Abstract
Background: Breast cancer recurrences in previously irradiated areas are treated with reirradiation (reRT) and hyperthermia (HT). The aim of this retrospective study is to quantify the toxicity of HT in breast cancer patients with reconstruction.
Methods: Between 1992 and 2009, 36 patients were treated with reRT with a scheme of 8 fractions of 4.0 Gy in 4 weeks, and HT on a total of 37 tissue reconstructions. The types of reconstructions were: split-thickness skin graft (15), transverse rectus abdominis myocutaneous flap (1), latissimus dorsi flap (14), rhomboid flap (1) or a combination of grafts and flaps (6). Toxicities were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0. Patient, tumour, and treatment characteristics predictive for the endpoints were identified in univariate and multivariate analyses. The primary endpoint was HT toxicity. Secondary endpoints were acute and late radiotherapy (RT) toxicity, complete response (CR), local control (LC) and overall survival (OS).
Results: The median follow-up time was 64 months. Grade 2 HT toxicity occurred in four patients and grade 3 in three. The three patients with grade 3 HT toxicity required reoperation. None of the evaluated parameters showed a significant relationship with HT toxicity. The CR rate in 15 patients with macroscopic disease was 80%. The 3 and 5 year LC rates were 74% and 69%; the median OS was 55 months.
Conclusions: Combined reRT and HT in breast cancer patients with reconstruction is safe and effective.
Introduction
The treatment of breast cancer recurrences can involve a multimodal therapy that includes surgery and radiotherapy (RT), and, in the event of reirradiation (reRT), hyperthermia (HT). RT of breast or chest wall is indicated for patients on the basis of the following criteria: (1) recurrent tumour; (2) inoperable tumour; (3) microscopically incomplete excision. The standard therapy offered to patients with locoregional recurrent breast cancer in previous irradiated area in the Netherlands is reRT combined with HT Citation[1], Citation[2]. The therapeutic benefit of HT when combined with RT has been documented in randomised comparative studies in various tumour types, including breast cancer Citation[3–8].
After mastectomy or local excision, surgical defects may be covered using grafts or flaps. To cope with the psychological and aesthetic consequences of mastectomy, some patients choose a form of breast reconstruction. The options for reconstruction include myocutaneous flaps or tissue expander with subsequent placement of a prosthetic expander/implant, and, if necessary, reconstructions with grafts to cover wounds as a result of the surgical resection.
When post-operative RT is required, many surgeons avoid breast reconstruction for fear of wound complications Citation[9–13]. Published experience on the tolerance of reconstructions with grafts and flaps in previously or subsequently irradiated regions suggest that significant complications are limited, and that the cosmetic results are acceptable Citation[14–23]. Reports in the literature on the tolerance of reconstructions with a graft or flap to HT in combination with reRT are scarce and are mostly single patient reports. We are aware of three studies that mention side effects from the combination therapy on reconstruction with a graft Citation[24–27]. In this retrospective study we analysed the occurrence of HT toxicity solely in patients with breast reconstruction suffering breast cancer recurrence after treatment with a combination of reRT and HT in an attempt to inform the oncology committee on the benefit and toxicity risks in a larger patient group.
Patients and methods
Patients
We retrospectively analysed 36 patients with 37 breast reconstructions, 7% of all the patients who had received post-operative reRT combined with HT at the Erasmus MC/Daniel den Hoed Cancer Centre between 1992 and 2009. Thirty-five patients had recurrent adenocarcinoma of the breast and one patient had Angiosarcoma; 14 patients (39%) had undergone surgery with breast reconstruction after their first diagnosis of breast cancer, 21 (58%) had undergone breast reconstruction after their recurrence and one patient (3%) had undergone two breast reconstructions, for the left- and right-sided chest wall after both recurrences. The median age at time of diagnosis was 59 years (range: 39–74 years).
Patient files were reviewed with regard to relevant medical history, details on breast reconstruction, use of chemotherapy and hormonal therapy, details of tumour and previously applied RT. Indication for reRT was recurrent tumour, inoperability or microscopically incomplete excision and systemic therapy was either inadequate or was deemed inappropriate, in the absence of systemic disease. Distant metastasis was diagnosed before the start of the treatment in four patients. summarises patient and tumour characteristics.
Table I. Patient and tumor characteristics in relation to time period between reconstruction and HT and reRT
Surgery
In all patients, a breast reconstruction was placed after mastectomy or local excision. Three patients had their reconstruction before the initial radiation. The surgical technique was not the same in all patients, because they had been operated on in different hospitals. The following breast reconstructions were included: split-thickness graft (15), latissimus dorsi flap (14), transverse rectus abdominis myocutaneous flap (1), rhomboid flap (1), a combination: latissimus dorsi flap and split-thickness graft (1), or a combination: omentum and split-thickness graft (5). A brief explanation will follow to show the diversity in tissue vascularity and perfusion in the treated areas which may influence the risk of toxicity.
Explanation of different surgical techniques
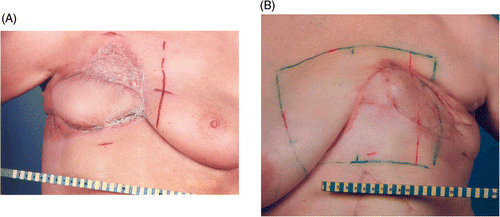
Split-thickness graft (described by the German surgeon Karl Thiersch in 1874) is a sheet of tissue containing epidermis and some dermis taken from a donor site. It is obtained by shaving the skin with an appropriate knife or blade. A skin graft depends for its survival on receiving adequate nutrition from the recipient bed. As it will contract to a certain extent, it will provide a far less aesthetic and durable form of coverage than a vascularised flap. Skin grafts may also be used as an adjunct for coverage of large defects (, and ).
Figure 1. a. Split-thickness graft, b. Latissimus dorsi flap, c. Transverse rectus abdominis muscle, d. Rhomboid flap.
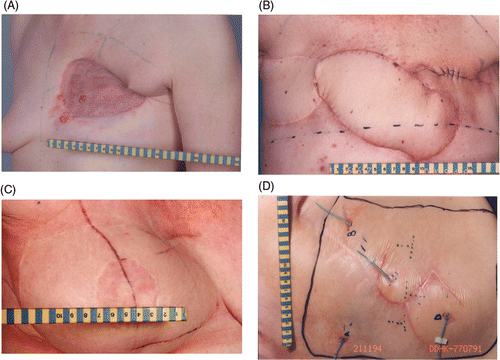
Figure 2. a. Combination split-thickness skin graft and latissimus dorsi flap, b. Combination split-thickness skin graft and omentum flap.
The mesh graft is a partial or split-thickness skin graft that has had multiple slits cut into it. These allow the graft to be stretched to several times its original size, and thus to cover a larger area on the recipient. They also facilitate acceptance of the graft by permitting fluids to escape from beneath the graft.
A latissimus dorsi flap takes advantage of the thoracodorsal vessels, which enter the muscle just below its insertion into the humerus; as a consequence their vascularity is generally robust. Even if these vessels have been ligated, the latissimus dorsi flap may survive on collateral blood flow between the thoracodorsal and serratus branches. The great advantages of the latissimus dorsi flap chest wall reconstruction are that it can be used as a muscle flap alone to cover a large defect, or the muscle can be used to transplant a small island of skin. It can be used as a pedicled flap for breast or chest wall reconstruction. It can also be used as a free flap. In our study only pedicled flaps were used ( and ).
The transverse rectus abdominis myocutaneous provides an alternative flap to the latissimus dorsi muscle. It has the advantage that it can normally transplant sufficient autologous tissue to avoid the need for an implant. The flap can also be used to reconstruct the chest-wall defects ().
A rhomboid (or Limberg) flap, is as its name suggests, a flap with the shape of an equilateral parallelogram – a lozenge shape. It is designed to contain the maximal blood supply, and takes advantage of well-known principles of flap surgery. The base of the flap should be centred over a regional blood supply source, and the length of the flap should be aligned along the principal direction of the subcutaneous vessel work. Rotation and transposition flaps should also be designed to be large enough to enable adequate movement and closure without tension ().
The highly vascularised omentum flap is a large flap and can be used as a pedicle or a free flap to cover chest wall and sternal wounds. It is usually folded as an apron utilising the left gastroepiploic vessels, but occasionally, if additional length is needed, either of the gastroepiploic vessels can be used. It has been used as a pedicled flap covered with a skin graft to treat RT injury to the chest wall ().
Treatment
Radiation
The post-operative external beam irradiation therapy of eight fractions of 4 Gy, twice weekly, was given to 35 patients on 36 breast reconstructions. In one patient a higher total dose of 60 Gy, 30 fractions of 2 Gy five times a week, was given because of an inoperable third recurrence of breast cancer. There was one patient with subclinical disease on the left and a macroscopic tumour on the right side of the chest wall for which she received combined treatment in different time periods. This patient was evaluated as two different breast reconstructions.
Radiation techniques included electrons (n = 12), photons (n = 11) or a combination (n = 14) depending on the tumour location and depth. The RT field included the full-thickness chest wall. A summary of treatment characteristics is given in .
Table II. Treatment characteristics.
Hyperthermia
HT for breast cancer recurrences is given four times once weekly after a RT fraction. During the early part of this study HT was administered twice a week with a total of eight treatments, but since 1996 it was administered once a week with a total of four treatments. This is now the standard scheme Citation[28]. HT was delivered using lucite cone applicators (LCA) with a 433 MHz technique as previously described Citation[29], Citation[30]. The applicator set-up was chosen to heat the whole reRT volume Citation[2]. The maximum area that can be treated in one session, using six LCAs, is 20 × 30 cm2 (n = 27). Three fields were treated alternately by standard waveguide applicators and LCAs to test the performance of both waveguide types in the clinical setting. In 1996 LCAs replaced the standard waveguides primarily because of better temperatures in the periphery of the treatment field Citation[31]. To cover larger than 600 cm2 fields, the treatment was carried out with two (n = 7) or four (n = 1) applicator set-ups. The HT treatment was started as soon after the RT treatment as possible (usually within 30–60 min). Surface temperature control was performed by using a perfused water bolus. The temperature of the circulating water in the bolus is selected according to the desired heating depth over the whole treatment volume as published in a previous paper Citation[32]. The standard prescribed duration of a treatment was 60 min, including a heating-up period of 10 min, during which temperatures were increased to as high and homogeneous as patient tolerance and normal tissue temperatures permitted using the independent power control for each LCA. In addition to measured temperatures, patients were carefully instructed and repeatedly questioned during the treatments to mention any pain or unpleasant sensation suggestive of a hot-spot. In cases of too high temperature, the power output of the concerning applicator and/or the applicator position was adjusted Citation[33].
Thermometry
For thermometry, a 24-channel scanning fibre-optic system (FT1210 and FT1310, Takaoka, Japan) was used. Five multisensory probes (up to four sensors) and four single-sensor probes were available to measure skin and interstitial temperatures continuously during treatment. Under local anaesthesia, up to four thermometry catheters were inserted subcutaneously in the treatment volume, with at least one interstitial temperature measuring point aimed below each antenna. The catheters were fixed in place with Histoacryl (B. Braun, Melsungen AG, Germany) and Tegaderm adhesive (3M, USA). They were left in place for the duration of the full reRT and HT treatment course, provided there were no signs of infection or pain. The multisensory probes were placed in the closed-end interstitial thermometry catheters and the single-sensor probes were placed superficially, prior to each HT treatment Citation[34].
Endpoints
The primary endpoint of this study was acute HT toxicity. Acute side effects of HT occur during or within 24 h after completion of a treatment. Acute toxicity was registered according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE v3 2006) Citation[35], Citation[36] () and the WHO scoring system (). For acute HT-related toxicity analysis, the highest CTCAE grade toxicity a patient developed was included.
Table III. CTCAE v3 scoring of hyperthermia toxicity.
Table IV. World Health Organization scoring of thermal burns.
Secondary study endpoints were acute and late RT toxicity (CTCAE v3), complete response (CR), duration of local control (LC) and overall survival (OS). Acute RT-induced toxicity was defined as toxicity developing during treatment or in the three months thereafter (). Late RT-induced toxicity was defined as toxicity occurring at least three months after the last fraction of RT. CR was defined according to WHO criteria: clinical disappearance of all tumours in the irradiated volume for at least two measurements separated by visits of at least four weeks. LC duration in patients treated for subclinical disease and in patients in whom CR was achieved, was defined from the start of treatment until the first in-field tumour progression. Patients who did not show a CR were considered local failures. OS was defined as the time from the start of treatment to death from any cause or was censored at the time of the last follow-up visit in patients who survived. Assessment was based on the registered information in the files, telephone interviews and letters from medical professionals.
Table V. CTCAE v3 scoring of acute radiotherapy toxicity.
Temperature parameters
From the measured temperatures, Tmax, Tave, and T90 were derived. Tmax and Tave are the maximum and the average temperature of all invasive temperature probes and T90 represents the temperature exceeded by 90% of all invasive temperature points during steady state (from 10 min after start of heating). For this analysis, the means of the maximum, average and T90 temperatures for each HT session were selected. The thermal dose parameter used in this study, CEM43°CT90, has been used extensively and has been previously described Citation[6], Citation[33], Citation[37–41]. In a mathematical description all time–temperature data are converted to an equivalent number of minutes at 43°C, where CEM43°C is cumulative equivalent minutes at 43°C. The sum of CEM43°C (CEM43°CT90tot) acquired per treatment (CEM43°CT90i) was calculated from the number of treatments actually given, and normalised using the number for which time–temperature data was available Citation[33]. The analysis of thermal dose was limited to interstitially measured temperatures because the temperatures measured from the thermometry probes placed on the skin were influenced by the water bolus and therefore uncertain ().
Table VI. Thermal parameters in relation to hyperthermia toxicity.
Other treatment and patient-related parameters
In addition to temperature parameters, a number of other treatment and patient-related parameters were evaluated for prognostic value regarding toxicity ().
Table VIb. Patient and treatment characteristics included in the evaluation of prognostic factors for toxicity.
Statistical methods
Toxicity and response rates were evaluated for each treatment field. The association between toxicity and thermal parameters was evaluated for each HT session. Pearson's chi-squared test was used to determine which parameters associated with acute toxicity were caused by the combined treatment. The association between toxicity and thermal dose parameters Tmax, Tave, T90 and CEM43T90, was tested using the non-parametric Kruskal-Wallis test. All data were tabulated in Excel spreadsheets and were further processed using Statistica® for Windows. Actuarial probability of LC and OS was plotted from the time of initiation of treatment using the Kaplan-Meier product-limit method. A relationship was defined to be statistically significant when the p-value was ≤0.05. The statistical analysis was performed using Stata statistical software Citation[42].
Results
Toxicity
All patients were eligible for toxicity evaluation. For patients still alive at last follow-up (n = 13) the median follow-up time was 64 months (range 4–188 months).
HT toxicity occurred in 17 patients (46%; split-skin (Thiersch) graft 19%, latissimus dorsi flap 16%, combination of split-thickness graft and omentum 8% or transverse rectus abdominis myocutaneous flap 3%). The burns were located inside the breast reconstruction (n = 6), outside the breast reconstruction (n = 8), or on the margin (n = 2), and one patient had two burns, one inside and one outside the breast reconstruction. The maximum skin toxicities are shown in .
Table VII. Maximum acute HT toxicity per patient and reconstruction.
HT CTCAE grade 3 toxicity was observed in three patients (8%). Two grade 3 HT toxicities developed in a latissimus dorsi flap and one in a transverse rectus abdominis myocutaneous flap. Removal of the latissimus dorsi flap was required in both patients. In one patient the skin defect was repaired by primary closure and the wound healed without event. The other patient required composite reconstruction of the chest wall. Four months afterwards she required removal of the composite reconstruction because of an infection and the wound was closed primarily. The last patient developed fever and appeared to have fat necrosis which was removed. She required transposition of transverse rectus abdominis myocutaneous tissue with a latissimus dorsi flap.
Acute grade 3 RT toxicity appeared after 2.7 months in one patient (3%). She required wound debridement of the chest wall without removal of her split skin graft and healed with conservative treatment.
Late RT toxicity was observed in two patients (5%) other than those with maximum HT toxicity. One patient had a large (14.5 cm) ulcerating tumour mass before the start of the combined treatment. As a result of capsular contracture, her prosthesis and latissimus dorsi flap had to be removed after 12 months and the wound was closed primarily. The second patient developed skin necrosis and required hyperbaric oxygen therapy after 5.5 years. The breast reconstruction, a combination of a flap and a graft remained in situ. All patients subsequently recovered well and required no further intervention.
Patient and treatment characteristics in relation to hyperthermia toxicity
shows patient- and treatment-related parameters according to maximum toxicity. All parameters are summarised in order of their p-value. We did not find a relationship with toxicity for any of the parameters.
The relation between acute HT toxicity and thermal dose parameters was evaluated for 46 HT applicator set-ups; for one treatment field temperature data were not available. HT-induced toxicity was not correlated with any of the thermal dose parameters, (p values varying between 0.108 and 0.408) (). The mean thermal dose parameters for patients who had less than grade 2 toxicity were T90 = 39.8°C, Tmax = 43.5°C, Tave = 41.1°C, and CEM43°CT90tot = 5.19 min; for grade 2 toxicity T90 = 40.3°C, Tmax = 43.5°C, Tave = 41.6°C, and CEM43°CT90tot = 8.25 min; for grade 3 toxicity T90 = 39.7°C, Tmax = 43.5°C, Tave = 41.3°C, and CEM43°CT90tot = 3.65 min.
Tumour response, duration of local control and overall survival
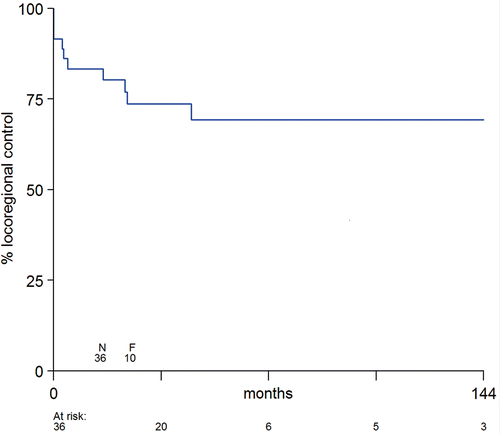
The patient with angiosarcoma of the breast was excluded from the response analysis. A CR was observed in 12 out of 15 (80%) patients with a macroscopic tumour (6 patients with a graft, 5 with a flap and 1 with a combination of a flap and a graft). All 3 patients with less than complete response had a reconstruction with a split-thickness skin graft. Including the 21 patients with subclinical disease, the LC rate was 92%. Local tumour control rate was 83% after 1 year, 74% after 3 years and 69% after 5 years with seven local recurrences included to date (). One local recurrence occurred in a field with a latissimus dorsi flap after 3 months, one in a field with a transverse rectus abdominis myocutaneous flap after 17 months, and five in a field with a split-thickness skin graft after 3, 5, 24, 25 and 46 months. Depth of target volume had no effect on duration of local control (p = 0.367).
Figure 3. Duration of local tumor control for 36 tissue transfers in 35 patients from the time of initiation of treatment treatment (N = number of tissue transfer, F = number of failures).
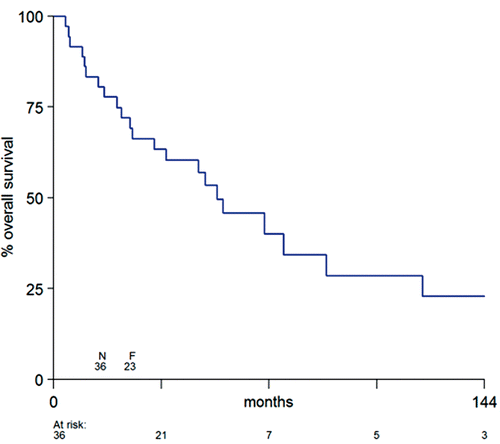
Fourteen patients died with local tumour control, after a median survival of 50 months, whereas twelve patients were free of local disease after a follow-up period of 22–188 months (median 65 months). The OS rate was 83% after 1 year, 63% after 3 years and 46% after 5 years with a median survival of 55 months ().
Discussion
In our study we retrospectively evaluated the results of reRT and HT in a group of 36 patients with 37 breast reconstructions, treated between October 1992 and March 2009. We found no HT toxicity in 20 patients (54%). CTCAE grade 1 toxicity was seen in 10 patients (27%), grade 2 in four (11%) and grade 3 in three (8%). In 12 of the 15 patients (80%) with macroscopic disease a CR was achieved and the 5-year LC rate in all patients was 69%.
Breast reconstructions are sometimes considered to be a contraindication for treatment with RT and/or HT. A problem in patients with reconstruction can be that sensitivity is disturbed, and that therefore they cannot report too high temperatures. From published results and clinical experiences with reRT and HT, however, it is unclear how high the incidence of severe toxicity in breast reconstructions really is. Kim et al. Citation[24], Citation[26] reports on 54 patients treated post-operatively with combined RT and HT, among whom only one patient had received a skin graft. This patient was treated with two series of RT and HT to a grafted area for a locally recurrent malignant melanoma. The patient developed an acute moist reaction in the grafted area which healed within four weeks. Nishimura et al. Citation[27] reports as late toxicity two patients who developed ulcers in grafted skin. Hehr et al. Citation[43] treated six patients with flap reconstructions among a total of 39 patients, but does not report specifically on severe toxicity in this subgroup. Zagar et al. Citation[44] reports on one patient with a third-degree burn in a transverse rectus abdominis myocutaneous flap reconstruction that healed with conservative measures.
In our institute, patients with breast reconstruction are not excluded from HT treatment. Compared to results of a previous study in patients with recurrent breast cancer Citation[1], acute hyperthermia toxicity was similar: WHO second-degree 31% compared to 27% and WHO third-degree 14% compared to 10%. We further found that the sensitivity impairment in patients with breast reconstruction did not stop our patients from complaining during hyperthermia treatments. The number of complaints for which power was adjusted was 0–13 (mean 5) in patients with reconstruction (0–13 (mean 5) in patients with CTCAE grade 0 or 1 toxicity, and 3–8 (mean 5) in patients with CTCAE grade 2 or 3 toxicity) while it was 0–10 (mean 4.2) in patients without reconstruction. The side effects were usually grade 2 or less according to the CTCAE scoring system. The HT-induced burns generally caused no pain because these preferentially developed at sites of decreased sensitivity. We considered only CTCAE grade 3 as severe toxicity. HT caused a CTCAE grade 3 burn of the thoracic wall in three (8%) patients. Removal of the latissimus dorsi flap was required in two patients and the transverse rectus abdominis myocutaneous flap stayed in situ. CTCAE grade 3 late radiation toxicity developed in two patients which is not significantly different from what we found in our previous study in which the majority of patients had no graft Citation[1]. One of these grade 3 toxicities resulted from tumour ulceration before treatment and the other was due to skin necrosis. Data on the tolerance of breast reconstructions to adjuvant RT are limited. The side effects in the tissue grafts and flaps after RT have resulted in acceptable rates of complications and reconstruction failures; the need for major corrective surgery was 0–11% Citation[14–23]. The grafts and flaps in these published studies were placed on a skin that had not been previously irradiated. In an experimental study on the effects of reRT on vascularised breast reconstruction in 100 rats, none of the transferred flap or reirradiated receiver musculature developed radiation necrosis after 72 Gy in 8 fractions in 10 days Citation[45].
Combined treatment in our patients with 32 Gy and HT resulted in an 80% CR in the 15 patients with macroscopic tumours and a 92% LC rate in the whole group of patients. After five years the LC rate was 69%.
In patients with breast cancer recurrences, reRT combined with HT is an effective treatment. The ESHO 5-88 study Citation[4] which compared reRT alone (with reRT schedule of 8 × 4 Gy) with reRT plus HT has shown a large improvement by additional HT. The CR rate after combined treatment was 78%, and 38% after reRT without HT. The tripling of the CR rate in various superficial tumours for reRT and high-dose HT versus RT and low-dose HT as demonstrated in a randomised study by Jones et al. Citation[6] made the National Comprehensive Cancer Network (NCCN) to include RT plus HT in its 2007 Breast Cancer Guidelines for recurrent breast cancer and other localised cancer recurrences. Although patients with recurrent breast cancer often are beyond cure, the achievement of local tumour control is important for the quality of life. Bedwinek et al. Citation[46] reported that in 62% of the patients, who experienced local recurrence, uncontrolled symptomatic local tumour can result in serious deterioration in quality of life. Liu et al. Citation[47] also reported that achievement of LC results in an improvement of quality of life. There has been no randomised trial investigating whether HT is beneficial in the subgroup of breast cancer patients with subclinical disease. Nevertheless, we have reasons to believe that HT is effective in this situation as well.
In the first place, a larger RT dose is required to achieve high LC rates. Bedwinek et al. Citation[46] advised applying at least 50 Gy in 2 Gy fractions in the elective situation, and 60 Gy after microscopic irradical tumour excision. The scheme of eight fractions of 4 Gy in 4 weeks is biologically less effective than 50 Gy in 2 Gy daily fractions (biologically effective dose (BED) = 44.8 Gy with α/β = 10 for an acutely responding tumour). In our experience, two types of observations suggest that eight fractions of 4 Gy indeed are insufficient to control subclinical disease Citation[1].
Secondly, at the time we were still developing our HT system and had only a few applicators, the aim was to apply HT to the macroscopic tumour only, or, after resection, to the site of surgery. Five patients that had HT applied to part of the reRT field showed tumour progression within the RT field but outside the HT field, while the combined treated region remained controlled.
Another finding was the difference in tumour control between patients treated for subclinical disease with 2450 MHz or 433 MHz equipment. An important difference between the 2450 and 433 MHz technique is that the homogeneity of heating is much better with the latter technique; with 2450 MHz a large part of the treatment volume receives an insufficient heat dose. The three patients treated with 2450 MHz all had in-field tumour regrowth after 10–12 months. Only two of 12 patients treated with 433 MHz had a recurrence at 10 and 13 months after treatment, while 10 of 12 patients remained locally controlled after average 32 months. Although these numbers are small, this difference was significant.
All patient, tumour and treatment characteristics, including thermal dose parameters, have been analysed concerning their impact on the toxicity of breast reconstructions and no statistically significant differences were found. Of course, the retrospective nature and relatively small sample size of this study do not allow firm conclusions, but this lack of correlation makes it difficult to prevent HT toxicity by thermometry. In the near future, HT treatment planning may become a tool to prevent the occurrence of burns. Prediction of the energy distribution in the tissues will allow better power control procedures and minimise the risk of toxicity. A thorough analysis of predicted 3D-SAR (specific absorption rate) coverage as a prognostic indicator for treatment outcome and hot-spots during treatment is the subject of an ongoing study in our department.
Conclusion
Based upon our results, breast reconstructions in previously irradiated areas are not a contraindication for treatment with reRT and HT, in view of the incidence of severe acute (8%) and late (5%) normal tissue reactions and the high LC rate (92%).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Van der Zee J, van der Holt B, Rietveld PJM, Helle PA, Wijnmaalen AJ, van Putten WL, van Rhoon GC. Reirradiation combined with hyperthermia in recurrent breast cancer results in a worthwhile local palliation. Br J Cancer 1999; 79: 483–490
- Van der Zee J, De Bruijne M, Mens JW, Ameziane A, Broekmeyer-Reurink MP, Drizdal T, Linthorst M, van Rhoon GC. Reirradiation combined with hyperthermia in breast cancer recurrences: Overview of experience in Erasmus MC. Int J Hyperthermia 2010; 26: 638–648
- Van der Zee J, Vujaskovic Z, Kondo M, Sugahara T. Kadota Fund International Forum 2004 – Clinical group consensus. Int J Hyperthermia 2008; 24: 111–122
- Vernon CC, Hand JW, Field SB, Machin D, Whaley JB, van der Zee J, van Putten WL, van Rhoon GC, van Dijk JD, González González D, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int Radiat Oncol Biol Phys 1996; 35: 731–744
- Overgaard J, González González D, Hulshof MC, Arcangeli G, Dahl O, Mella O, Bentzen SM. Hyperthermia as an adjuvant to radiation therapy of recurrent or metastatic malignant melanoma. A multicentre randomized trial by the European Society for Hyperthermic Oncology. Int J Hyperthermia 2009; 25: 323–334
- Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW. Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 2005; 23: 3079–3085
- Hua Y, Ma S, Fu Z, Hu Q, Wang L, Piao Y. Intracavity hyperthermia in nasopharyngeal cancer: A phase III clinical study. Int J Hyperthermia 2011; 27: 180–186
- Zagar TM, Oleson JR, Vujaskovic Z, Dewhirst MW, Craciunescu OI, Blackwell KL, Prosnitz LR, Jones EL. Hyperthermia combined with radiation therapy for superficial breast cancer and chest wall recurrence: A review of the randomised data. Int J Hyperthermia 2010; 26: 612–617
- Lans TE, van der Pol C, Wouters MW, Schmitz PI, van Geel AN. Complications in wound healing after chest wall resection in cancer patients; A multivariate analysis of 220 patients. J Thorac Oncol 2009; 4: 639–643
- Chen SA, Hiley C, Petusksiri J, Andic F, Riesterer O, Torres M. Influence of breast reconstruction on post-mastectomy radiotherapy: Global perceptions and practice patterns. Int J Radiat Oncol Biol Phys 2010; 78: S235–236
- Hameed A, Akhtar S, Naqvi A, Pervaiz Z. Reconstruction of complex chest wall defects by using polypropylene mesh and a pedicled latissimus dorsi flap: A 6-year experience. J Plast Reconstr Aesthet Surg 2008; 61: 628–635
- Lin KY, Johns FR, Gibson J, Long M, Drake DB, Moore MM. An outcome study of breast reconstruction: Presurgical identification of risk factors for complications. Ann Surg Oncol 2001; 8: 586–591
- Hadad I, Johnstone BH, Brabham JG, Blanton MW, Rogers PI, Fellers C, Solomon JL, Merfeld-Clauss S, DesRosiers CM, Dynlacht JR, et al. Development of a porcine delayed wound-healing model and its use in testing a novel cell-based therapy. Int J Radiat Oncol Biol Phys 2010; 78: 888–896
- Cram RW, Weder CH, Watson TA. Tolerance of skin grafts to radiation: A study of post-mastectomy irradiated grafts. Ann Surg 1959; 149: 65–67
- Mehta VK, Goffinet D. Postmastectomy radiation therapy after TRAM flap breast reconstruction. Breast J 2004; 10: 118–122
- Lawrence WT, Zabell A, McDonald HD. The tolerance of skin grafts to postoperative radiation therapy in patients with soft-tissue sarcoma. Ann Plast Surg 1986; 16: 204–210
- Spierer M, Alektiar K, Zelefsky M, Brennan MF, Cordiero PG. Tolerance of tissue transfers to adjuvant radiation therapy in primary soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 2003; 56: 1112–1116
- Bui DT, Chunilal A, Mehrara BJ, Disa JJ, Alektiar KM, Cordeiro PG. Outcome of split-thickness skin grafts after external beam radiotherapy. Ann Plast Surg 2004; 52: 551–557
- Anderson PR, Hanlon AL, McNeeley SW, McNeeley SW, Freedman GM. Low complication rates are achievable after postmastectomy breast reconstruction and radiation therapy. Int J Radiat Oncol Biol Phys 2004; 59: 1080–1087
- Jhaveri JD, Rush SC, Kostroff K, Derisi D, Farber LA, Maurer VE, Bosworth JL. Clinical outcomes of postmastectomy radiation therapy after immediate breast reconstruction. Int J Radiat Oncol Biol Phys 2008; 72: 859–865
- Spiegel A, Eldor L, Blanco A, Teh B. Effect of radiation on partial breast reconstruction in patients undergoing mini-abdominal free flaps. Int J Radiat Oncol Biol Phys 2010; 3: S246–247
- Adesiyun TA, Lee BT, Yueh JH, Chen C, Colakoglu S, Anderson KE, Nguyen MD, Recht A. Impact of sequencing of postmastectomy radiotherapy and breast reconstruction on timing and rate of complications and patient satisfaction. Int J Radiat Oncol Biol Phys 2011; 80: 392–397
- Claβen J, Nittzsche S, Wallwiener D, Kristen P, Souchon R, Bamberg M, Brucker S. Fibrotic changes after postmastectomy radiotherapy and reconstructive surgery in breast cancer. Strahlenther Onkol 2010; 186: 630–636
- Ehmann M, One year experience and results with surface hyperthermia. Paper presented at the 25th Annual Meeting of the European Society for Hyperthermic Oncology, Verona, Italy, 4–6 June 2009
- Kim JH, Hahn EW, Tokita N, Nisce LZ. Local tumor hyperthermia in combination with radiation therapy. Cancer 1977; 40: 161–169
- Kim JH, Hahn EW. Clinical and biological studies of localized hyperthermia. Cancer Res 1979; 39: 2258–2261
- Nishimura Y, Hiraoka M, Mitsumori M, Okuno Y, Li YP, Masunaga S, Koishi M, Akuta K, Abe M. Thermoradiotherapy of superficial and subsurface tumours: Analysis of thermal parameters and tumour response. Int J Hyperthermia 1995; 11: 603–613
- Van der Zee J, van Rhoon GC, Treurniet-Donker AD, Broekmeyer-Reurink MP, Kuijs AEM, Rietveld RJM, van den Berg AP, Reinhold HS. Local hyperthermia in recurrent breast cancer treatment. Eur Surg 1992; 24: 218–222
- Van Rhoon GC, Rietveld PJ, van der Zee J. 433 MHz Lucite cone waveguide applicator for superficial Hyperthermia. Int J Hyperthermia 1998; 14: 13–27
- De Bruijne M, Wielheesen DH, van der Zee J, Chavannes N, van Rhoon GC. Benefits of superficial hyperthermia treatment planning: Five case studies. Int J Hyperthermia 2007; 23: 417–429
- Rietveld PJM, Van Putten WLJ, Van der Zee J, van Rhoon GC. Comparison of the clinical effectiveness of the 433MHz lucite cone applicator with that of a conventional wave guide applicator in applications of superficial hyperthermia. Int J Radiat Oncol Biol Phys 1999; 43: 681–687
- Van der Gaag ML, De Bruijne M, Samaras T, van der Zee J, van Rhoon GC. Development of a guideline for the water bolus temperature in superficial hyperthermia. Int J Hyperthermia 2006; 22: 637–656
- De Bruijne M, van der Zee J, Ameziane A, van Rhoon GC. Quality control of superficial hyperthermia by treatment evaluation. Int J Hyperthermia 2011; 27: 199–213
- Van der Zee J, Rietveld PJM, Broeckmeyer-Reurink MP, Wielheesen DHM, van Rhoon GC. Hyperthermia in recurrent breast cancer from experimental oncology to standard practice. Exp Oncol 2002; 24: 45–50
- National Cancer Institute. December 12, 2003. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Available from: http://www.krebsgesellschaft.de/download/CTCAEv3_Kriterien.pdf
- Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003; 13: 176–181
- Hand JW, Machin D, Vernon CC, Whaley JB. Analysis of thermal parameters obtained during phase III trials of hyperthermia as an adjunct to radiotherapy in the treatment of breast carcinoma. Int J Hyperthermia 1997; 13: 343–364
- Sherar M, Liu FF, Pintilie M, Levin W, Hunt J, Hill R, Hand J, Vernon C, van Rhoon G, van der Zee J, et al. Relationship between thermal dose and outcome in thermoradiotherapy treatments for superficial recurrences of breast cancer: Data from a phase III trial. Int J Radiat Oncol Biol Phys 1997; 39: 371–380
- Oleson JR, Samulski TV, Leopold KA, Clegg ST, Dewhirst MW, Dodge RK, George SL. Sensitivity of hyperthermia trial outcomes to temperature and time: Implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys 1993; 25: 289–297
- Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 2003; 19: 267–294
- Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984; 10: 787–800
- Stata Statistical Software 11.1. College Station, TX: StataCorp, 2010.
- Hehr T, Lamprecht U, Glocker S, Classen J, Paulsen F, Budach W, Budach W, Bamberg M. Thermoradiotherapy for locally recurrent breast cancer with skin involvement. Int J Hyperthermia 2001; 17: 291–301
- Zagar TM, Higgins KA, Miles EF, Vujaskovic Z, Dewhirst MW, Clough RW, Prosnitz LR, Jones EL. Durable palliation of breast cancer chest wall recurrence with radiation therapy, hyperthermia, and chemotherapy. Radiotherapy Oncology 2010; 97: 535–540
- Narayan K, Ashton MW, Taylor GI, The effects of vascularised tissue transfer on re-irradiation. Paper presented at the 38th Annual Meeting of the American Society for Therapeutic Radiology and Oncology, Los Angeles, USA, October 1996
- Bedwinek JM, Fineberg B, Lee J, Ocwieza M. Analysis of failures following local treatment of isolated local-regional recurrence of breast cancer. Int J Radiat Oncol Biol Phys 1981; 7: 581–585
- Liu F-F, Bezjak A, Levin W, Cooper B, Pintilie M, Sherar MD. Letter to the Editor. Assessment of palliation in women with recurrent breast cancer. Int J Hyperthermia 1996; 12: 925–926