amount of ATP in those cells and is also proportional to the number of living cells. After Pluronic and/or low-grade hyperthermia treatment at 37°C or 43°C (± 0.05°C) for 20 min, 100 µL of reagent was added to each well. The plates were shaken for 2 min to promote cell lysis, and relative luminescence was measured using a plate reader (Tecan, Durham, NC). At the same time points, cells were trypsinised and a single cell suspension was obtained by well mixing with a pipette. The cell suspension was mixed with 0.4% trypan blue in 1 : 1 ratio and after ∼3 min viable cells were counted using a haemocytometer (Fisher Scientific, Pittsburgh, PA). All studies were repeated in triplicate.
Protein analysis
Cells were treated with heat (43° ± 0.05°C) with or without Pluronic for 20 min. At different time points (0, 2, 4, 6, 9, 24, 48, 72 h post treatment), cells were lysed on ice for 30 min in a lysis buffer (Cell Signaling Technology, Beverly, MA) containing a protease inhibitor cocktail (Dimethyl sulphoxide, benzenesulphonylfluoride, trypsin inhibitor, bestatin, leucine, pepstatin A) (Sigma, MO) and centrifuged at 10,000 g for 10 min at 4°C. A Bio-Rad (Hercules, CA) protein assay kit was used to determine protein concentration in the supernatant. Protein was electrophoresced on sodium dodecyl sulphate/polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat dry milk in TBST buffer (0.1% Tween-20, 20 mM of Tris-HCl; pH 7.5, and 140 mM of NaCl). Membranes were then incubated with primary antibodies against Hsp70 and β-actin (Assay Designs/Stressgen, Ann Arbor, MI), followed by secondary antibody/horseradish peroxidase conjugates (Pierce, IL). The SNAP i.d. system (Millipore, MA) was used for the antibody incubation. Horseradish peroxidase (HRP) substrate-luminal reagent (Millipore, MA) was used to detect chemiluminescence signal and photographed by Alpha Imager HP (Cell Biosciences (proteinsimple),Santa Clara, CA).
For in vivo Hsp70 analysis, tumours were excised from each group of BDIX rats and tissue samples were prepared by homogenising the piece of dissected tumour (radially sectioned) in lysis buffer, followed by centrifugation at 4°C and collecting the supernatant. Western blot analysis was carried out as described above.
In vivo assessment of Hsp70 analysis
Pilot in vivo experiments were performed as approved by the Institutional Animal Care and Use Committee at Case Western Reserve University and were in accordance with all applicable protocols and guidelines in regard to animal use. BDIX rats 12 weeks old carrying subcutaneous tumours were used in this study. Tumours were created by injecting 1.0 × 105 rat colorectal adenocarcinoma cells (DHD/K12/TRb). The tumours were treated with a combination of L61 pretreatment and hyperthermia. L61 was chosen for these experiments because it had demonstrated sensitising efficacy in previous studies in the same rodent model of cancer (unpublished data). Therefore, in this study we used L61 for in vivo analysis of Hsp70.
After 5 weeks the rats were randomly divided into three groups. Animals were anaesthetised using 1% isoflurane with an oxygen flow rate of 1 L/min (EZ150 Isoflurane Vaporizer; EZ Anesthesia, Palmer, PA). The first group received 0.1 mg/mL of L61 in saline intravenously. Four hours after L61 treatment, the tumours were subjected to hyperthermia at 45°C for 2 min with an abdominal grounding pad. Application of local hyperthermia was performed using a 480-kHz RF generator (Radionics, Burlington, MA) and a custom-designed 21-gauge monopolar needle electrode. The other group received the same amount of saline instead of L61 followed by hyperthermia at 45°C for 2 min. The control group received only saline. The animals were euthanised 5 h after hyperthermia and tumours were excised and stored at −80°C for Hsp70 analysis.
Statistical analysis
All data are presented as mean ± STDEV (standard deviation) unless otherwise noted. Cell viability data are normalised with respect to untreated control. Two-tailed unpaired student's t-test with unequal variants and a one-way ANOVA were used to determine the significance of the outcome. Data analysis was performed with Microsoft Excel and ANOVA.
Results
Effect of Pluronic on ATP recovery and cell growth in vitro
The effects of low-grade hyperthermia alone, Pluronic treatment alone, and combined treatment of heat plus Pluronic on ATP depletion are shown in . The data is normalised to the untreated control and reported as a percentage (n = 3). Subsequent to heating at 43° ± 0.05°C for 20 min, ATP decreased to 85% ± 15.5% (P = 0.18) relative to the untreated control. Twenty-four hours after low-grade hyperthermia alone, ATP recovered to 96% ± 4.5% (P = 0.18). ATP decreased immediately after Pluronic treatment to 62% ± 19%, 63% ± 6%, and 78% ± 3% (P < 0.03) of untreated control for L10, L61, and L64 respectively. Parallel to the heat alone treatment, after 24 h, ATP of Pluronic-treated cells recovered gradually to 100% ± 7%, 96% ± 2%, and 94% ± 7% (P < 0.3). A significant loss of cell viability was observed when cells were treated with both low-grade hyperthermia and Pluronic. The synergic treatments with L10 demonstrated significant ATP depletion compared to the treatments with other Pluronic polymers used in this study reaching a level of less than half of the untreated control and heat alone treatments immediately after L10 plus low-grade hyperthermia treatment (42% ± 13%, P = 0.002 and 37% ± 8%, P = 0.02). Five hours after treatment, ATP returned to 67% ± 18% (P < 0.05) of untreated control, demonstrating a high production of energy. Subsequently, 24 h after treatment, the ATP level decreased to 8% ± 4% (P < 0.001) of untreated control.
Figure 1. Intracellular ATP in DHD/K12/TRb cells treated with heat only (43° ± 0.05°C for 20 min), Pluronic L10 (A), L61 (B), L64 (C) alone and combined (Pluronic + heat) treatment. *Indicates statistically significant difference (P < 0.05) compared to the untreated control (n = 3 ± STDEV).
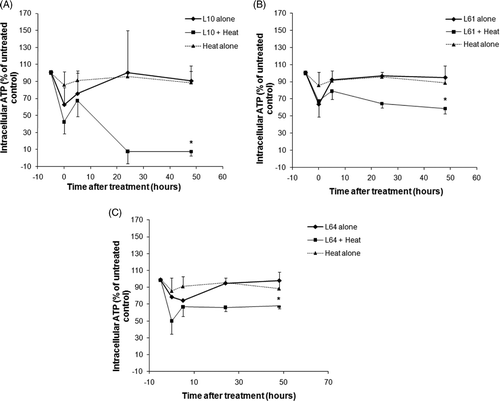
The amount of ATP after the synergic treatment of L61 and L64 plus low-grade hyperthermia displayed similar behaviour to the L10 with a drop of ATP to 67% ± 18% (P = 0.03) and 50% ± 10% (P < 0.01) of heat alone, respectively, and a slight increase was observed within 5–10 h. However, in contrast to L10, ATP recovered to 64% ± 4% (P < 0.001) and 66% ± 18% (P = 0.08) of untreated control after 24 h for L61 and L64, respectively.
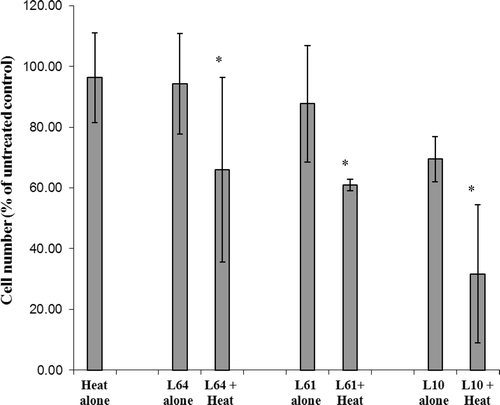
Pluronic effect on cell proliferation was investigated 72 h after treatment and is presented in . Cells treated with heat alone or with L64, L61, and L10 showed proliferation levels of 96% ± 14%, 94% ± 16%, 87% ± 19%, 69% ± 7% (P < 0.001) of untreated control respectively. In contrast, when combined with heat, the number of live cells decreased to 68% ± 25%, 63% ± 7%, and 33% ± 10% (P < 0.05) for L64, L61, and L10 respectively.
Effect of Pluronic on heat shock protein 70 expression in vitro
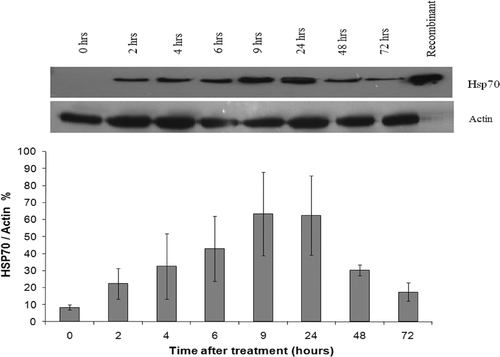
Hsp70 expression in confluent DHD/K12/TRb monolayer cells after low-grade hyperthermia treatment (43° ± 0.05°C) was determined by immunoblot analysis. After 20 min of heat exposure, cells were returned to the 37°C incubator and, at different time points, cell lysates were collected and analysed for Hsp70. shows the heat-induced increase in Hsp70 protein expression at different post treatment time points (n = 3). The Hsp70 expression was up-regulated to a detectable level (38% ± 6% of actin, P < 0.05) within 2 h after heat treatment alone. Maximal Hsp70 expression (80% ± 23% of actin amount, P < 0.05) was detected between 9 and 24 h after treatment and decreased gradually afterwards for up to 72 h.
Figure 3. Up-regulation of Hsp70 expression in DHD/K12/TRb cells treated with 43°C low-grade hyperthermia for 20 min (top). Representative western blot of Hsp70 expression with heat stress (bottom). Hsp70 expression at different time points normalised versus actin. Hsp70 expression is elevated 2 h after hyperthermia treatment and lasts 72 h. Each peak is the mean of measurements of 3 ± STDEV.
Low-grade hyperthermia combined with Pluronic decreased Hsp70 expression (). Pluronic L10 and low-grade hyperthermia completely suppressed Hsp70 expression (n = 3). Here, the ‘heat control’ is defined as the Hsp70 expression of heat only treatment at the same time points. Pluronic L61 and L64 synergic treatment resulted in suppression of Hsp70 for up to 6 h. After 6 h, Hsp70 expression returned to a detectable level. A less remarkable inhibition of Hsp70 protein was noted with Pluronic P85. In all cases, the corresponding β-actin analysis was conducted (figures are not shown) and each band normalised to β-actin. and summarise the Pluronics’ effects on Hsp70 expression compared to those of the heat control. Finally, shows the representative immunoblot outcome for Hsp70 analysis of Pluronic-only treatments for cancer cells. The results showed that there was an insignificant increase of intracellular Hsp70 expression compared to the heat control.
Figure 4. Inhibition of Hsp70 expression in DHD/K12/TRb cells treated with different Pluronics and 43°C low-grade hyperthermia for 20 min. Western blots reveal that the Hsp70 expression was totally diminished by L10 + heat treatment. Pluronic L61, L64, and P85 + heat treatment down-regulate Hsp70 for 6 h.
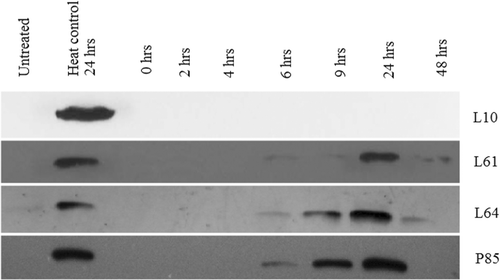
Figure 5. Effect of Pluronic L10, L61, L64, and P85 on the inhibition of Hsp70 with the hyperthermia treatment at 43°C for 20 min. The peaks are normalised to actin and calculated as a percentage relative to heat only treatment at same time points. Results presented as the mean of 3 ± STDEV. *The Hsp70 expression is significantly different from the expression of heat control with P < 0.001.
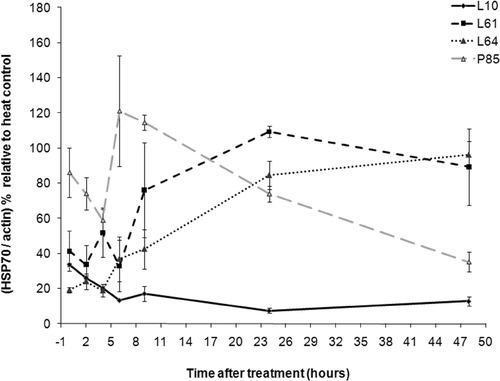
Figure 6. Expression of Hsp70 in DHD/K12/TRb cells treated with Pluronic L10, L61, L64, or P85 for 20 min at 37°C. Western blot analysis indicated an insignificant level of Hsp70 expression with Pluronic only treatment.
Table I. Summary of Hsp70 expression following treatment with low-grade hyperthermia in conjunction with Pluronic.
In vivo expression of Hsp70
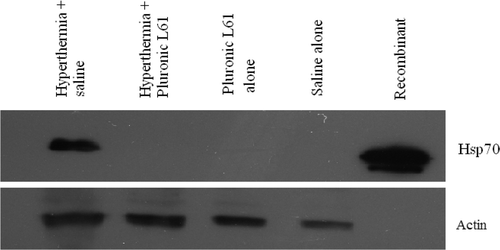
In vivo Hsp70 expression in hyperthermia-treated tumours with or without Pluronic L61, and subsequent control groups are shown in . Hsp70 expression in the L61 pretreated with hyperthermia group (n = 8), the L61 alone group (n = 3), and the saline treated group (n = 2) were undetectable. An average of 88% ± 10% (normalised to constitutively expressed β-actin) Hsp70 expression was observed in tumours, 5 h after hyperthermia alone treatment.
Figure 7. Hsp70 expression 5 h after hyperthermia (for 20 min at 45°C) in subcutaneous colorectal tumour model. A total of 50 µg of tissue lysate was analysed by western blot against anti- Hsp70 antibody. Actin was used as the internal control. Hyperthermia-treated tumours show Hsp70 expression, but with Pluronic L61 the protein expression diminished.
Discussion
Radiofrequency (RF) ablation has recently gained recognition as a minimally invasive surgical modality for treating a variety of unresectable solid tumours Citation[37]. One challenge facing RF ablation is local tumour recurrence, particularly in lesions exceeding 3 cm in diameter Citation[12], Citation[38], Citation[39]. Several strategies have been developed to improve the outcomes of ablation. Goldberg et al. have demonstrated the synergistic effect of RF ablation and liposomal doxorubicin in enhancing tumour necrosis [Citation40–44]. Krupka et al. administered polymer implants loaded with carboplatin with a chemosensitiser to increase the efficacy of RF ablation Citation[26]. Haaga et al. demonstrated effectiveness of a similar approach with 5-FU loaded implants Citation[45]. To overcome the problem from an instrumentation perspective, multiple array needle electrodes have also been explored Citation[46]. Although these strategies increased the volume of tumour necrosis in some cases, new paradigms which perform this task without device modification or use of toxic chemotherapeutics present a compelling alternative Citation[47]. One possible cause of marginal recurrence and a potential target for a complementary therapy is up-regulation of Hsp70 expression observed in various tumour models after sublethal RF ablation Citation[14], Citation[48], Citation[49]. Several mechanistic studies have been performed to explain the upstream effect of Hsp70 in apoptosis and cell proliferation in diverse organs [Citation20–25].
Our previous studies showed that Pluronic P85 combined with hyperthermia decreased cell viability and proliferative ability of DHD/K12/TRb cell line in vitro and in vivo. The synergistic treatment with Pluronic P85 plus hyperthermia demonstrated a 22–28% decrease in cell viability in vitro and a 59% reduction in tumour volume when P85 was combined with RF ablation compared to an increase of 16% with RF ablation alone Citation[35]. In this current study we investigated the synergic effect of various other Pluronics with low-grade hyperthermia and its impact on ATP depletion and Hsp70 expression in vitro using DHD/K12/TRb cell line and in vivo using subcutaneous tumours in rats using the same cell line. The optimum doses of Pluronic L61 and P85 used in this study were obtained from previous studies Citation[50] and optimum doses of Pluronic L10 and L64 were obtained based on the mitochondrial succinate dehydrogenase assay (data not shown). Our current findings show that Pluronics L10, L61, and L64 play a role in reducing intracellular ATP levels immediately after treatment when combined with low-grade hyperthermia; and their effectiveness appears to be dependent on Pluronic structure Citation[51]. For example, after treatment of hyperthermia and Pluronic L10 (calculated hydrophilic-lipophilic balance (HLB) = 2, molecular weight 3200 Da) resulted in complete lack of recovery of cell viability. However, Pluronic L61, which also has low HLB value (2) but a lower molecular weight (2000 Da) demonstrated a less intense effect on ATP depletion compared to L10. Pluronic L64 with the calculated HLB value of 8 and higher molecular weight (2900 Da) showed even less effect on ATP depletion even at 48 h after treatment.
Similarly, the phenomenon can be related to the number of lipophilic and hydrophilic segments of the block polymers. Pluronics with longer lipophilic propylene oxide (PO) and shorter hydrophilic ethylene oxide (EO) segments have a greater ability to interact with cell membranes Citation[32]. The high lipophilic nature of L10 (49.7 of PO and 7.3 of EO segments) may have enhanced capacity to change the membrane fluidity, resulting in decreased mitochondrial activity and demonstrate higher effect for ATP reduction. Pluronic L61 (31 PO and 4.55 EO segments) with lower molecular weight shows lesser effects in ATP reduction compared to L10. Pluronic L64 with high PO units (30) and high EO units (26.36) also showed less effectiveness for ATP depletion. Finally, hydrophilic Pluronic P85, which has a higher number of EO units than PO units (39.66 of PO and 52.27 of EO segments) and high molecular weight (4600 Da) contributes much less to the ATP depletion activity in our model. In addition, ATP levels in cells treated with Pluronic alone were nearly restored to original levels after 24 h, suggesting low inherent effects of the polymer.
Electron paramagnetic resonance (EPR) technique results Citation[52] and fluorescence results Citation[53] demonstrated that Pluronic has the ability to alter the mitochondrial membrane permeability and hence has an effect on energy production. Krupka et al. reported on the mitochondrial succinate dehydrogenase activity assay (WST-1) results after treatment of L61 and heat for DHD/K12/TRb cells. These data showed a significant decrease of cell viability when cells were treated with L61 plus heat, compared to the heat alone treatment. By day 3, no enzyme activity was observed with the L61 and heat treatment Citation[50]. Rapoport et al. demonstrated the cytostatic action of Pluronic in MDR cells and explained the inhibitory effect of Pluronic in cell growth Citation[52], Citation[54]. Our live cell data showed suppression of cellular growth in L61, L64, and L10-treated cells 72 h after the treatment, but the inhibitory effect was increased considerably with the combination of Pluronic and heat. Hyperthermia has been known to increase the cell membrane permeability for enhanced drug uptake Citation[55], Citation[56]. It is possible that the low-grade hyperthermia could increase the internalisation of Pluronic into cells and affect the cytostatic action rather than have a cytotoxic effect.
The most noteworthy finding in the current study was that Pluronic has the ability to reduce Hsp70 expression both in vitro and in vivo. In the cytoplasm and the nucleus of most mammalian cells, Hsp70 is up-regulated when cells experience stress Citation[57]. The production of Hsp70 assists in repairing and stabilising damaged proteins by correcting their 3D folding, permitting continued functions under stress [Citation57–59]. In this scenario, cancer cell survival is increased and a greater focus is placed on the inhibition of Hsp70 for therapeutic approach Citation[60].
In our model Hsp70 is undetectable under normal baseline conditions but the heat stress caused up-regulation of Hsp70 in DHD/K12/TRb cells 2 h after treatment and the maximum expression was observed 9–24 h after low-grade hyperthermia. We then investigated the systematic expression of Hsp70 after synergic treatment of Pluronic and low-grade hyperthermia showing that L61, L64, and P85 combined with heat decrease Hsp70 expression for 6 h followed by recovery of Hsp70 to or above pre-treatment levels. The P85 group shows relatively high percentage of Hsp70 expression after 6 h compared to the other Pluronics and relative to heat alone but the difference is not statistically significant and is likely due to experimental variability. In contrast, synergic treatment of L10 fully diminished Hsp70 for the entire duration of observation. Although the exact mechanism of this phenomenon is not yet known, the findings may be valuable for the growing interest in the identification of Hsp70 modulators for cancer therapy.
Hsp70 expression has been examined in various models Citation[14], Citation[48], Citation[49]. Since Pluronic L61 used for previous studies showed efficacy in the same animal model with low doses (0.1 mg/mL) [51], to be consistent with past studies, L61 was also selected here for in vivo analysis. In the current study, colorectal adenocarcinoma tumours treated with local, intratumoural application of hyperthermia showed Hsp70 expression after 5 h post treatment. Since L61 demonstrated decrease of Hsp70 at 0 to 6 h post treatment in vitro, the 5 h time point was selected for the in vivo studies. In contrast, the tumours pretreated with L61 showed no significant expression after the hyperthermia treatment. These early results suggest that the reduction of Hsp70 expression by Pluronic L61 can be translated to in vivo systems, but an in-depth study of the time course and spatial distribution of this phenomenon is required to validate the approach.
Conclusion
The current study investigated the utility of various Pluronic triblock copolymers in conjunction with hyperthermia and low-grade hyperthermia cancer treatment. As an ideal sensitiser, Pluronic shows relatively low inherent toxicity to the cancer cells examined here, yet the killing effect is increased considerably with low-grade hyperthermia in vitro, suggesting a synergistic effect. Our studies revealed that the synergy of Pluronic and low-grade hyperthermia depleted intracellular ATP and reduced Hsp70 expression, which ultimately led to increased cytotoxicity of cancer cells in vitro. The effects appear to be dependent on the molecular weight and the HLB of the polymers. Most notably, Pluronic L61 reduced Hsp70 expression both in vitro and in vivo when cells or tissues were exposed to mild elevated temperature (43° and 45°C). These findings support further examination of the thermal sensitisation effects of Pluronic and their potential use as a tumour thermal ablation modulator.
Declaration of interest: This study was supported by the National Cancer Institute of the National Institutes of Health (R01CA136857 to AAE). The authors alone are responsible for the content and writing of the paper.
References
- Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: Results in 123 patients. Ann Surg 1999; 230: 1–8
- Zerbini A, Pilli M, Laccabue D, Pelosi G, Molinari A, Negri E, Cerioni S, Fagnoni F, Soliani P, Ferrari C, et al. Radiofrequency thermal ablation for hepatocellular carcinoma stimulates autologous NK-cell response. Gastroenterology 2010; 138: 1931–1942
- Poulou LS, Ziakas PD, Xila V, Vakrinos G, Malagari K, Syrigos KN, Thanos L. Percutaneous radiofrequency ablation for unresectable colorectal liver metastases: Time for shadows to disperse. Rev Recent Clin Trials 2009; 4: 140–146
- Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: Long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005; 234: 961–967
- Lees WR, Gillams A. Radiofrequency ablation: Other abdominal organs. Abdom Imaging 2005; 30: 451–455
- Varshney S, Sewkani A, Sharma S, Kapoor S, Naik S, Sharma A, Patel K. Radiofrequency ablation of unresectable pancreatic carcinoma: Feasibility, efficacy and safety. J Pancreas 2006; 7: 74–8
- McAchran SE, Lesani OA, Resnick MI. Radiofrequency ablation of renal tumors: Past, present, and future. Urology 2005; 66: 15–22
- Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: Part 1, Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. Am J Roentgenol 2005; 185: 64–71
- Dupuy DE, DiPetrillo T, Gandhi S, Ready N, Ng T, Donat W, Mayo-Smith WW. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest 2006; 129: 738–745
- Solbiati L, Goldberg SN, Ierace T, Livraghi T, Meloni F, Dellanoce M, Sironi S, Gazelle GS. Hepatic metastases: Percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology 1997; 205: 367–373
- Solbiati L, Ierace T, Tonolini M, Osti V, Cova L. Radiofrequency thermal ablation of hepatic metastases. Eur J Ultrasound 2001; 13: 149–158
- Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005; 103: 1201–1209
- Calderwood SK, Ciocca DR. Heat shock proteins: Stress proteins with Janus-like properties in cancer. Int J Hyperthermia 2008; 24: 31–39
- Solazzo SA, Ahmed M, Schor-Bardach R, Yang W, Girnun GD, Rahmanuddin S, Levchenko T, Signoretti S, Spitz DR, Torchilin V, et al. Liposomal doxorubicin increases radiofrequency ablation-induced tumor destruction by increasing cellular oxidative and nitrative stress and accelerating apoptotic pathways. Radiology 2010; 255: 62–74
- Vanagas T, Gulbinas A, Pundzius J, Barauskas G. Radiofrequency ablation of liver tumors (I): Biological background. Medicina (Kaunas) 2010; 46: 13–17
- Li GC, Mak JY. Re-induction of Hsp70 synthesis: An assay for thermotolerance. 1988. Int J Hyperthermia 2009; 25: 249–257
- Price BD, Calderwood SK. Ca2+ is essential for multistep activation of the heat shock factor in permeabilized cells. Mol Cell Biol 1991; 11: 3365–3368
- Shpund S, Gershon D. Alterations in the chaperone activity of Hsp70 in aging organisms. Arch Gerontol Geriatr 1997; 24: 125–131
- Creagh EM, Carmody RJ, Cotter TG. Heat shock protein 70 inhibits caspase-dependent and independent apoptosis in Jurkat T cells. Exp Cell Res 2000; 257: 58–66
- Mehlen P, Mehlen A, Godet J, Arrigo AP. Hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J Biol Chem 1997; 272: 31657–31665
- Horman S, Galand P, Mosselmans R, Legros N, Leclercq G, Mairesse N. Changes in the phosphorylation status of the 27 kDa heat shock protein (Hsp27) associated with the modulation of growth and/or differentiation in MCF-7 cells. Cell Prolif 1997; 30: 21–35
- Ghassemi M, Heydari AR, Richardson A. Induction of heat shock proteins in lymphocytes increases with mitogen stimulation. Immunol Lett 1991; 30: 333–337
- Yun JK, McCormick TS, Villabona C, Judware RR, Espinosa MB, Lapetina EG. Inflammatory mediators are perpetuated in macrophages resistant to apoptosis induced by hypoxia. Proc Natl Acad Sci USA 1997; 94: 13903–13908
- Karlseder J, Wissing D, Holzer G, Orel L, Sliutz G, Auer H, Jaattela M, Simon MM. Hsp70 overexpression mediates the escape of a doxorubicin-induced G2 cell cycle arrest. Biochem Biophys Res Commun 1996; 220: 153–159
- Ciocca DR, Fuqua SA, Lock-Lim S, Toft DO, Welch WJ, McGuire WL. Response of human breast cancer cells to heat shock and chemotherapeutic drugs. Cancer Res 1992; 52: 3648–3654
- Krupka TM, Weinberg BD, Ziats NP, Haaga JR, Exner AA. Injectable polymer depot combined with radiofrequency ablation for treatment of experimental carcinoma in rat. Invest Radiol 2006; 41: 890–897
- Exner AA, Krupka TM, Scherrer K, Teets JM. Enhancement of carboplatin toxicity by Pluronic block copolymers. J Control Release 2005; 106: 188–197
- Krupka TM, Weinberg BD, Wu H, Ziats NP, Exner AA. Effect of intratumoral injection of carboplatin combined with pluronic P85 or L61 on experimental colorectal carcinoma in rats. Exp Biol Med (Maywood) 2007; 232: 950–957
- Alakhov V, Moskaleva E, Batrakova EV, Kabanov AV. Hypersensitization of multidrug resistant human ovarian carcinoma cells by pluronic P85 block copolymer. Bioconjug Chem 1996; 7: 209–216
- Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release 2002; 82: 189–212
- Kabanov AV, Batrakova EV, Alakhov VY. An essential relationship between ATP depletion and chemosensitizing activity of Pluronic block copolymers. J Control Release 2003; 91: 75–83
- Batrakova E, Lee S, Li S, Venne A, Alakhov V, Kabanov A. Fundamental relationships between the composition of pluronic block copolymers and their hypersensitization effect in MDR cancer cells. Pharm Res 1999; 16: 1373–1379
- Batrakova EV, Li S, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: Contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther 2001; 299: 483–493
- Batrakova EV, Kabanov AV. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release 2008; 130: 98–106
- Weinberg BD, Krupka TM, Haaga JR, Exner AA. Combination of sensitizing pretreatment and radiofrequency tumor ablation: Evaluation in rat model. Radiology 2008; 246: 796–803
- Chiappetta DA, Sosnik A. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: Improved hydrosolubility, stability and bioavailability of drugs. Eur J Pharm Biopharm 2007; 66: 303–317
- Mirza AN, Fornage BD, Sneige N, Kuerer HM, Newman LA, Ames FC, Singletary SE. Radiofrequency ablation of solid tumors. Cancer J 2001; 7: 95–102
- Kuvshinoff BW, Ota DM. Radiofrequency ablation of liver tumors: Influence of technique and tumor size. Surgery 2002; 132: 605–611, discussion 11–12
- Bleicher RJ, Allegra DP, Nora DT, Wood TF, Foshag LJ, Bilchik AJ. Radiofrequency ablation in 447 complex unresectable liver tumors: Lessons learned. Ann Surg Oncol 2003; 10: 52–58
- Ahmed M, Lukyanov AN, Torchilin V, Tournier H, Schneider AN, Goldberg SN. Combined radiofrequency ablation and adjuvant liposomal chemotherapy: Effect of chemotherapeutic agent, nanoparticle size, and circulation time. J Vasc Interv Radiol 2005; 16: 1365–1371
- Ahmed M, Goldberg SN. Combination radiofrequency thermal ablation and adjuvant IV liposomal doxorubicin increases tissue coagulation and intratumoural drug accumulation. Int J Hyperthermia 2004; 20: 781–802
- Ahmed M, Monsky WE, Girnun G, Lukyanov A, D’Ippolito G, Kruskal JB, Stuart KE, Torchilin VP, Goldberg SN. Radiofrequency thermal ablation sharply increases intratumoral liposomal doxorubicin accumulation and tumor coagulation. Cancer Res 2003; 63: 6327–6333
- Ahmed M, Liu Z, Lukyanov AN, Signoretti S, Horkan C, Monsky WL, Torchilin VP, Goldberg SN. Combination radiofrequency ablation with intratumoral liposomal doxorubicin: Effect on drug accumulation and coagulation in multiple tissues and tumor types in animals. Radiology 2005; 235: 469–477
- Goldberg SN, Girnan GD, Lukyanov AN, Ahmed M, Monsky WL, Gazelle GS, Huertas JC, Stuart KE, Jacobs T, Torchillin VP, et al. Percutaneous tumor ablation: Increased necrosis with combined radio-frequency ablation and intravenous liposomal doxorubicin in a rat breast tumor model. Radiology 2002; 222: 797–804
- Haaga JR, Exner AA, Wang Y, Stowe NT, Tarcha PJ. Combined tumor therapy by using radiofrequency ablation and 5-FU-laden polymer implants: Evaluation in rats and rabbits. Radiology 2005; 237: 911–918
- Curley SA. Radiofrequency ablation of malignant liver tumors. Oncologist 2001; 6: 14–23
- Hoffman AL, Wu SS, Obaid AK, French SW, Lois J, McMonigle M, Ramos HC, Sher LS, Lopez RR. Histologic evaluation and treatment outcome after sequential radiofrequency ablation and hepatic resection for primary and metastatic tumors. Am Surg 2002; 68: 1038–1043
- Liu GJ, Moriyasu F, Hirokawa T, Rexiati M, Yamada M, Imai Y. Expression of heat shock protein 70 in rabbit liver after contrast-enhanced ultrasound and radiofrequency ablation. Ultrasound Med Biol 2010; 36: 78–85
- Yang WL, Nair DG, Makizumi R, Gallos G, Ye X, Sharma RR, Ravikumar TS. Heat shock protein 70 is induced in mouse human colon tumor xenografts after sublethal radiofrequency ablation. Ann Surg Oncol 2004; 11: 399–406
- Krupka TM, Dremann D, Exner AA. Time and dose dependence of Pluronic bioactivity in hyperthermia-induced tumor cell death. Exp Biol Med (Maywood) 2009; 234: 95–104
- Krupka TM EA. Structural parameters govering activity of Pluronic triblock copolymers in hyperthermia cancer therapy. Int J Hyperthermia 2011, In press
- Rapoport N, Marin AP, Timoshin AA. Effect of a polymeric surfactant on electron transport in HL-60 cells. Arch Biochem Biophys 2000; 384: 100–108
- Batrakova EV, Li S, Alakhov VY, Miller DW, Kabanov AV. Optimal structure requirements for Pluronic block copolymers in modifying P-glycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J Pharmacol Exp Ther 2003; 304: 845–854
- Rapoport N, Pitt WG, Sun H, Nelson JL. Drug delivery in polymeric micelles: From in vitro to in vivo. J Control Release 2003; 91: 85–95
- Hahn GM, Strande DP. Cytotoxic effects of hyperthermia and adriamycin on Chinese hamster cells. J Natl Cancer Inst 1976; 57: 1063–1067
- Shivers RR, Wijsman JA. Blood–brain barrier permeability during hyperthermia. Prog Brain Res 1998; 115: 413–424
- Helbig D, Bodendorf M, Anderegg U, Simon J, Paasch U. A human skin explant model to study molecular changes in response to fractional photothermolysis: Spatio-temporal expression of HSP70. Med Laser Appl 2010; 25: 173–180
- Charveron M, Calvo M, Gall Y. Cell stress and implications of the heat-shock response in skin. Cell Biol Toxicol 1995; 11: 161–165
- Laplante AF, Moulin V, Auger FA, Landry J, Li H, Morrow G, Tanguay RM, Germain L. Expression of heat shock proteins in mouse skin during wound healing. J Histochem Cytochem 1998; 46: 1291–1301
- Sahin E, Sahin M, Sanlioglu AD, Gumuslu S. KNK437, a benzylidene lactam compound, sensitises prostate cancer cells to the apoptotic effect of hyperthermia. Int J Hyperthermia 2011; 27: 63–73