Abstract
Purpose: The present study was designed to elucidate the role of endothelial nitric oxide (NO) synthase (eNOS), inducible NOS (iNOS)-derived NO and heat-shock protein (Hsp70) in a rat model of whole-body hyperthermia (WBH)-induced liver injury.
Materials and methods: Real-time polymerase chain reaction, immunohistochemistry and western blot were used to observe the mRNA and protein expression of eNOS, iNOS and Hsp70. Rats were exposed to hyperthermia by immersion for 60 min at a conscious state in a water bath maintained at 41°C. Plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were used to assess liver injury 15 h after the hyperthermia challenge. Nitrosative and oxidative mediators, particularly NO and hydroxyl radical were measured.
Results: Plasma AST, ALT, hydroxyl radical, and NO were significantly increased after WBH. There were 4.14 ± 0.42, 2.82 ± 0.34 and 2.91 ± 0.16-fold increases in the mRNA expression of eNOS, iNOS and Hsp70. Immunohistochemistry and western blot showed up-regulation of eNOS, iNOS and Hsp70 protein. An eNOS inhibitor (Nω-nitro-L-arginine methyl ester (L-NAME)), or an iNOS inhibitor (aminoguanidine (AG)), significantly aggravated the liver injury. On the contrary, administration of NO precursor, L-arginine (L-ARG), attenuated the liver injury. Hsp70 inhibitor quercetin reduced Hsp70, while aggravating the WBH-induced hepatic changes.
Conclusions: WBH induces increases in eNOS, iNOS and Hsp70 expression with increase in NO release. The deleterious effects of L-NAME and AG and the protective effects of L-ARG and Hsp70 inhibitor on the liver function and pathology suggest that NO and heat shock protein play a beneficial role in the WBH-induced hepatic injury.
Introduction
Heat stress or heat shock is a life-threatening disorder characterised by hyperpyrexia, multiple organ failure and neurological dysfunction Citation[1], Citation[2]. Redistribution of blood flow under heat stress condition leads to low perfusion and ischaemia in the splanchnic vascular beds. Subsequently, injury and increased microvascular permeability ensue in the mesenteric beds Citation[3], Citation[4]. The pathological changes induce release of endotoxin, nitric oxide (NO), proinflammatory cytokines, free radical and other mediators Citation[5–7]. We have reported that NO overproduction may be detrimental in the hepatic injury caused by ischaemia reperfusion Citation[8].
Controversy exists with respect to the role of NO in liver injury. It appears that NO plays paradoxical or dual effects on the hepatic injury due to various causes, depending on the experimental conditions, amount of NO production and NO synthase isoforms Citation[9–11]. In mice subjected to whole body hyperthermia (WBH), increase and decrease in NO production by pre-treatment with L-arginine and Nω-nitro-L-arginine methyl ester (L-NAME) affected the survival rate depending on the doses and plasma level of NO metabolites Citation[12]. Administration of L-NAME after heat stress did not affect the core temperature and systemic hypotension Citation[13].
On the other hand, the formation of peroxynitrite as a consequence of NO combined with superoxide anion is detrimental to cells and organs Citation[14–16]. The presence of abundant nitrotyrosine in liver tissue may contribute to the hepatic injury induced by whole body hyperthermia. An in vitro study has indicated the NO increases hydrogen peroxide toxicity against rat liver endothelial cells and hepatocytes through inhibition of hydrogen peroxide degradation Citation[17].
The present study was designed to test whether heat stress-induced eNOS and iNOS mRNA and protein expression in liver tissue lead to increased NO production. In addition, the effects of eNOS and iNOS inhibitors Nω-nitro-L-arginine methyl ester (L-NAME) and aminoguanine (AG) and NO precursor L-arginine (L-ARG) on hyperthermia-induced liver injury were evaluated in order to elucidate the beneficial or detrimental role of NO in this type of liver injury. Hsp70 mRNA and protein expression were also examined to determine the role of heat shock protein in the hyperthermia-induced hepatic pathology. We used a Hsp70 inhibitor, quercetin to define the role of HSP.
Materials and methods
Preparation of animals
Male Sprague-Dawley rats (300 to 350 g, 12–16 wks old, pathogen-free) were purchased from the National Animal Centre. They were housed in a controlled environment at a temperature of 22° ± 1°C under a 12 h/12 h light/dark cycle. Food and water were available ad libitum. Care and use of the animals followed the National Animal Center guidelines. Rats were fasted overnight prior to the operation but had access to water. Conscious rats state were exposed to hyperthermia. They were immersed in a water bath with their heads above the water. The temperature of the water was maintained at 41°C, and the rat remained in the 41°C water for 1 h. The rats were then taken out of the water bath and allowed to recover for 15 h. Blood was collected at 15 h after hyperthermia challenge and plasma samples were analysed to assess levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), methyl guanidine (MG) and NO metabolites (nitrate/nitrite).
Quantification of liver injury by measuring AST and ALT activities in plasma
Blood samples were immediately centrifuged. Plasma levels of AST and ALT were measured using a Kodak Ektachem DT60 analyser (Rochester, NY, USA) and expressed in IU/L Citation[8].
Measurement of methyl guanidine by spectrofluorometry
Because the formation of MG is an index of hydroxyl radical production in the blood Citation[18], we measured MG levels as a reflection of hyperthermia-induced hydroxyl radical production. A spectrofluorometer (Jusco 821-FP, Hachioji, Japan) was used, and fluorescence spectra were obtained with an emission maximum at 500 nm and excitation maximum at 395 nm. Blood samples were diluted 1:100 with distilled water. Liver tissue samples were homogenised by Kontes sonicator (KT 50, Ultrasonicator, Atlantic City, NJ, USA) with ice-cold phosphate buffer in a ratio of 1:10. Following 10 min of incubation at 4°C, cell debris was removed by centrifugation at 12,000 rpm for 20 min, and the supernatant was diluted by 1:40 to measure the MG. The assay was calibrated with authentic MG (Sigma M0377, St Louis, MO, USA).
Measurement of nitrate/nitrite by HPLC
Plasma levels of nitrate/nitrite were determined using a high-performance liquid chromatographic (HPLC) method. This method has a sensitivity of 30 pmol for both anions. As little as 0.05–0.1 mL of sample volume is required, and linearity is observed up to 60 nmol for each anion. At concentrations above 60 nmol, the detection curve was essentially linear. The non-linearity of the curve in pmol/L concentration may result in some variations in data collection. Before injection into the chromatographic system (ENO-20, Eicom Nox analyser, Kyoto, Japan), the samples were diluted and subjected to suitable clean-up procedures, and serum samples were deproteinised by ultrafiltration through membranes with a molecular mass cut-off of 3000. The samples were separated on a strong anion-exchange column (Spherisorb SAX, 250 × 4.6 mm internal diameter, 5 µm) and this separation was followed by two on-line post-column reactions. The first involved nitrate reduction to nitrite on a copper-plated cadmium-filled column. The second reaction involved a diazotisation-coupling reaction between nitrite and the Griess reagent (0.05% naphthylenediamine dihydrochloride plus 0.5% sulphanilamide in 5% phosphoric acid). The absorbance of the chromophore was read at 540 nm.
RNA isolation
Isolation of mRNA from liver tissues was performed using an mRNA isolation kit (QIAGEN RNeasy kits, Valencia, CA, USA). The mRNA isolated from each liver tissue sample was reverse-transcribed to cDNA following the manufacturer's recommended procedures. The integrity of mRNA was validated by RNA gel electrophoresis. The resolution of 18S and 28S RNA would indicate the quality of mRNA isolation.
Real-time PCR
PCR primers and TaqMan-MGB probes () were designed using Primer Express V.2.0 software (Applied Biosystems, Foster, CA, USA) based on the sequences from GenBank. TaqMan-MGB probes were labelled with 6-carboxy-fluorescein (FAM) as the reporter dye. Real-time PCR was performed in a two-step process: In the first step, sample RNA (100 ng) was reverse-transcribed with 50 ng random hexamers in a volume of 20 µL using 200 U of SuperScript III reverse transcriptase and 40 U of RNaseOUT recombinant RNase inhibitor (both from Invitrogen, Carlsbad, CA, USA). In the second step, real-time PCR was carried out in a MicroAmp Optical 96-well plate using TaqMan Master Mix (Applied Biosystems), with 5 µL cDNA in each well. PCR reactions were monitored in real time using the ABI PRISM 7000 Sequence Detector (Applied Biosystems, Foster, CA, USA). The thermal cycling conditions for real-time PCR were 1) 50°C for 2 min, 2) 95°C for 10 min, and 3) 40 cycles of melting (95°C, 15 s) and annealing/extension (60°C, 60 s). The relationship between the initial amount A of target present and the amount Xn of DNA produced after n PCR cycle can be expressed as Xn = A × (1 + E)n, where E is the amplification efficiency of one PCR step. Threshold cycle (Ct) indicates the fractional cycle number at which the amount of amplified target reaches a fixed threshold. The variation in gene expression of candidate genes A and B is shown by △Ct. The relative gene expression of target, normalised to an endogenous reference (18 s rRNA; supplied by Applied Biosystems) and relative to a calibrator, was determined by 2−△△Ct in various tissues. Less △Ct means higher target mRNA expression before amplification.
Table I. Sequence of PCR primers and TaqMan probes for putative NOS target genes.
Immunohistochemistry
Liver tissues were dissected 15 h after hyperthermia challenge for immunochemical analysis of the protein expression of eNOS and iNOS.
Liver tissues from hyperthermia-treated and sham-operated rats were fixed in tissue fix buffer, embedded in Super-Tek OCT compound (PS0001 and PS0002 Gene Research Laboratory, Taipei, Taiwan), and frozen in liquid nitrogen. Sections (5 -µm thickness) were cut on a cryostat (Leica CM1900), then thawed and mounted onto gelatin-coated slides. All 5 -µm frozen liver sections from the WBH and sham groups were used for immunohistochemical staining.
Liver sections were first incubated with blocking reagent, then with the appropriate dilution of primary antibody (mouse anti-rat eNOS or anti-rat iNOS monoclonal antibody at a titer of 1:50; Chemicon MAb, 13421, Temecular, CA, USA), and finally with an anti-mouse IgG-horseradish peroxidase (HRP) secondary antibody at a titre of 1:100. Sections were labelled and developed with HRP substrate solution and counterstained with a haematoxylin stain kit (PS003, Gene Research Laboratory, Taiwan).
To quantify immunohistochemical differences in rat liver sections without relying on subjective assessments, we used digital imaging and the Image-Pro Plus (Media Cybernetics, Silver Spring, MD, USA) microimaging package. Data were collected and analysed by the method described in the user guide in the counting, measuring and classifying sections. The overall fields from each section of each tissue were digitally captured with a high-resolution cooled CCD camera (ProgRes C14, Jenoptik Laser, Optik, System, Jena, Germany) and stored as 8-bit colour images. The immunostained tissue cells were automatically highlighted by Image-Pro Plus, and the area covered by immunohistochemically positive cells (with red colour) was scored as positive and divided by the total area.
Western blot analysis
Pieces of liver tissue (5 g) were washed twice with ice-cold phosphate-buffered saline and resuspended in 1 mL of ice-cold tissue lysis buffer (containing 15 mM NaCl, 100 µM ethylene diamine tetraacetic acid (EDTA), 100 µM ethylene glycol tetraacetic acid (EGTA), 0.1% Triton X-100, 250 µM sodium pyrophosphate, 100 µM β-glycerolphosphate, 100 µM Na3VO4, 0.1 µg/mL leupeptin and 1 mM phenylmethylsulfonyl fluoride in 2 mM Tris-HCl buffer, pH 7.5). After 5-min incubation on ice, tissues pieces were homogenised to liquid form and transferred to microcentrifuge tubes. Following 20 to 30 min incubation at 4°C, cell debris was removed by centrifugation at 12,000 rpm for 10 min, and the supernatant was used as cell lysate and stored at −80°C when necessary. An aliquot was used to determine protein concentration using the protein quantitation kit (Gene Research, Taiwan) with bovine serum albumin as standard. A total of 30 µg of each extract was separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis with a 10% polyacrylamide gel (SDS-PAGE) and transferred to PVDF membrane (0.2 -µm polyvinylidine fluoride, Amersham Life Science, Arlington Heights, IL, USA). Membranes were blocked in TTBS (10% non-fat milk, 0.1% Tween-20 in Tris buffer solution). Immunodetection of eNOS, iNOS and Hsp70 were done using Gene-RL western blot assay kit (Gene Research, Taiwan). Primary antibody was used at a 1:500 dilution at 4°C overnight. Blots were washed with TTBS and incubated with horseradish peroxidase- anti-IgG antibody for 45 min. Immunocomplexes were viewed by chemiluminescence using Gene-RL western blot assay kit (Gene Research, Taiwan) and Biomax film (Kodak, Rochester, NY). Relative expressions of eNOS, iNOS and Hsp70 were normalised by an internal standard of β-actin.
Liver histology
At 15 h after WBH challenge, the animal was euthanised by an overdose of pentobarbital. A lobe of liver was taken for histological examination. The tissues were immersed in a 10% formaldehyde fixative for 24 h. The liver lobe was then washed for 8 h with tap water to remove the formaldehyde. For light microscopy the liver tissues were dehydrated with graded alcohol (70, 80, 90, 95% or absolute alcohol, each concentration for 45 min) and put into xylene for 1 h and then embedded in paraffin at 60°C. A series of 5 -µm sections was cut and stained with haematoxylin and eosin (H&E). The histological changes were observed under an Axioplan microscope (Zeiss, Oberkochen, Germany). The liver injury was scored in a blind fashion. Each observer gave a grade from 0 to 6 according to the severity of hepatic changes. There were six pathologists and students. The score was blind to the observers and the average value was used for the hepatic injury.
Experimental design
The animals were randomly divided into five groups. In the WBH group (n = 12), rats were given saline only prior to induction of hyperthermia. In the L-NAME group (n = 12), rats received 5 mg/kg L-NAME (Sigma) by intraperitoneal injection 30 min prior to the induction of hyperthermia. In the AG group (n = 12), rats received 5 mg/kg AG (Sigma) by intraperitoneal injection 30 min before hyperthermia. In the L-ARG group (n = 12), rats received 2 mg/kg L-ARG (Sigma) by intraperitoneal injection 30 min before hyperthermia. The doses for NO inhibitors and precursor were based on the dose range described previously Citation[16], Citation[18], Citation[19]. Rats in the pre-WBH group (n = 12) were prepared in the same manner as the control group without hyperthermia challenge, but were exposed to 37°C water in a water bath.
In the second series of experiments, we tested the effects of quercetin (QUE) on the WBH liver injury. Quercetin is an agent of Hsp70 inhibitor or antisense. QUE was given intraperitoneally at a dose of 50 mg/kg before and after WBH. The drug was kindly provided by Y.L. Yang at Chiayi University, Taiwan. In the pre-WBH group (n = 12), saline was administered, and in the QUE group (n = 12), QUE was introduced. The experimental protocol basically followed those described previously Citation[20].
Data analysis
Data were expressed as means ± SE. Comparisons within and among groups were made using one-way analysis of variance with repeated measures followed by a post hoc comparison with the Newman–Keuls test. Values of p < 0.05 were considered statistically significant.
Results
Real-time PCR analysis revealed marked increases in the expression of eNOS (2.8 ± 0.4-fold increase), iNOS (3.1 ± 0.5-fold increase) and Hsp70 (3.2 ± 0.8-fold increase) in the WBH-challenged group compared with the pre-WBH group (*p < 0.05, ). Western blot analysis disclosed the increases in eNOS, iNOS and Hsp70 expression. The eNOS, iNOS and HSP expression was increased 2.1 ± 0.6, 2.3 ± 0.9, and 2.2 ± 0.7-fold for eNOS, iNOS and Hsp70, respectively (). Immunohistochemical examination of eNOS, iNOS and Hsp70 expression in liver tissue disclosed marked increases in iNOS and Hsp70 with a moderate increase in eNOS in the WBH group. There was essentially no immunostaining for eNOS, iNOS and Hsp70 in the pre-WBH group ().
Figure 1. A real-time PCR 2.8 ± 0.4, 3.1 ± 0.5 and 3.2 ± 0.8 analysis of eNOS, iNOS and Hsp70 expressions of the liver tissues from the pre-WBH and WBH groups (A). The increases in eNOS, iNOS and Hsp70 mRNA were 2.8 ± 0.4, 3.1 ± 0.5 and 3.2 ± 0.8-fold, respectively (B). There were 12 rats in each group, *p < 0.05, pre-WBH versus WBH. Pre-WBH and WBH denote before and 15 h after whole body hyperthermia. Glyceraldehyde phosphate dehydrogenase (GAPDH) serves as contrast.
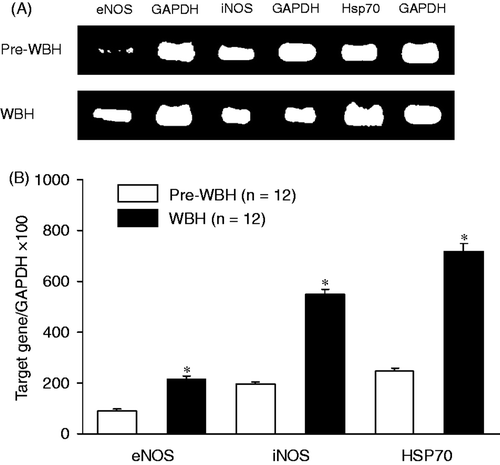
Figure 2. Western blot analysis of the eNOS, iNOS and Hsp70 protein expressions in liver tissues after WBH compared with pre-WBH group. The results indicate significant increases in eNOS, iNOS and Hsp70 expressions (*p < 0.05) in the WBH group. Representative western blot with an immunodetection of eNOS, iNOS and Hsp70 were done using Gene-RL western-blot assay kit (Gene Research, Taiwan). β-actin is an internal standard (n = 12 for each group). The abbreviations are the same as .
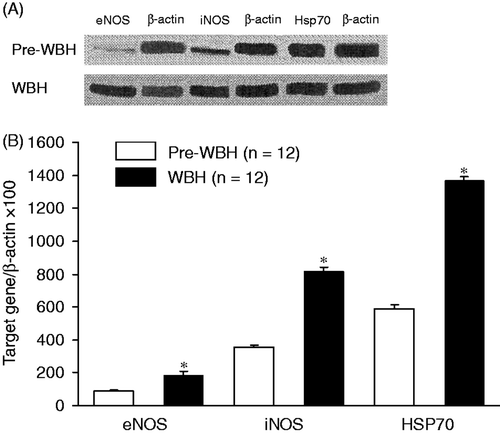
Figure 3. Immunohistochemical stain of eNOS (A), iNOS (B) and Hsp70 (C) in pre-WBH and WBH groups. The illustrative micrographs show changes in eNOS, iNOS and Hsp70 activities (arrows) after whole body hyperthermia. The enhancement was 3.1 ± 0.9-fold for eNOS, 10.6 ± 2.6-fold for iNOS, and 6.5 ± 1.4-fold for Hsp70 (*p < 0.05) (D).
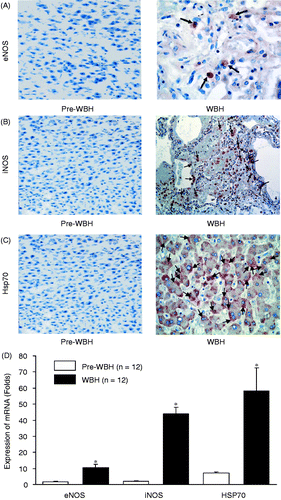
showed that nitrate/nitrite, the NO metabolists increased significantly after hyperthermia (***p < 0.001, significantly different between WBH and pre-WBH group). Inhibitors of eNOS and iNOS (L-NAME and AG) significantly attenuated the hyperthermia-induced NO release. NO precursor (L-arginine) increased the NO release (*p < 0.05, WBH versus pre-WBH). The changes of plasma methyl guanidine (representing hydroxyl radical) were similar to those for nitrite/nitrate. NOS inhibitors potentiated hydroxyl radical release, while NO precursor attenuated the hydroxyl radical production (*p < 0.05, WBH versus pre-WBH, ).
Figure 4. High-performance liquid chromatographic method was used to measure the changes in nitrate/nitrite, the metabolites of nitric oxide, in the pre-WBH and WBH-challenged groups. To evaluate the effects of nitric oxide synthase inhibition, L-NAME and AG were used. Treatment of nitric oxide precursor L-arginine (L-ARG) was also tested. (*p < 0.05, significantly different between pre-WBH and WBH-challenged groups; *p < 0.05, significantly different between pre-WBH versus WBH group with and without nitric oxide inhibitors and precursor, n = 12 for each group). A spectrofluorometer was used to measure methyl guanidine, which represents formation of hydroxyl radicals. The results were similar to those for nitrate/nitrite (*p < 0.05, significantly different between pre-WBH and WBH-challenged groups; *p < 0.01, significantly different between pre-WBH and WBH group with and without nitric oxide inhibitors and precursor, n = 12 for each group).
showed that there were significant increases in plasma AST and ALT after exposure to hyperthermia (***p < 0.001). Administration of eNOS and iNOS inhibitors (L-NAME and AG) significantly increased the levels of AST and ALT. In contrast, administration of NO precursor, L-ARG reduced the levels of AST and ALT (*p < 0.05, WBH versus pre-WBH). Quantitation of the liver injury score indicated that WBH induced hepatic injury. L-NAME, AG and L-ARG did affect the liver injury score in control rats without WBH (pre-WBH or sham group). On the contrary, L-NANE, AG and QUE aggravated the WBH-induced hepatic injury, while L-ARG reduced the liver injury score ().
Figure 5. Plasma levels of aspartate aminotransferase (A, AST) and alanine aminotransferase (B, ALT) were measured before and 15 h after exposure to whole body hyperthermia. Significant increase in plasma AST and ALT after exposure to hyperthermia (*p < 0.05) compared with the pre-WBH group. Administration of eNOS and iNOS inhibitors (L-NAME and AG) significantly increased the AST and ALT (*p < 0.001). However, NO precursor, L-ARG attenuated the increase in AST and ALT (*p < 0.05, n = 12 for each group).
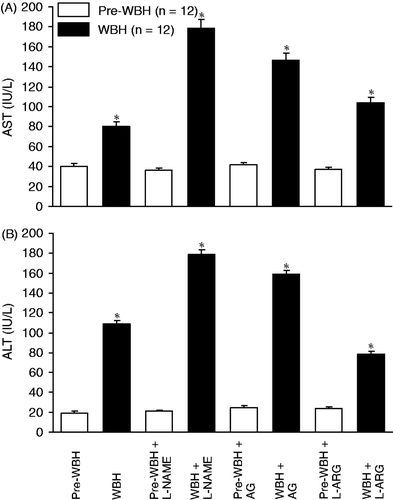
Table II. The liver injury score. The values are means ± SEM (n = 12 in each group).
Western blot analysis of Hsp70 activity revealed that WBH increased the Hsp70 (*p < 0.05). Administration of Hsp70 inhibitor, quercetin significantly enhanced the WBH-induced liver injury. This agent also attenuated the WBH-induced increase in Hsp70 activity (). Plasma nitrate/nitrite, methyl guanidine, AST, ALT, the hepatic pathological change and white cell infiltration were greatly elevated (). The results strongly support the protective effects of Hsp70 in the hyperthermia hepatic injury.
Figure 6. Western blots of Hsp70 in pre-WBH, WBH and WBH with quercetin (QUE). WBH enhanced the Hsp70 activity (*p < 0.05). Quercetin reduced the Hsp70 activity after WBH (+p < 0.05).
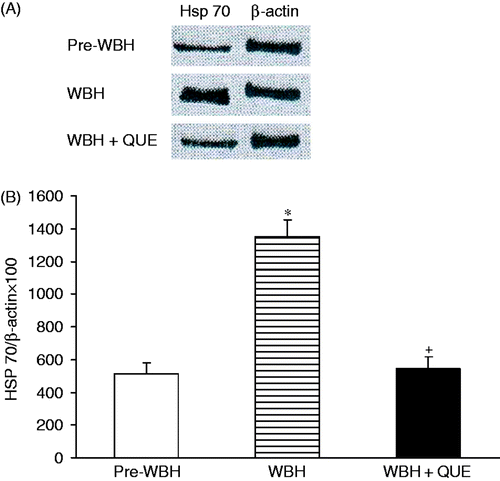
Table III. The plasma NOx (nitrate/nitrite), MG (methyl guanidine), aspartate aminotransferase (AST), alanine aminotransferase (ALT), liver injury score (LIS) and white cell infiltration (WBC) in the sham (pre-WBH) and quercetin (QUE) groups (n = 12 in each group).
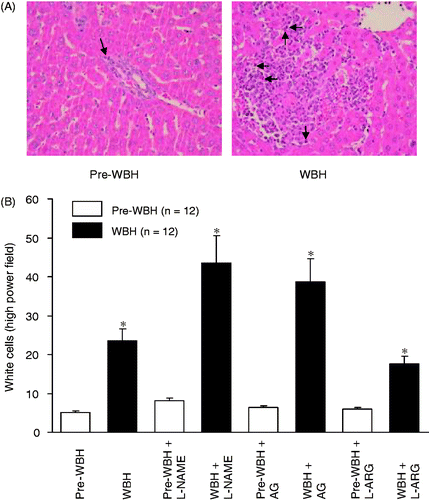
showed the WBH-induced pathological changes in the liver (upper panel). Histological examination revealed that WBH produced hepatocytes degradation and necrosis with increase of inflammatory cells sequestration in the liver tissue. In the pre-WBH group, little pathological lesion was observed. Comparison of the number of inflammatory cell sequestrations in the liver tissue under microscope by 10 high power fields showed significant increase of white cell sequestration in the liver of WBH group (21.3 ± 1.8 versus 3.0 ± 0.6, p < 0.05). Treatment with L-NAME and AG increased the number of WBC sequestration in the liver. Administration of L-ARG decreased the increase of white cells (, lower panel).
Figure 7. A section of liver tissues 15 h after WBH challenge. Inflammation was evidenced by inflammatory cell infiltration with hepatocytes degradation and necrosis (A, arrows). In the pre-WBH group, there were essentially no hepatocyte degradations or necrosis. Panel B shows the white cell count in liver histology under high power field. WBH challenge induced significant white cell sequestration in liver tissues (*p < 0.001). NOS inhibitors aggravated the white cells sequestration (*p < 0.05). However, NO precursor attenuated the cell sequestration (*p < 0.05, n = 12 for each group).
Discussion
In the present study, rats in a conscious state were subjected to whole body hyperthermia. Examinations taken 15 h after WBH revealed a significant up-regulation of inducible NO synthase (iNOS) with a significant albeit slight increase in endothelial NO synthase (eNOS) by real-time polymerase chain reaction and immunobiochemical staining. Western blot analysis disclosed that WBH significantly elevated the protein of iNOS, eNOS and Hsp70 in liver tissue. WBH significantly increased the plasma nitrate/nitrite, methyl guanidine (MG), alanine aminotransferase (ALT) and aspartate aminotransferase (AST). These biochemical changes were aggravated by pretreatment with L-NAME and AG, while attenuated by L-ARG. Histopathological examination observed liver cell injury with sequestration of white blood cells. The hepatic pathology with inflammatory cell sequestration was enhanced by L-NAME and AG pretreatment, whereas reduced by L-ARG. Our results suggest that NO, NO synthases and Hsp70 are involved in the WBH hepatic injury. It appears that NO plays a beneficial role.
It has been a debate with respect to the role of NO in the liver injury induced by WBH. Hyperthermia may stimulate xanthine oxidase production of reactive species that activate metals and limit heat tolerance. Overproduction of NO may contribute to splanchnic dilatation leading to circulatory and intestinal barrier dysfunction Citation[3]. On the other hand, many studies have shown that WBH results in increased NO production Citation[12], Citation[13], Citation[15], Citation[16]. In support of the contention that NO exerts therapeutic effects on the WBH hepatic injury, Chatterjee and co-workers have reported that post-treatment with L-arginine could rescue the mice from heat-induced death and reduce the hyperthermia. L-arginine also abrogates the WBH-induced aptosis, mRNA iNOS expression, NO level and inflammatory cytokines Citation[19], Citation[21].
The present study has shown the benefical effects of NO on the WBH-induced hepatic injury. The protective effects of NO may be attributed to its vasodilatory effect that reduces the splanchnic low perfusion and improves organ ischaemia. Other mechanisms involved in the beneficial effects of NO may be possible. WBH might enhance the iNOS gene transcription mediated through NF-κB Citation[19], Citation[22], Citation[23]. Our and other laboratories have demonstrated the detrimental role of NO through the iNOS isoform in the lung injury caused by various causes Citation[24–26]. Further investigations are required to elucidate the mechanisms by which NO and NO isoforms exert different effects on the organ injury. WBH induces a 6.4-fold increase in heat shock protein expression in liver tissue, a similar observation in our previous study Citation[16], Citation[25]. HSPs have been considered to be beneficial to heat-induced organ injury. Yang et al. found that induction of Hsp72 with arenite reduced the cerebral ischaemia, neural damage, and systemic hypotension, and increased the survival rate in rats exported to heat stress Citation[23]. Hsp70 also exerted protective effects on the hepatic injury caused by ischaemia-reperfusion in mice Citation[23], Citation[26], Citation[27] and acted to stimulate the immunological system under conditions of oxidative stress. In HSP knockout mice, the hepatic toxicity of acetaminophen was enhanced Citation[28]. Furthermore, induction of heat shock proteins, inhibition of NF-kB, and proinflammatory cytokines with reduction in liver injury and mortality in rat following endotoxaemia Citation[27–31]. In the present study we used quercetin, an inhibitor of Hsp70. The findings that inhibition of HSP enhanced the WBH liver damage support the contention that HSP exerts a protective role in this model of hyperthermia. These studies have provided evidence for the protective role of heat shock proteins in organ injury induced by different challenges. In a previous study using alteration in the perfusion, we found that the major site of NO production through the iNOS system was from the lung Citation[32]. Whether WBH-hepatic injury is induced by NO generation in the liver remains a subject to be determined.
Conclusions
In summary, the present study has demonstrated that WBH increases NO production, iNOS, eNOS and Hsp70 expression. It elevates the plasma nitrate/nitrite, methyl guanidine, AST and ALI. The WBH-hepatic injury is associated with white cell sequestration. These biochemical and pathological changes are exacerbated by NOS inhibitors with L-NAME and AG, while attenuated by L-arginine. The results suggest that NO production through the iNOS isoform is protective to this type of organ injury. A Hsp70 inhibitor, quercetin aggravated the WBH liver injury, indicating the beneficial role of HSP.
Acknowledgements
The authors appreciate the kind supply of quercetin and technical assistance in the real-time PCR analysis from Y.L. Yang at Chiayi University, Taiwan. We are also grateful to A. Huang and W.H. Wang for the editing of this manuscript.
Declaration of interest: This study was supported in part by a grant from the National Science Council (NSC 99-2320-B-320-010-MY3). The authors alone are responsible for the content and writing of the paper.
References
- Alzeer AH, Arifi AI, Warsy AS, Ansari Z, Zhang H, Vincent JL. Nitric oxide production is enhanced in patients with heat stroke. Intensive Care Med 1999; 25: 58–62
- Bouchama A, Konchel JP. Heat stroke. N Engl J Med 2002; 346: 1978–1988
- Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechansims of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circ Physiol 2001; 280: H509–H521
- Lambert GP, Gisolfi CV, Berg DJ, Moseley PL, Oberley LW, Kregel KC. Selected contribution: Hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J Appl Physiol 2002; 92: 1750–1761
- Arnaud C, Joyeux M, Garrel C, Godin-Ribuot D, Demenge P, Ribuot C. Free-radical production triggered by hyperthermia contributes to heat stress-induced cardioprotection in isolated rat hearts. Br J Pharmacol 2002; 135: 1776–1782
- Lu KC, Wang JY, Lin SH, Chu P, Lin YF. Role of circulating cytokines and chemokines in exertional heatstroke. Crit Care Med 2004; 32: 399–403
- Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol 1998; 508: 949–953
- Chen CF, Wang D, Hwang CP, Liu HW, Wei J, Chen HI. The protective effect of niacinamide on ischemia-reperfusion-induced liver injury. J Biomed Sci 2001; 8: 446–452
- Hsu CM, Wang JS, Liu CH, Chen LW. Kupffer cells protect liver from ischemia-reperfusion injury by an inducible nitric oxide synthase-dependent mechanism. Shock 2002; 17: 280–285
- Kimura H, Katsuramaki T, Iobe M, Nagayama M, Meguro M, Kukita K, et al. Role of inducible nitric oxide synthase in pig liver transplantation. J Surg Res 2003; 111: 28–37
- Lee VG, Johnson ML, Baust J, Laubach VE, Watkins SC, Billiar TR. The roles of iNOS in liver ischemia-reperfusion injury. Shock 2001; 16: 355–360
- Poduval TB, Chatterjee S, Sainis KB. Effect of nitric oxide on mortality of mice after whole body hyperthermia. Int J Hyperthermia 2003; 19: 35–44
- Ryan KL, Inhrany MR, Jauchem JR. Nitric oxide does not contribute to the hypotension of heatstroke. J Appl Physiol 2001; 90: 961–970
- Szabó C, Cuzzocrea S, Zingarelli B, O'Connor M, Salzman AL. Endothelial dysfunction in a rat model of endotoxic shock. Importance of the activation of poly (ADP-ribose) synthetase by peroxynitrite. J Clin Invest 1997; 100: 723–735
- Grogan H, Hopkins PM. Heat stroke: Implications for critical care and anaesthesia. Br J Anaesth 2002; 88: 700–707
- Lee JF, Wang D, Hsu YH, Chen HI. Oxidase and nitrosative mediators in hepatic injury caused by whole body hyperthermia in rats. Chin J Physiol 2008; 51: 85–93
- Rauen U, Li T, Ioannidis I, de Groot H. Nitric oxide increases toxicity of hydrogen peroxide against rat liver endothelial cells and hepatocytes by inhibition of hydrogen peroxide degradation. Am J Physiol 2007; 292: C1440–C1449
- Nakamura K, Ienaga K, Yokozawa T, Fujitsuka N, Oura H. Production of methylguanidine from creatinine via creatol by active oxygen species: Analyses of the catabolism in vitro. Nephron 1991; 58: 42–46
- Chatterjee S, Premachandran S, Sharma D, Bagewadikar RS, Podural TB. Therapeutic treatment with L-arginine rescues mice from heat stroke-induced death: Physiological and molecular mechanisms. Shock 2005; 24: 341–347
- Carlstrom J, Symons JD, Wu TC, Bruno RS, Litwin SE, Jalili T. A quercetin supplemented diet does not prevent cardiovascular complications in spontaneously hypertensive rats. J Nutr 2007; 137: 628–633
- Chatterjee S, Premachandran S, Bagewadikar RS, Bhattacharga S, Chattopadhyay S, Poduval TB. Arginine metabolic pathways determine its therapeutic benefit in experitmental heatstroke: Role of Th1/Th2 cytokine balance. Nitric Oxide 2006; 15: 408–416
- Remick DG, Colletti LM, Scales WA, McCurry KR, Jr, Campbell DA. Cytokines and extrahepatic sequelae of ischemia-reperfusion injury to the liver. Ann N Y Acad Sci 1994; 723: 271–283
- Yang YL, Lu KT, Tsay HJ, Lin CH, Lin MT. Heat shock protein expression protects against death following exposure to heatstroke in rats. Neurosci Lett 1998; 252: 9–12
- Chen HI, Chung HR, Wu CY, Kao SJ, Wang D, Hsieh NK, et al. Nitric oxide in the cardiovascular and pulmonary circulation – A brief review of literatures and historical landmarks. Chin J Physiol 2007; 50: 43–50
- Chen HI, Yeh DY, Kao SJ. The detrimental role of inducible nitric oxide synthase in the pulmonary edema caused by hypercalcemia in conscious rats and isolated lungs. J Biomed Sci 2008; 15: 227–238
- Yang YL, Huang KL, Liou HL, Chen HI. The involvement of nitric oxide, nitric oxide synthase, neutrophil elastase, myeloperoxidase and proinflammatory cytokines in the acute lung injury caused by phorbol myristate acetate. J Biomed Sci 2008; 15: 499–507
- Kuboki S, Schuster R, Blanchard J, Pritts TA, Wong H, Lentsch AB. Role of heat shock protein 70 in hepatic ischemia/reperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol 2007; 292: G1141–G1149
- Tolson JK, Dix DJ, Voellmy RW, Robert SM. Increased hepatoxicity of acetaminophen in Hsp70 knockout mice. Toxicol Appl Pharmacol 2006; 210: 157–162
- Menoret A, Chaillot D, Callahan M, Jacuquin C. Hsp70, an immunological actor playing with the intracellular self under oxidative stress. Int J Hyperthermia 2002; 18: 490–505
- Chen D, Pan J, Du B, Sun D. Induction for the heat shock response in vivo inhibits NF-kappaB activity and protects murine liver from endotoxemic injury. J Clin Immunol 2005; 25: 452–461
- Multhoff G. Hyperthermia classic commentary: Activation of natural killer (NK) cells by heat shock protein 70, Gabriele Multhoff. Int J Hyperthermia, 2002; 18: 576–585, Int J Hyperthermia 2009;25:176–179
- Lee RP, Wang D, Kao SJ, Chen HI. The lung is the major site that produces nitric oxide to induce acute pulmonary oedema in endotoxin shock. Clin Exp Pharmacol Physiol 2001; 28: 315–320
