Abstract
Purpose: This study assessed the relationship between time, power and ablation size using a novel high-frequency 14.5 GHz microwave applicator in ex vivo human hepatic parenchyma and colorectal liver metastases. Previous examination has demonstrated structurally normal but non-viable cells within the ablation zone. This study aimed to further investigate how ablation affects these cells, and to confirm non-viability.
Materials and methods: Ablations were performed in ex vivo human hepatic parenchyma and tumour for a variety of time (10–180 s) and power (10–50 W) settings. Histological examination was performed to assess cellular anatomy, whilst enzyme histochemistry was used to confirm cellular non-viability. Transmission electron microscopy was used to investigate the subcellular structural effects of ablation within these fixed cells. Preliminary proteomic analysis was also performed to explore the mechanism of microwave cell death.
Results: Increasing time and power settings led to a predictable and reproducible increase in size of ablation. At 50 W and 180 s application, a maximum ablation diameter of 38.8 mm (±1.3) was produced. Ablations were produced rapidly, and at all time and power settings ablations remained spherical (longest:shortest diameter <1.2). Routine histological analysis using haematoxylin-eosin (H&E) confirmed well preserved cellular anatomy despite ablation. Transmission electron microscopy demonstrated marked subcellular damage. Enzyme histochemistry showed complete absence of viability in ablated tissue.
Conclusions: Large spherical ablation zones can be rapidly and reproducibly achieved in ex vivo human hepatic parenchyma and colorectal liver metastases using a 14.5 GHz microwave generator. Despite well preserved cellular appearance, ablated tissue is non-viable.
Introduction
Ablation of colorectal liver metastases relies on the focal delivery of energy to a lesion causing tumour destruction. Targeted ablations minimise the removal of healthy parenchyma, and are useful in patients with borderline parenchymal volume and function, or anatomically difficult lesions which may not be amenable to formal resection but accessible by antenna ablation. Much attention has focused on radiofrequency ablation (RFA) and there is now growing clinical evidence to support the use of ablation for the treatment of irresectable lesions Citation1–2. RFA relies on current transmission, and tissue desiccation and charring can lead to an exponential increase in tissue impedance causing unpredictable ablation size and a maximum theoretical size of ablation that can be achieved Citation3. Current work investigating RF antennas with a cooling mechanism aims to overcome this obstacle by reducing the desiccation of tissue immediately around the antenna, allowing the creation of a larger electric pole and improving electrical conductivity within tissue leading to larger ablation zones Citation4–5.
Microwave ablation offers several theoretical advantages over RFA. These include higher intratumoural temperatures, faster ablation times, improved heating of cystic lesions and reduced procedural pain Citation6. Microwave radiation causes polarised water molecules to oscillate or vibrate and this active heating mechanism means microwave energy is not reduced by transmission through charred and desiccated tissue, leading to a more clearly delineated histopathological ablation zone Citation7. This active heating mechanism also means all tissue within the treatment zone is heated simultaneously, leading to reduced ablation times. The absence of a ‘ground patch’ on patient skin reduces the risk of patient burning and also allows multiple simultaneous ablations resulting in rapid treatment of large areas Citation6.
An ex vivo porcine liver study directly compared ablation by internally cooled bipolar microwave and multiple RFA antennas and demonstrated more rapid heating and larger ablation zones after microwave treatment Citation8.
One of the major drawbacks associated with existing microwave ablation systems is that a high portion of the energy from the source is reflected as the tissue impedance changes, making the ablation process increasingly unpredictable. This is of particular importance in the treatment of lesions adjacent to structures that are susceptible to thermal injury Citation9. However, the clinical application of higher frequency microwave systems which overcome these problems have been limited by the need for expensive and unwieldy generators. Advances in the telecommunications sector have now led to the development of mass produced solid state microwave generators which operate at frequencies higher than 2.45 GHz, allowing the development of affordable systems that produce focused and controlled heating.
This study assessed a system that includes a stable (phase locked) and controllable solid state source operating at a frequency within the super high frequency (SHF) band of 14.5 GHz, an IEC approved frequency. The system incorporates a novel and proprietary automatically adjustable impedance matching system in which the reflected signal (caused by the reflection coefficient, produced by the difference between the impedance at the tip of the antenna and the impedance of the contact tissue) is transferred back to the generator using a low loss microwave transmission cable. Phase and magnitude information are extracted using a double heterodyne detector that down-converts the 14.5 GHz signal to a 10 MHz measurement signal that still contains the necessary tissue impedance information, but can be processed using a standard digital to analogue (DAC) converter and microprocessor/digital signal processing unit. This phase and magnitude information is used to calculate the movement of actuators contained within a triple stub tuning network to ensure that the impedance of the generator is matched to the impedance of the biological tissue load, ensuring that the power available at the output of the source (less the insertion loss of the low loss microwave cable and the insertion loss of the applicator shaft) is transferred into the tumour or other biological tissue in contact with the tip of the applicator Citation10. In essence, a resonant tuning cavity is created using the triple stub tuning network, the low loss co-axial cable and the antenna. This tuning cavity allows the power available at the output of the source (the power demanded by the user) to be delivered to the tissue, even when the tissue impedance changes during the ablation process. In addition to matching the output of the source to the tissue load, the phase and magnitude information can also be used to characterise the tissue at the antenna tip. The ability to locate the tip of the antenna at the centre of a lesion would allow more accurate ablation, as well as the potential for identifying clinically relevant changes in tissue behaviour, e.g. tissue popping and bleeding during ablation.
Correct identification of antenna location in a three-dimensional organ using two-dimensional imaging techniques remains a problem, and inappropriate probe placement may be responsible for high rates of local recurrence after ablation. The ability of the antenna to provide further information would greatly assist the clinician in siting the antenna in the correct location.
Previous work has demonstrated that 14.5 GHz is an optimum frequency for high power ablation and low power measurement Citation10–12. The limited depth of the microwave field (defined as the distance into tissue at which the value of the electric field has decreased to 37% of its maximal or initial value) at 14.5 GHz ensures that the phase and magnitude of the reflected signal is due to the tissue directly at the antenna tip and adjacent tissue causes minimal perturbations.. It also ensures that the energy propagates quickly and in a focused manner when performing ablations. For example, the depth of penetration of the microwave field into blood at 14.5 GHz is 1.6 mm, producing a sphere of coagulation from an omni-directional antenna of 17 mm3, compared to 12.5 mm in fat, resulting in a sphere of coagulation of 7606 mm3. If the energy at the source is the same in both instances then the temperature of the fat will increase only slightly, whereas the temperature of the blood will increase to a value whereby instant coagulation occurs. The loaded wavelength of normal hepatic parenchyma and corresponding depth of penetration at 14.5 GHz is 3.8 mm and 2 mm respectively Citation13, meaning the temperature of the tissue surrounding the tip of the antenna increases rapidly but in a controlled manner. This limited depth of penetration, coupled with the tissue matching mechanism, enables the temperature of the tissue to be elevated much more quickly than is possible using other lower frequency systems, i.e. 915 MHz or 2.45 GHz, where the spread of the energy means that the antenna has to deliver energy for longer, leading to shaft heating. By minimising antenna heating, the current system offers advantages for percutaneous use where skin burns from heated antennas can be problematic.
Previous histopathological examination of microwave ablated tissue has identified an area of structurally normal but non-viable tissue within the ablation zone, which does not demonstrate thermal damage, e.g. coagulative necrosis. It has been suggested that ablation leads to protein fixation and enzymatic denaturation within this zone, preventing autolysis. The body is unable to break down this fixed tissue, and so the classical wound healing pathway is not followed. The fixed zone becomes surrounded by an area of fibrotic scar tissue, and the fixed zone can remain stable for many years after ablation Citation14. The well preserved cellular anatomy of this tissue on haematoxylin-eosin (H&E) staining can make distinguishing between viable and non-viable tissue difficult. With local recurrence rates of between 3.6–60% after microwave ablation Citation2, it becomes vitally important to confirm that ablated tissue is completely destroyed. Preliminary assessment of cellular viability using enzyme histochemical analysis Citation15 has suggested that ablated parenchyma and tumour are non-viable, and has led to the suggestion that thermal energy may lead to a novel type of cell death, distinct from apoptosis and oncosis. The mechanism of cell death after microwave ablation was further explored using in vivo modelling by Mori et al., who demonstrated that it not only gave rise to thermal cell fixation, but also gave rise to a distinct pattern of DNA damage caused by non-thermal microwave effect Citation16. The precise mechanism of this microwave cell death is not well understood.
This pilot study aimed to assess the efficacy of a novel 14.5 GHz microwave source, with proprietary dynamic impedance matching and tissue type/state recognition, in performing ablations in ex vivo human liver model. The study aimed to assess the size and reproducibility of ablation zones in normal hepatic parenchyma, as well as colorectal liver metastases, data which would be vital when planning future clinical studies. Enzyme histochemistry was used to assess post-ablation cellular viability and confirm cell death, whilst transmission electron microscopy was used to assess the effects of ablation on subcellular structure. 2D gel electrophoresis and isobaric tagging relative and absolute quantification (ITRAQ) were used to perform preliminary investigations into downstream variation in protein expression caused by microwave-induced DNA damage.
Materials and methods
Surgical procedures
This was a treat and resect study, where patients received normal standard of care with their ex vivo specimens being used for experimentation. A hepatobiliary multidisciplinary team identified sequential patients with easily resectable colorectal liver metastases as suitable for the study. Northern and Yorkshire Research Ethics Committee gave ethical approval for the study (09/H0903/21) in accordance with the 1975 Declaration of Helsinki. Eight patients were recruited into the study (3 men, 5 women), with a median age of 67 (range 45–78). All patients underwent uneventful liver resection. Patients received a reverse L laparotomy. In all cases, intraoperative ultrasound was performed to exclude further lesions. To reduce intraoperative blood loss, low venous pressure anaesthesia was used, with CVP (central venous pressure) consistently maintained below 5 cm H2O. Parenchymal transaction was performed using a Cavitron ultrasonic surgical aspirator (CUSA, Valleylab, Boulder, CO). The Pringle manoeuvre was not used routinely, and was decided on by the operating surgeon. When used, this followed a cycle of 10 min preconditioning, 5 min perfusion, followed by alternating cycles of 15 min exclusion and 5 min perfusion. Pringle was applied in four patients, with a median duration of 8 min (range 5–17). Patient temperature was maintained between 36–38°C throughout the procedure. The operating theatre was maintained at a constant 21°C. Immediately following delivery of the specimen from the patient, the tissue was transferred to a bench in a room adjacent to the operating suite (typical time to first ablation <60 s). Sequential microwave ablations were then performed.
Ablation protocol
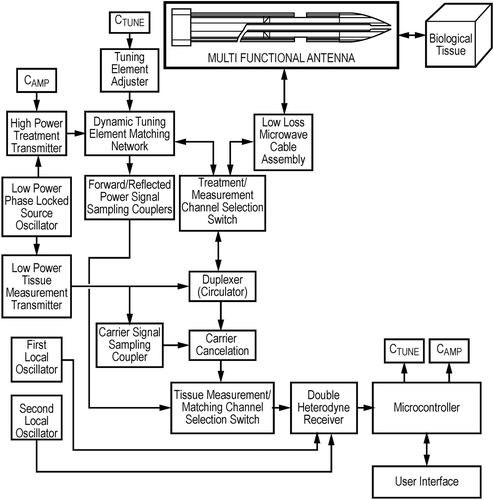
The microwave ablator consisted of a MicroBlate microwave treatment and measurement system (Medical Device Innovations, Daresbury, Cheshire, UK) with a maximum power output of 50 W. The system is based on a solid state power source, driven by a double phase locked oscillator, which produces low power of around 10 mW at a spot frequency of 14.5 GHz, with a variation of less than ±100 KHz. The output from the power source is connected to a tuning network that is dynamically adjusted based on phase and magnitude information, measured at the tip of the antenna and extracted using a double heterodyne transceiver, which also forms a proprietary element of the MicroBlate treatment system. The double heterodyne transceiver is also used to make in situ measurements of biological tissue that is in contact with the tip of the antenna. In order to be able to make valid tissue measurements, the tip of the antenna is calibrated, using a proprietary calibration technique, prior to use. provides a high level diagram of the MicroBlate treatment and measurement system.
The output of the MicroBlate system was connected to a 2.2-mm diameter × 120-mm long disposable antenna () by a 1.5 metre flexible low loss microwave coaxial cable assembly. The design of the system has been reported previously Citation10. Power and duration of ablation were programmed into the MicroBlate system, which was activated by a foot pedal.
Figure 2. The 2.2-mm antenna. The white ceramic tip is the microwave antenna, and the impedance matching mechanism of the generator ensures that energy is released from the tip of the antenna leading to spherical ablations.

All antenna placements were performed under direct vision to a depth of 30 mm. Ablations were performed in normal hepatic parenchyma using powers of 10 W, 30 W and 50 W. Energy was delivered for 10, 20, 30, 60, 120, 150 and 180 s at each power setting. All ablations were performed six times, with two ablations performed in three different liver resections. Ablations were also performed in the metastatic tissue, with time and power varying dependent on the size of the lesion. Immediately after ablation, the resected specimen was sectioned and two assessors measured the maximal cross-sectional length and width of the ablation zone using calipers. The ablation zone was defined as the blanched area (so called white zone), up to but not including the haemorrhagic zone (red zone) (see ). Maximal ablation diameter for each power setting was compared at each time point. The ratio of maximal length:width ratio was calculated to describe how spherical an ablation was.
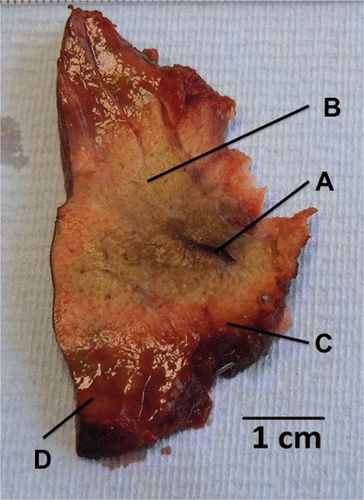
Figure 3. Macroscopic appearance of ablated parenchyma. After 30 s at 50 W, a mean ablation diameter of 21.3 mm ± 1 was achieved. Note the antenna track with some evidence of charring (A), white zone, consisting of blanched parenchyma within the ablation zone (B), red zone, the rim of haemorrhagic tissue (C) and the surrounding normal-looking parenchyma (D).
Tissue sampling
Immediately after ablation, the specimen was sectioned. Samples of tissue from within the white zone and samples of untreated tissue distant from the ablation were excised and placed in a Cryotube vial (Thermo Scientific, Rochester, NY, USA), then immediately snap frozen in liquid nitrogen and stored at −80°C for proteomic analysis. This was repeated for two parenchymal ablations and one tumour ablation in each patient (apart from one patient who had no detectable tumour after resection).
Multiple sections of ablated parenchyma and ablated metastatic tumour were sampled for H&E pathological analysis. Specimens were specifically chosen to include the interface between the white zone, red zone and normal-looking tissue, as well as the antenna track. Immediately after sampling, they were placed in sampling cassettes and immersed in formalin.
Cellular viability was assessed by staining fresh tissue (gross specimens and frozen sections) for mitochondrial NADH function, a surrogate marker for cellular viability Citation15. Gross specimens were chosen to encompass all of the ablation zone, including an area of surrounding normal tissue, and were stored at −80°C until staining. Frozen sections were chosen to include the boundary between the white zone, red zone and surrounding normal tissue. Frozen sections were produced by mounting tissue on a cork disc with cryoglue, freezing to −20°C and taking sequential 5 µm sections using a cutting cryotome. Sections were then mounted on glass slides, and stored at −80°C until staining. Staining was performed by defrosting specimens and incubating with ß-NADH (Sigma, St Louis, MO) in tetrazolium solution (0.2 M Tris buffer, 1 mg mL−1 ß-nitroblue tetrazolium) at 37°C for 30 min.
Tissue for transmission electron microscopy was taken from ablated parenchyma and ablated tumour in one patient. Tissue was sampled from within the white zone and outside the red zone in normal appearing parenchyma, then immediately placed in a light-proof container with iced 3% glutaraldehyde and refrigerated at 3°C until analysis (<30 days).
2D gel electrophoresis
2D gel analysis was performed on samples from three patients (Patients 2, 4, 5). Ablated and non-ablated parenchyma and tumour were prepared by homogenising tissue in lysis buffer (7.0 M urea, 2.0 M thiourea, 4% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulphonate (CHAPS), 40 mm Tris base ±1% dithiothreitol (DTT)), followed by freeze-thaw cycling and ultrasonification. DTT was excluded for non-reducing conditions. After centrifugation, the supernatant was removed and 1 mg of protein was made up to 350 µL using rehydration solution (9 M urea, 2% (w/v) CHAPS, bromophenol blue, 2% IPG). A non-linear IPG (Immobilized pH gradient) strip (GE Healthcare Life Sciences, Buckinghamshire, UK) was immersed in the sample overnight, before running the first dimension in a water-cooled IEF-multiphor (GE Healthcare, Amersham, Bucks). After the first dimension had completed, IPG strips containing the sample underwent equilibration using first buffer (50 mm Tris, 6 M urea, 30% (v/v) glycerol, 2% (w/v) SDS(sodium dodecyl sulphate), bromophenol blue, ddH2O ±0.1%DTT) followed by second buffer (50 mm Tris, 6M urea, 30% (v/v) glycerol, 2% (w/v) SDS, bromophenol blue, ddH2O ± 0.25% iodoacetamide). After equilibration, the second dimension was run on a 12% SDS-polyacrylamide gel electrophoresis (PAGE) gel using an Invitrogen ZOOM®system (Invitrogen, Carlsbad, CA, USA) in running buffer. Proteins were fixed in 7% glacial acetic acid in 40% (v/v) methanol, then stained overnight using freshly prepared Coomassie stain. Gels were de-stained using 10% acetic acid in 25% (v/v) methanol, prior to scanning using a Biorad GS800 scanner (BioRad Laboratories, Hemel Hempstead, UK), and analysed and interpreted using Progenesis SameSpots (Non Linear Dynamics, Newcastle, UK).
Isobaric tag for relative and absolute quantification analysis
Ablated and non-ablated liver parenchyma and tumour from one patient (7) were homogenised in dissolution buffer (0.5 M tetraethylammonium bromide (TEAB)/0.1% SDS in H2O). Following centrifugation, the supernatant was removed and protein concentration was determined using sequential Bradford assay. A 75 µg aliquot of protein was placed in 20 µL of dissolution buffer (Applied Biosystems, Carlsbad, CA, USA). Aliquots of 1 µL of denaturant and 2 µL of reducing agent (Applied Biosystems) were added followed by incubation at 60°C for 1 h. An aliquot of 1 µL of cysteine blocking agent (Applied Biosystems) was added and incubated at room temperature for 10 min. Trypsin was reconstituted using 25 µL of Milli-Q water. 10 µL of the reconstituted trypsin solution was then added to each sample followed by incubation at 37°C overnight. ITRAQ labelling reagents were reconstituted as per the manufacturer's protocol using 50 µL of isopropanol. Each freshly prepared labelling reagent was then added to the sample tube. The pH was adjusted to 7.5–8.5 by adding further dissolution buffer. All labelled samples were combined and immediately underwent cation exchange, followed by LC-MS/MS using a QqTOF QStar XL mass spectrometer (Applied Biosystems). The accumulated LC-MS/MS data was analysed using Protein Pilot (Applied Biosystems).
Results
Two patients had received systemic chemotherapy prior to resection, both FOLFOX (5-FU, leucovorin, oxaliplatin) (see ). Both patients had stopped chemotherapy at least 12 weeks prior to resection. All patients had normal preoperative liver function tests. There was no statistically significant difference in size of ablation between chemonaïve and post-chemotherapy tissue (p = 0.26).
Table I. Patient information, neoadjuvant therapy and tumour characteristics. Ablations performed in tumour showed similar size to ablations performed in normal parenchyma.
Ablation size
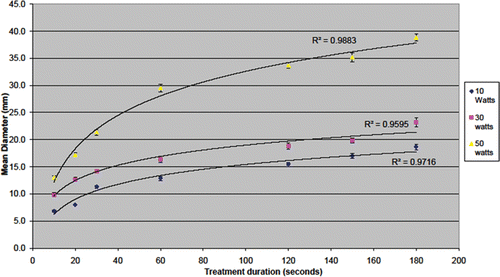
Ablations performed in normal parenchyma were of consistently uniform size, with a maximum standard deviation of 1.7 mm for all times and power settings. Size of ablation increased with time in a sinusoidal fashion (R2 > 0.96) at all three power settings. All ablations had a length:width ratio of <1.2, suggesting consistently spherical ablation zones. The curves did plateau during longer applications, but did not level out completely suggesting larger ablations would be possible with longer applications (see ). Antenna temperature was measured 5 cm from the tip after an ablation of 180 s at 50 W, and demonstrated a rise of 19°C.
Figure 4. Maximum ablation diameter achieved in ex vivo human modelling. Increased time and power leads to increased ablation diameter. Although the curves appear to plateau, increasing treatment time continues to increase ablation zone diameter.
One ablation was performed on each individual metastasis (n = 8, see ). The necrotic centre of tumour metastases meant the zone of ablation within the tumour was less clear than within parenchyma, but it was still possible to define the edge of the ablation area. Because the antenna was inserted under direct vision it was not always placed directly within the centre of the lesion (patients 1 and 7), and the zone of ablation included some normal hepatic parenchyma. The zone of ablation was circular in both cases, with a ratio <1.2. Although the number and size of metastases included in this study meant only a small number of tumour ablations were performed, the sizes of these ablations were broadly comparable with the size of ablations in normal parenchyma. Again, these lesions were consistently spherical with a length:width ratio of <1.2.
Histological analysis
Ablations in normal parenchyma showed a consistent macroscopic appearance (). There was a central cavity where the antenna had been inserted, which demonstrated some charring at higher power settings (30 W and 50 W) with ablations lasting longer than 120 s. Surrounding this was an area of marked blanching (white zone). Around this was a further haemorrhagic zone (red zone), surrounded by normal looking parenchyma. A similar pattern was evident in ablated colorectal metastases, although the white zone appeared less evident in necrotic tissue () and there was an almost complete absence of red zone. In these cases, the edge of the ablation zone was identifiable by a more subtle change in appearance, with the ablated tissue appearing lighter.
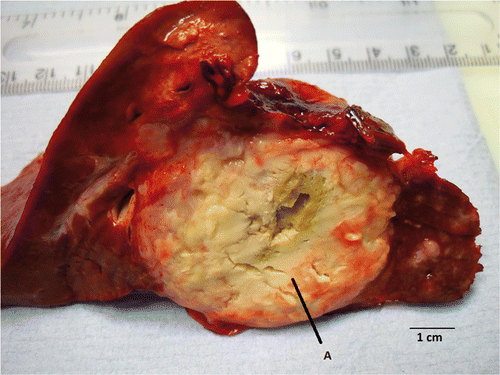
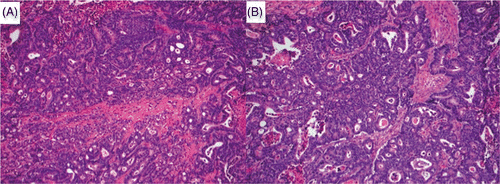
Figure 5. Macroscopic appearance of the ablation zone within tumour. The necrotic tumour tissue shows the edge of the ablation zone less clearly (A), although the antenna track is clearly visible within the tumour. Histological examination of the resected tumour showed apparently viable-looking adenocarcinoma within the ablation zone.
Microscopic examination of ablated parenchyma showed marked disruption around the site of antenna insertion. There was evidence of thermal damage around the antenna site, with cavitation between cells, possibly as a result of steam artefact. Surrounding this area was tissue with normal cellular anatomy (), although these cells appeared to more avidly take up haematoxylin stain than untreated control tissue. There was a degree of nuclear smearing, and in some areas cellular cytoplasm appeared to be more granular. Biliary and arterial vessels within the ablation zone had an abnormal appearance, with tracts close to the centre of the ablation zone demonstrating a loss of endothelium. Outside the macroscopic ablation zone, parenchyma appeared completely normal. Ablated tumour followed a similar pattern, with areas of viable looking adenocarcinoma within the ablation zone (). Nuclear smearing was apparent within some cells, although it is impossible to comment on whether this is normal tumour appearance. In all cases, the clear border between the macroscopically identifiable zones was not readily apparent on microscopic analysis.
Figure 6. Normal untreated parenchyma (A) and ablated parenchyma (B) (magnification ×20, H&E stain). Hepatocytes within the white zone exhibit central nuclear smearing (arrow), have increased density (hyperchromasia), and have taken on a slightly shrunken appearance. The characteristic cellular anatomy is well maintained between ablated and non-ablated tissue.
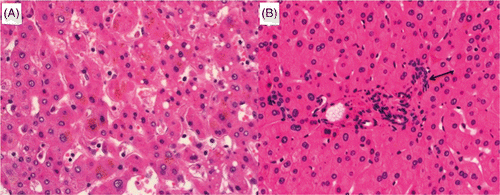
Figure 7. Untreated (A) and ablated (B) colorectal liver metastasis ×20 (H&E stain). Cells have large nuclei and disorganised glandular structure, appearances consistent with viable well differentiated adenocarcinoma. Further investigation using enzyme histochemistry and transmission electron microscopy confirmed that despite well preserved cellular anatomy this tumour was not viable.
Enzyme histochemistry
NADH staining of macroscopic tissue blocks showed a clear loss of cellular viability around the edge of the lesion, correlating with the border between the white and red zone (). Frozen section analysis demonstrated a sudden change from viable to non-viable tissue over a distance of 1 mm (). Sequential frozen slices showed no obvious structural change on H&E changes to correlate with this change in viability.
Figure 8. Macroscopic resection of ablation zone showing fresh tissue (A) and NADH-stained tissue with black staining demonstrating viability (B). The absence of staining with the white zone of the ablation site confirms lack of tissue viability. There is some staining within the red zone, and so ablation size was calculated on the size of the white zone (white arrow).
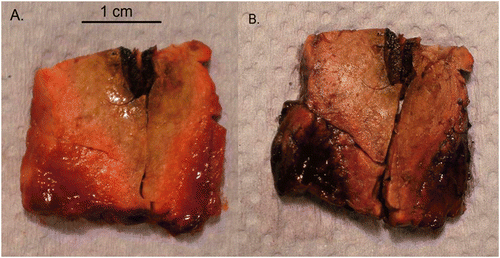
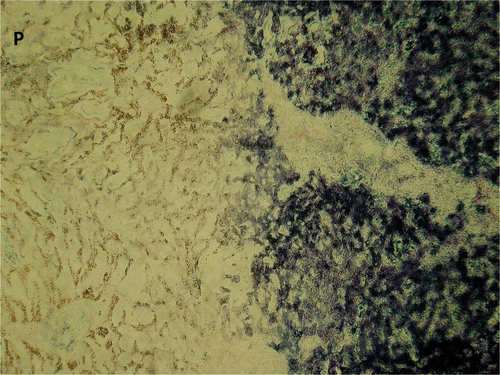
Figure 9. Parenchyma (magnification ×10) from the edge of the white zone sampled as a frozen section, stained for NADH. P indicates the site of antenna insertion. The sudden change from viable to non-viable tissue on NADH staining appears to correlate with the border between the white zone and the surrounding red zone on macroscopic section. Identifying this transition from treated to untreated tissue on routine H&E analysis was more difficult, with a more subtle change in cellular appearance.
Transmission electron microscopic analysis
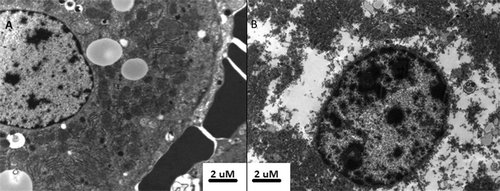
Electron microscopy of tissue with the white zone showed marked organelle disruption. Nuclei appeared distorted, with an irregular appearance of the nuclear membrane. Nuclear contents appeared to have clumped together (). Cytoplasmic content was clumped together, with occasional remnants of organelles visible. In contrast, tissue from outside the white zone showed well preserved nuclear morphology and a clearly defined nuclear envelope. Cytoplasmic content was well structured, with easily identifiable organelles (). As previously described by Yamaguchi et al. Citation20, erythrocytes within the white zone were destroyed, whereas those in surrounding tissue showed well maintained structure.
Figure 10. Transmission electron microscopy of untreated (A) and ablated (B) tumour. Tissue from untreated adenocarcinoma demonstrates well preserved nuclear structure and clearly identifiable mitochondrion and rough endoplasmic reticulum within the cytoplasm. The presence of intact erythrocytes was associated with untreated tissue, whereas erythrocytes within the ablation zone had ruptured cellular membranes. By contrast, the ablated tumour demonstrates atypical nuclear and cytoplasmic appearance, suggesting that despite normal cellular appearance, subcellular structure is markedly altered by ablation.
2D PAGE
Gel electrophoresis of ablated tissue protein was conducted under reducing and non-reducing conditions, as it has previously been suggested that thermal ablation results in protein fixation via cross-linking through disulphide bridge formation Citation9. Visual inspection of the Coomassie-stained gel images revealed little discernible difference between ablated and non-ablated tumour and parenchyma. Furthermore, repeating the analysis under non-reducing conditions failed to show a differential expression pattern between ablated and non-ablated samples (not shown). This was supported by automated image analysis of gels using SameSpots software, which detected a similar number of discreet spots in both ablated and non-ablated tissues, irrespective of the presence of a reducing agent (, coefficient of variation between samples <13.2%). These data suggest that microwave treatment does not result in pronounced protein denaturation through consistent cross-linking.
Table II. Number of discreet spots identified on SDS-PAGE using Progenesis SameSpots analysis. SD, standard deviation, CoV, % co-efficient of variation. A similar number of spots were detected between ablated and non-ablated tissues run under both reducing and non-reducing conditions.
ITRAQ analysis
A single 4-plex ITRAQ analysis was performed comparing ablated and non-ablated parenchyma and ablated and non-ablated tumour. Using a false discovery rate of 1% with 95% confidence, 1650 unique proteins were identified as present in all four samples. Although ITRAQ data did suggest a significant variation in protein quantification (carboxylesterase-2, fatty acid synthase) between samples, this was not confirmed on immunoblotting (data not shown).
Discussion
The study demonstrated that a 14.5 GHz MicroBlate system and 2.2-mm diameter antennae can develop spherical zones of ablation of consistent size in ex vivo human hepatic parenchyma and colorectal liver metastases. Ablations were performed rapidly, with a zone of 38.8 mm ± 1.3 mm produced after only 180 s. The maximum ablation diameter achieved in this series was 38.8 mm although the gradient of the curve suggests that further gains in ablation zone size could easily be achieved by prolonging the time course. The development of a next generation system with a maximum power output of 100 W also offers the prospect of increased ablation size. Recent ASCO guidelines Citation1 highlighted the higher recurrence rates associated with ablations greater than 30 mm diameter, and our unit currently adopts a policy of only ablating lesions with a maximal diameter of less than 30 mm (with a circumferential rim of 5–10 mm of normal parenchyma). The system tested in this study is capable of ablating lesions of this size.
The flattening of the curve of ablation size compared to time/power observed in this ex vivo study likely reflects a non-linear reduction in penetration of the microwave through tissue as distance from the antenna increases. The magnitude of the electric field reduces exponentially with distance from the radiating tip of the antenna. The temperature profile inside tissue as a function of time is proportional to the square of the electric field, and so size of ablation will reduce exponentially. Ablations performed in tumour and parenchyma were of similar size. However, in vivo ablation behaviour may be different, with arterially fed hypervascular tumours experiencing a far larger perfusion-mediated cooling effect and so smaller sized ablations than healthy hepatic parenchyma perfused by the venous supply. Further in vivo experimentation is necessary to explore this clinically important question.
This study considered ablations in both post-chemotherapy and chemonaïve patients. Neoadjuvant chemotherapy is associated with drug-induced histopathological change including steatosis, steatohepatitis and sinusoidal obstructive syndrome Citation17. The size of the ablation achieved in each of these scenarios was consistent with the active heating ablating the same volume of tissue in both post-chemotherapy and chemonaïve patients.
The reproducibility of ablation size (coefficient of variation <7.7% at all settings) and consistently spherical shape (ratio <1.2) are promising. The dynamic tissue impedance matching system ensures that the desired quantity of energy demanded by the user is actually delivered into the tissue, which helps ensure consistency of ablation size. This feature should also reduce the treatment time and overcome the effects of perfusion as the same amount of energy is delivered into the tissue irrespective of changes in the impedance of the tissue – this is unlike other lower frequency microwave systems where the changing load impedance causes the energy to be reflected back along the microwave cable to the source. As with resectional surgery, the aim of ablation is to achieve a negative margin surrounding the ablation zone, and accurate prediction of ablation zone size and shape would assist the pre-intervention planning of the treatment to ensure adequate tumour destruction and minimise unnecessary parenchymal damage. The consistent length:width ratio is in contrast to an ex vivo study Citation18 which used a similar sampling technique. They demonstrated an ellipsoid ablation zone in ex vivo tissue, in contrast to a more spherical zone in perfused tissue. The authors suggested that heat is transmitted along the shaft leading to ellipsoid ablations ex vivo, whilst perfusion-mediated cooling maintains spherical ablations in vivo. The current system demonstrated excellent length:width ratio in ex vivo tissue, possibly as a result of the tissue impedance matching system as well as the narrow diameter of the antenna shaft providing a relatively small conductor for thermal energy to track away from the antenna tip. This is supported by the relatively minor rise in shaft temperature during ablation. We believe the accuracy and precision of the system, as well as highly and reproducibly spherical ablations offer an advantage if the system is used for ablating near major biliary and vascular structures.
Ablations were rapid, with 79% of the largest ablation produced in this series achieved within 60 s. This rapid ablation time offers a distinct theoretical advantage in the percutaneous ablation of liver metastases, where sedated patients are more likely to move during the procedure. In these cases, short ablation times should result in more accurate ablations. The narrow diameter of the antenna used with this system would also be ideal for the development of a percutaneous antenna. The narrow antenna also produces minimal tissue distortion during insertion, which helps improve the accuracy of placement. The low rise in shaft temperature is a result of the tissue matching system, which reduces energy reflection into the antenna. This removes the need for high pressure water cooling systems to be engineered into the antenna, making the antenna smaller and simpler, as well as reducing the cost of what would be a single-use item.
This study only considered ex vivo ablations in freshly excised human liver tissue. Previous work has suggested that in vivo ablations are smaller than ex vivo, an effect attributed to perfusion-mediated tissue cooling. However, data produced by Hines-Peralta et al. Citation18 suggested that in vivo ablations may be larger than ex vivo and hypothesised that the higher water content of in vivo tissue or coagulation induced by hyperthermia led to large areas of secondary ischaemic necrosis. Previous histopathological examination of in vivo Citation19 ablations has also demonstrated a complete loss of vascular endothelium within ablated tissues, which would lead to thrombosis and increased tissue ischaemia. The precise relationship between in vivo and ex vivo ablation size will require further clarification before clinical application can begin.
Microwave ablation is known to cause tissue fixation, with cellular anatomy well preserved within the ablation zone Citation19. H&E microscopic analysis of ablated tissue from this study demonstrated the same effect, with well preserved cellular morphology in ablated parenchyma and tumour. NADH staining confirmed lack of cellular viability within the ablation zone, suggesting that although structure is preserved, function has been lost and the ablated tissue is not viable. The boundary between treated and untreated tissue was very sharp (<1 mm), suggesting that thermal damage outside the ablation zone was limited. Electron microscopy showed that despite the well preserved structural morphology on light microscopy, subcellular structure was profoundly distorted with an almost complete absence of identifiable cytoplasmic organelles within the ablation zone. This phenomenon has been reported before Citation20 and it has been suggested that organelle membranes are significantly damaged by microwave energy Citation21. The combination of H&E analysis, enzyme histochemistry and electron microscopy presented in this study confirms that ablated tumour and hepatic parenchyma is non-viable, despite well preserved cellular anatomy. Yamaguchi et al. Citation20 hypothesised that microwave energy may cause protein cross-linking, fixing cellular structure whilst preventing enzyme function and therefore autolysis. Ablation energy would be expected to cause random cross-linking between proteins. Extensive denaturation of proteins through covalent cross-linking would therefore be expected to result in a clear alteration of the image pattern obtained; this was not the case, and a similar number of discrete proteins were identified between different samples. To check that the cross-linking was not due to disulphide bridge formation that was reversed in the presence of strong reducing agents, repeat gels were run under non-reducing conditions. Again, no major differences were observed, suggesting that widespread cross-linking of protein is not the principal mechanism underlying the microwave-induced fixation process. Preliminary ITRAQ investigation did not demonstrate variation in protein expression between ablated and non-ablated tissue. However, the ex vivo model used in this study may limit these findings, and further work will be necessary using in vivo ablations and delayed resection to further assess the downstream effects of microwave-mediated DNA changes.
Conclusions
This work demonstrates that ablation with a controllable 14.5 GHz solid state source with innovative dynamic tissue matching and tissue type/state measurement leads to the rapid creation of consistently reproducible sized and spherical zones of coagulation. This study achieved a maximum ablation diameter of 39 mm in 180 s with potential for larger zones of coagulation by applying longer time courses, and confirmed that despite ambiguous appearances on H&E staining, ablated tissue is non-viable.
Declaration of interest: R.J. received an unrestricted educational grant from Medical Device Innovations. Medical Device Innovations had no input into the design, data collection, analysis or interpretation of data, nor any input into the writing of the manuscript. The authors alone are responsible for the content and writing of the paper.
References
- Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, III, Dorfman GS, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol 2010; 28: 493–508
- Pathak S, Jones RP, Tang JM, Parmar C, Fenwick S, Malik H, et al. Ablative therapies for colorectal liver metastases (CRLM): A systematic review. Colorectal Dis Sep, 2011; 13(9)e252–65
- Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: Radiologic–pathologic correlation. Cancer 2000; 88: 2452–2463
- Rempp H, Scharpf M, Voigtländer M, Schraml C, Schmidt D, Fend F, et al. Sustained growth of the ex vivo ablation zones’ critical short axis using gas-cooled radiofrequency applicators. Cardiovasc Intervent Radiol 2011; 34: 149–155
- Rempp H, Voigtländer M, Clasen S, Kempf S, Neugebauer A, Schraml C, et al. Increased ablation zones using cryo-based internally cooled bipolar RF applicator in ex vivo bovine liver. Invest Radiol 2009; 44: 763–768
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: Principles and applications. Radiographics 2005; 25: S69–83
- Gravante G, Ong SL, Metcalfe MS, Strickland A, Dennison AR, Lloyd DM. Hepatic microwave ablation: A review of the histological changes following thermal damage. Liver Int 2008; 28: 911–921
- Li X, Zhang L, Fan W, Zhao M, Wang L, Tang T, et al. Comparison of microwave ablation and multipolar radiofrequency ablation, both using a pair of internally cooled interstitial applicators: Results in ex vivo porcine livers. Int J Hyperthermia 2011; 27: 240–248
- Chen JC, Moriarty JA, Derbyshire JA, Peters RD, Trachtenberg J, Bell SD, et al. Prostate cancer: MR imaging and thermometry during microwave thermal ablation – Initial experience. Radiology 2000; 214: 290–297
- Hancock CP, Chaudry S, Wall P, Goodman AM. Proof of concept percutaneous treatment system to enable fast and finely controlled ablation of biological tissue. Med Biol Eng Comput 2007; 45: 531–540
- MicroOncology. Method of Calibration and Tissue Recognition Using the MicroBlate 2 System. MB2_TRP019_1_0_CALSystem, technical report, 31 July. Daresbury, Cheshire: Medical Device Innovations, 2007.
- MicroOncology. First Morbid Tissue Testing Carried Out Using the MicroBlate 2 System. MB2_TRP017_1_0_1st Tissue Testing, technical report, 3rd April. Cheshire: Medical Device Innovations, 2007.
- Italian National Research Council Institute for Applied Physics, Dielectric Properties of Body Tissues. Available at: http://niremf.ifac.cnr.it/tissprop/ (accessed 10 July 2011).
- Godwin BL, Coad J. Healing responses following cryothermic and hyperthermic tissue ablation. SPIE Proc 2009; 7181: 1–9
- Shen P, Geisinger KR, Zagoria R, Levine EA. Pathologic correlation study of microwave coagulation therapy for hepatic malignancies using a three-ring antenna. J Gastrointest Surg 2007; 11: 603–611
- Mori I, Ozaki T, Tabuse K, Utsunomiya H, Taniguchi E, Kakudo K. Microwave cell death: Molecular analysis using DNA electrophoresis, PCR amplification and TUNEL. Pathol Int 2009; 59: 294–299
- Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 2006; 24: 2065–2072
- Hines-Peralta AU, Pirani N, Clegg P, Cronin N, Ryan TP, Liu Z, et al. Microwave ablation: Results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology 2006; 239: 94–102
- Bhardwaj N, Strickland AD, Ahmad F, Atanesyan L, West K, Lloyd DM. A comparative histological evaluation of the ablations produced by microwave, cryotherapy and radiofrequency in the liver. Pathology 2009; 41: 168–172
- Yamaguchi T, Mukaisho K, Yamamoto H, Shiomi H, Kurumi Y, Sugihara H, et al. Disruption of erythrocytes distinguishes fixed cells/tissues from viable cells/tissues following microwave coagulation therapy. Dig Dis Sci 2005; 50: 1347–1355
- Dwivedi RS, Dwivedi U, Chiang B. Low intensity microwave radiation effects on the ultrastructure of Chang liver cells. Exp Cell Res 1989; 180: 253–265