Abstract
Purpose: The aim of this paper was to establish non-invasive CT-based temperature monitoring during hepatic radiofrequency (RF) ablation in an ex vivo porcine model followed by transfer of the technique into a feasibility in vivo experiment.
Materials and methods: Bipolar RF ablations were performed in 10 specimens of porcine liver. Parallel to the needle-shaped RF applicator three optical temperature probes were inserted into the liver specimens at fixed distances of 5, 10 and 15 mm from the RF probe. During energy application (20 W) unenhanced sequential MSCT scans were acquired using the following scan protocol: 140 kV tube voltage, 300 mAs/rotation tube current time product, collimation 24 × 1.2 mm, rotation time 0.5 s. Axial image data was reconstructed using a soft tissue convolution kernel. Temperature data was recorded during every CT scan. Using a circular 0.5 cm2 region of interest local CT values were measured at the tips of the temperature probes and matched with the measured temperatures. Regression analysis was performed to analyse the relationship between local temperatures and CT values for each temperature probe position. Furthermore, the same experimental design was used in four anaesthetised female pigs in order to investigate the potential of this technique for an in vivo application.
Results: A negative correlation was found for the relationship between temperature and CT value. Regression coefficients were −0.44 (5 mm), −0.35 (10 mm) and −0.37 (15 mm) for ex vivo data. Analysis of in vivo experiments showed regression coefficients between −0.025 and −0.434.
Conclusion: Multislice computed tomography is able to depict temperature changes in liver tissue during RFA.
Introduction
Hyperthermal ablation therapies offer a potentially curative treatment option for patients who are not candidates for surgical resection due to severe comorbidities. Besides microwave ablation (MWA) Citation1, Citation2, high intensity focused ultrasound (HIFU) Citation3 and laser induced thermometry (LITT) Citation4, radiofrequency (RF) ablation found its way into clinical routine during the last decade as a minimally invasive therapy for different malignancies of lung Citation5, kidneys Citation6 and bone Citation7. However, most often RF ablation is used for treating primary or secondary malignant hepatic tumours Citation8–11. In applying hepatic RF ablation with curative intent, complete tumour coagulation including a safety margin of at least 0.5–1.0 cm is crucial. In order to achieve nearly instantaneous protein denaturation a local temperature of 60°C must be achieved Citation12. Non-invasive thermometry during RF energy application would allow assessing the temperature distribution within the target volume and thereby help the interventionalist to decide whether complete tumour ablation is achieved. Furthermore, collateral thermal damage of structures located near the ablation volume could be avoided. So far, magnetic resonance imaging (MRI)-based thermometry, ultrasound thermometry, microwave-based thermometry and electrical impedance tomography were shown to have the potential of measuring temperature non-invasively Citation13. Lately, the temperature-dependent shift of CT numbers, which has been known for more than thirty years, was investigated in an in vitro study using a modern clinical CT scanner Citation14. The reported temperature dependency of the measured CT values may provide the base for a non-invasive temperature measurement during hyperthermal ablation therapies.
Therefore, the aim of our study was to investigate whether multi-slice CT is able to depict local temperature changes during hepatic RF-ablation in an ex-vivo and an in-vivo setting.
Materials and method
Ex vivo experiments
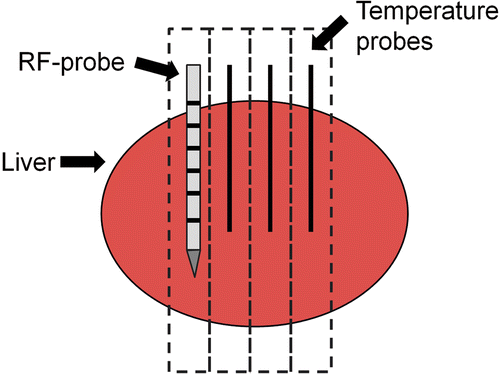
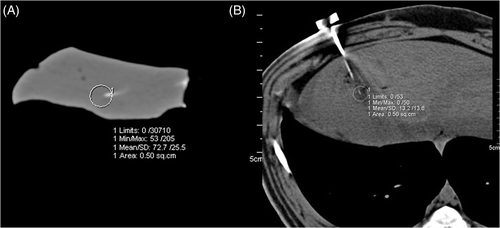
Freshly excised porcine livers, which were obtained from the Institute for Veterinary Medicine, were cut into ten pieces of approximately 10 × 5 × 4 cm. A single specimen was placed on a paper plate and the bipolar RF-applicator (Celon ProSurge T30, Celon AG, Teltow, Germany) was inserted at a depth of at least 3.5 cm. In order to measure spatial temperature changes during the RF ablation, three optical temperature probes (SFF-2 m, Luxtron Corporation, Santa Clara, CA, accuracy ±2°C) were additionally inserted into the liver specimen with their tips being located 5, 10 and 15 mm away from the middle of the active tip of the RF probe (). Thereafter, the prepared liver block was placed in the isocenter of a 64-slice dual source CT scanner (SOMATOM Definition, Siemens, Forchheim, Germany), with the RF applicator and the temperature sensors being placed perpendicular to the scanner's z-axis. During the RF ablation, which was performed with a generator output of 20 W as recommended by the vendor, sequential CT examinations without table movement (28.8 mm z-coverage) were performed every 15 s employing the following scan protocol: 120 kV tube voltage, 320 mAs/rotation tube current time product, 24 × 1.2 mm collimation, 0.5 s rotation time. Prior to each scan series the implemented check-up procedure including a scanner calibration in air was performed. After raw data acquisition a soft tissue convolution kernel (B 40 s) and a 512 × 512 matrix were used for reconstruction of axial images with a slice thickness of 2.4 mm. A slice thickness of 2.4 mm was chosen in order to reduce image noise. For image data analysis local CT numbers were measured using a circular 0.5 cm2 region of interest (ROI) that was placed centrally around the tip of each temperature probe at each image data acquisition time-point as shown in . The measured CT numbers were matched with the temperature measured at the same time. In total, 10 ex vivo experiments as described above were performed.
In vivo experiments
After approval from the official committee on animal affairs four female pigs with a weight of 70 kg were included in this study. After intramuscular premedication with a combination of atropine (Atropinum sulphuricum solution 1%, WDT, Garbsen, Germany), azaperone (Stresnil, Janssen-Cilag, Neuss, Germany) and ketamine (Ketamin 10%, Ceva Tiergesundheit, Düsseldorf, Germany) anaesthesia was induced using diluted pentobarbital (Narcoren, Merial, Hallbergmoos, Germany), which was injected using a 18 gauge venous access placed in an ear vein. The animals were intubated and mechanically ventilated (Sulla 808, Draeger Medical, Luebeck, Germany) with an oxygen–air mixture containing 1.0 vol% Isoflurane to maintain anaesthesia. For additional analgesia animals received a fentanyl drip via the venous access line and 1 L of 0.9% saline infusion in order to prevent dehydration.
All in vivo experiments were performed using the same CT system (SOMATOM) as for the previous ex vivo study. Animals were placed on the CT table in supine position and moved to the isocentre of the CT gantry. After acquisition of a non-enhanced spiral CT scan in end-expiratory breath hold (64 × 0.6 mm collimation, 120 kV tube voltage, 165 mAs/rotation tube current time product, 0.5 s gantry rotation time), RF probe placement via an anterior strictly in-plane approach was planned using the implemented CT scanner software. After cleaning of the skin the RF applicator was advanced along the planned puncture path under repetitive sequential unenhanced CT scans. Then three optical temperature probes were additionally inserted parallel to the RF probe with a fixed distance of 5, 10 and 15 mm to the middle of the active tip () using positioning device. In our experiments RF applicator and temperature probes were inserted mostly in the same part of the liver in all cases. After the start of the RF ablation, sequential CT scans were performed using 140 kV tube voltage and 300 mAs/rotation tube current time product, a collimation of 24 × 1.2 mm, and a rotation time of 0.5 s. Axial image data was reconstructed at a slice thickness of 1.2 mm using a B31 smooth kernel. During performance of CT scans temperature data were read out from the thermal sensors continuously throughout the RF ablation using a serial port and a custom-made software tool. As described above for the ex vivo study area, averaged CT numbers were measured at the tip of the temperature probes at every image data acquisition time-point using a 0.5 cm2 ROI as shown in and matched to the corresponding temperature data.
RF ablation system
All RF-ablations were performed with a bipolar RF system (CelonLabPower, Celon Medical Instruments, Teltow, Germany) which operates at a frequency of 470 kHz and provides a variable power output between 2 and 250 W. The used RF applicator features an active tip of 30 mm (Celon ProSurge T30) and an internal cooling with 0.9% saline solution delivered by a peristaltic pump at a flow rate of 30 mL/min. As recommended by the manufacturer the generator output was set to 20 W. The RF system was operated at the “resistance controlled automatic power mode”, which means that the power output automatically adjusted to the measured tissue impedance in order to avoid early tissue dehydration. During RF ablations the applied power, tissue resistance, and application time were monitored using a dedicated software tool (CelonPowerMonitor Version 2.6).
Statistical analysis
For ex vivo experiments a linear regression analysis was performed to analyse the relationship between temperature and measured CT values. Therefore, temperature data from the 10 ex vivo experiments was pooled for each temperature probe position (5, 10, 15 mm distance to the RF-applicator) and matched to the corresponding CT numbers.
For in vivo experiments a linear regression analysis was performed for each thermal sensor position in every animal. A p value <0.05 was considered statistically significant. All analyses were performed using SAS Software 9.1.3 (SAS Institute, Cary, NC).
Results
Ex vivo study
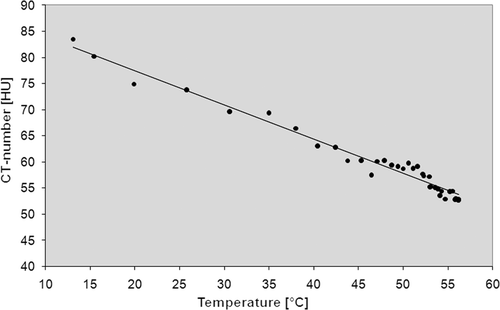
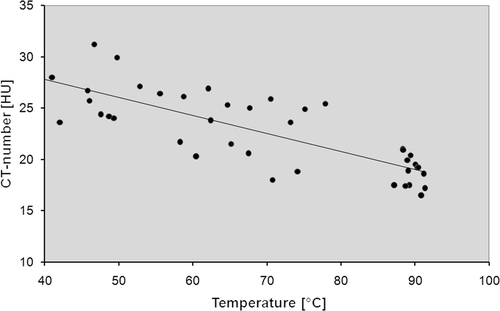
The regression analysis showed a negative relationship between measured temperature during RF-ablations and the area-averaged CT numbers. Results are summarised in . Regression coefficients varied between −0.44 (5 mm distance to the RF applicator and −0.35 (10 mm distance to the RF applicator) as shown in .
Table I. Results of the ex vivo study. Temperature data and measured CT-numbers derived from the 10 experiments were pooled for each temperature probe position.
In vivo study
The results of the in vivo study also show negative regression coefficients for the relationship between temperature and CT numbers for all temperature probes in every animal ranging from −0.025 (p value: 0.1721) to −0.432 (p value: 0.0050) as shown in . The CT value measurements at the tip of the temperature probe being located 5 mm away from the RF-probe were especially hampered by metal artefacts caused by the RF applicator in the in vivo experiments. Results are summarised in Tables .
Table II. Results of the in vivo study (pig 1).
Table III. Results of the in vivo study (pig 2).
Table IV. Results of the in vivo study (pig 3).
Table V. Results of the in vivo study (pig 4).
Discussion
As recently published by Frich Citation13, the ideal requirements for non-invasive temperature measurements during hyperthermal ablation therapies include a temperature accuracy of 1–2°C, a spatial resolution of <1–2 mm and an acquisition time of <10–30 s. Furthermore, the three dimensional temperature distributions should be presented in real time to allow for correction of RF applicator placement or adjustment of the RF energy delivery. In addition, the ideal non-invasive thermometry should be insensitive to motion of the patient and be compatible with commonly used ablation devices as well as with the used imaging modality.
So far, mainly MRI and ultrasound have been used for non-invasive temperature mapping during hyperthermal ablation therapies in the liver. Non-invasive MR thermometry is based on the temperature dependency of different MR parameters. Initially, the temperature-dependent change of longitudinal relaxation time was used for non-invasive temperature mapping Citation15. Other MR parameters which provide a potential base for non-invasive thermometry are the proton resonance frequency of tissue water, the diffusion coefficient, and the proton density Citation16. Also, temperature-sensitive contrast agents can be used for quantification of local temperature Citation17. In contrast to RF ablation, which suffers from the usage of metallic applicators and interferences between the RF generator and the MR scanner, LITT is a suitable hyperthermal ablation technique to be monitored with MR thermometry. But different technical solutions have also been explored for MR-guided RF-ablation to overcome the limitations mentioned, such as interleaving of RF ablation cycles and MR data acquisition Citation18, filtering Citation19, Citation20 and the use of MR-compatible RF-applicators Citation21. Several ultrasonic parameters provide a basis for non-invasive thermometry as well. These parameters include the temperature-dependent echo shifts due to changes in tissue thermal expansion and speed of sound, the attenuation coefficient and change in backscattered energy due to tissue in homogeneities Citation22. Other, more experimental techniques for non-invasive temperature measurements which could potentially be used during hyperthermal ablation procedures include microwave computed tomography and electrical impedance tomography.
A non-invasive thermometry during RF ablation based on the temperature dependency of CT numbers would be beneficial due to several reasons:
There is no need for special RF equipment because all commercially available RF systems are compatible with modern CT scanners and only one single case-report refers to a potential electromagnetic cross-talk between a CT system and an operating RF generator during hepatic RF ablation resulting in severe imaging artefacts Citation25.
CT guidance for the placement of RF applicators is widely used due to broad availability of CT scanners providing a high spatial resolution, an acceptable soft tissue contrast and the possibility to detect immediate complications like bleeding.
The additional option to perform a CT-based thermometry during RF ablation would allow improvement of the safety of the procedure by depiction of temperature around vital structures being located in the vicinity of the target volume. The three-dimensional visualisation of temperature distribution would also be helpful to decide whether the induced coagulation volume completely covers the target lesion including a safety margin of at least 0.5 cm and thereby reduces the risk for incomplete ablation.
First data concerning the temperature dependency of CT attenuation values in water and biological tissue was published in the late 1970 s Citation23. Fallone et al. published in vitro data about the decrease of CT numbers during the heating of water and muscle tissue Citation24. They postulated an achievable temperature discrimination of less than 1°C at a spatial resolution of the order of 1 cm based on CT value thermal shift of 0.4 and 0.45 HU/°C for water and muscle tissue, respectively. But due to technical CT scanner limitations this approach was not further investigated. The results of the presented ex vivo experiments using state-of-the-art CT technology show a temperature-dependent shift of CT values between −0.35 and −0.44 HU/°C. However, the transfer into the in vivo study showed some major problems as reflected by the regression coefficient (−0.03) which was calculated for the temperature probe at 5 mm distance from the RF probe in pig 1 () and pig 3 (). Metal artefacts and beam hardening () as well as vaporisation hamper a reliable measurement of local CT-numbers in the direct vicinity of the RF probe especially. In contrast to the ex vivo study, the image data of the in vivo experiments show severe beam hardening artefacts which distort the measurement of CT numbers. This observation is due to the fact that in ex vivo experiments the RF probe could be placed exactly in the scan plane. That is why significant beam hardening artefacts only occur in the slice showing the RF probe, and much less in the slices depicting the temperature probes. In contrast, in the in vivo experiments a strictly in-plane placement of the RF probe in the living animal was hard to achieve, which led to more severe beam-hardening artefacts. Newly developed algorithms for metal artefact reduction may help to overcome this issue Citation26. Furthermore, as reflected by and the scatter of CT numbers is clearly larger in the in vivo experiments when compared to the ex vivo situation. A reason for this observation may be that the ex vivo liver specimens provide a quite homogenous subject with a uniform heat distribution. In contrast, in the in vivo experiments blood flow leads to the typical heat-sink effect around vessels, resulting in irregular heat propagation. Although we tried to avoid placement of temperature probes in the vicinity of large vessels, it sometimes occurred, as only unenhanced CT scans were used for RF applicator and thermal sensor positioning. With regard to the measurements at the outer rim of the ablation zone, the induced local hyperaemia, which surrounds the ablation volume, may also contribute to inhomogeneous local temperature distribution and thereby hamper a reliable CT-based thermometry. This aspect may be the reason for the lowest regression coefficient being seen at the largest distance from the RF probe in pig 2 ().
Using multi-slice CT as the base for in-invasive thermometry in a clinical context will require an individual calibration process because of the significant inter- and intrascanner variability of CT numbers Citation27. Moreover, the individual composition of liver tissue, e.g. in patients suffering from liver cirrhosis or hepatic steatosis, will influence the temperature dependency of CT numbers. Therefore, the use of a RF applicator featuring an integrated thermocouple being located at a certain distance from the active tip can be a useful tool for an individual patient calibration process. Alternatively the insertion of a separate optical temperature probe into the target volume could be used for this purpose.
A visualisation of temperature-dependent changes of CT numbers during RFA may be realised as a colour-coded image based on image subtraction Citation28, Citation29 that is superimposed on a preinterventionally acquired diagnostic examination showing the target lesion. If a reliable registration of both scans can be assumed this would allow the evaluation of the local temperature distribution within the target volume. Local bleeding within the target volume due to RF applicator placement may lead to an increase of local CT numbers and thereby can inhibit a detection of temperature changes. Also the intravenous administration of contrast agent immediately before or during the RF ablation would hinder a CT-based temperature monitoring.
The study presented suffers from some limitations that need to be discussed. First, the study population includes only four animals which are only suited to show the feasibility of MSCT to depict temperature-dependent changes of hepatic tissue. Hence, further in vivo and clinical studies are needed to confirm the presented results. Furthermore, the temperature dependency of CT numbers was investigated in healthy porcine liver parenchyma which may differ from pathologically altered human liver tissue. This limits the transfer of the results into the clinical situation. Also, the manual measurement of CT numbers using a small circular ROI needs to be improved by a more accurate and robust software-based method which might also improve the achievable spatial resolution.
In conclusion, the presented data shows an inverse relationship between local temperature and area-averaged CT numbers during hepatic RF ablation in ex vivo and in vivo porcine models. This finding may provide the basic principle for CT-based non-invasive thermometry during hyperthermal ablation procedures.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. Bernhardt Schmidt is an emlpoyee of Siemens Healthcare.
References
- Bhardwaj N, Strickland AD, Ahmad F, El-Abassy M, Morgan B, Robertson GS, et al. Microwave ablation for unresectable hepatic tumours: Clinical results using a novel microwave probe and generator. Eur J Surg Oncol 2010; 36: 264–268
- Ryan TP, Turner PF, Hamilton B. Interstitial microwave transition from hyperthermia to ablation: Historical perspectives and current trends in thermal therapy. Int J Hyperthermia 2010; 26: 415–433
- Park MY, Jung SE, Cho SH, Piao XH, Hahn ST, Han JY, et al. Preliminary experience using high intensity focused ultrasound for treating liver metastasis from colon and stomach cancer. Int J Hyperthermia 2009; 25: 180–188
- Vogl TJ, Straub R, Eichler K, Söllner O, Mack MG. Colorectal carcinoma metastases in liver: Laser-induced interstitial thermotherapy—local tumor control rate and survival data. Radiology 2004; 230: 450–458
- de Baère T, Palussière J, Aupérin A, Hakime A, Abdel-Rehim M, Kind M, et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: Prospective evaluation. Radiology 2006; 240: 587–596
- Tracy CR, Raman JD, Donnally C, Trimmer CK, Cadeddu JA. Durable oncologic outcomes after radiofrequency ablation: Experience from treating 243 small renal masses over 7.5 years. Cancer 2010; 116: 3135–3142
- Dupuy DE, Liu D, Hartfeil D, Hanna L, Blume JD, Ahrar K, et al. Percutaneous radiofrequency ablation of painful osseous metastases: A multicenter American College of Radiology Imaging Network trial. Cancer 2010; 116: 989–997
- Pereira PL. Actual role of radiofrequency ablation of liver metastases. Eur Radiol 2007; 17: 2062–2070
- Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: Long-term results of percutaneous image-guided radiofrequency ablation. Radiology 2005; 234: 961–967
- Mulier S, Ruers T, Jamart J, Michel L, Marchal G, Ni Y. Radiofrequency ablation versus resection for resectable colorectal liver metastases: Time for a randomized trial? An update. Dig Surg 2008; 25: 445–460
- Kim HR, Cheon HS, Lee KH, Ahn JR, Jeung HC, Lee SS, et al. Efficacy and feasibility of radiofrequency ablation for liver metastases from gastric adenocarcinoma. Int J Hyperthermia 2010; 26: 305–315
- Goldberg SN, Dupuy DE. Image-guided radiofrequency tumor ablation: Challenges and opportunities—Part I. J Vasc Interv Radiol 2001; 12: 1021–1032
- Frich L. Non-invasive thermometry for monitoring hepatic radiofrequency ablation. Minim Invasive Ther Allied Technol 2006; 15: 18–25
- Bruners P, Levit E, Penzkofer T, Isfort P, Ocklenburg C, Schmidt B, et al. Multi-slice computed tomography: A tool for non-invasive temperature measurement?. Int J Hyperthermia 2010; 26: 359–365
- Parker DL, Smith V, Sheldon P, Crooks LE, Fussell L. Temperature distribution measurements in two-dimensional NMR imaging. Med Phys 1983; 10: 321–325
- Rieke V, Butts Pauly K. MR thermometry. J Magn Reson Imaging 2008; 27: 376–390
- Quesson B, de Zwart JA, Moonen CT. Magnetic resonance temperature imaging for guidance of thermotherapy. J Magn Reson Imaging 2000; 12: 525–533
- Botnar RM, Steiner P, Dubno B, Erhart P, von Schulthess GK, Debatin JF. Temperature quantification using the proton frequency shift technique: In vitro and in vivo validation in an open 0.5 Tesla interventional MR scanner during RF ablation. J Magn Reson Imaging 2001; 13: 437–444
- Gellermann J, Faehling H, Mielec M, Cho CH, Budach V, Wust P. Image artifacts during MRT hybrid hyperthermia – Causes and elimination. Int J Hyperthermia 2008; 24: 327–335
- Mertyna P, Dewhirst MW, Halpern E, Goldberg W, Goldberg SN. Radiofrequency ablation: The effect of distance and baseline temperature on thermal dose required for coagulation. Int J Hyperthermia 2008; 24: 550–559
- Mahnken AH, Buecker A, Spuentrup E, Krombach GA, Henzler D, Günther RW, et al. MR-guided radiofrequency ablation of hepatic malignancies at 1.5 T: Initial results. J Magn Reson Imaging 2004; 19: 342–348
- Arthur RM, Straube WL, Trobaugh JW, Moros EG. Noninvasive estimation of hyperthermia temperatures with ultrasound. Int J Hyperthermia 2005; 21: 589–600
- Bydder GM, Kreel L. The temperature dependence of computed tomography attenuation values. J Comput Assist Tomogr 1979; 3: 506–510
- Fallone BG, Moran PR, Podgorsak EB. Noninvasive thermometry with a clinical X-ray scanner. Med Phys 1982; 9: 715–721
- Brennan DD, Appelbaum L, Raptopolous V, Kruskal JB, Goldberg SN. CT artifact introduced by radiofrequency ablation. Am J Roentgenol 2006; 186S5: S284–286
- Mahnken AH, Raupach R, Wildberger JE, Jung B, Heussen N, Flohr TG, et al. A new algorithm for metal artifact reduction in computed tomography: In vitro and in vivo evaluation after total hip replacement. Invest Radiol 2003; 38: 769–775
- Birnbaum BA, Hindman N, Lee J, Babb JS. Multi-detector row CT attenuation measurements: Assessment of intra- and interscanner variability with an anthropomorphic body CT phantom. Radiology 2007; 242: 109–119
- Bentzen SM, Overgaard J, Jorgensen J. Isotherm mapping in hyperthermia using subtraction X-ray computed tomography. Radiother Oncol 1984; 2: 255–260
- Minhaj AM, Mann F, Milne PJ, Denham DB, Salas N, Jr, Nose I, et al. Laser interstitial thermotherapy (LITT) monitoring using high-resolution digital mammography: Theory and experimental studies. Phys Med Biol 2002; 47: 2987–2999